Diabetes mellitus (DM) is a metabolic disorder that causes chronic hyperglycemia with disturbances in the metabolism of carbohydrates, fats and proteins, and alterations in microcirculation. We evaluate the criteria for the interpretation of the oral glucose tolerance test (OGTT) in the Hospital Nacional Docente Madre-Niño “San Bartolomé” in Lima, Peru, and determine the percentages of pre-diabetic patients, diabetics that should not be included in the test, and those with alterations in their glucose curve during the biochemical determinations.
To this purpose, a non-experimental, prospective cross-sectional analytic study was performed in 1271 patients, included in the study to comply with the guidelines and recommendations of the ADA and the CLSI POCT12-A3 guide, which were processed in the Biochemical Autoanalyzer Biosystems A25. Data analysis was performed using SPSS version 20.0 statistical analyzer, and respecting ethical diabetics.
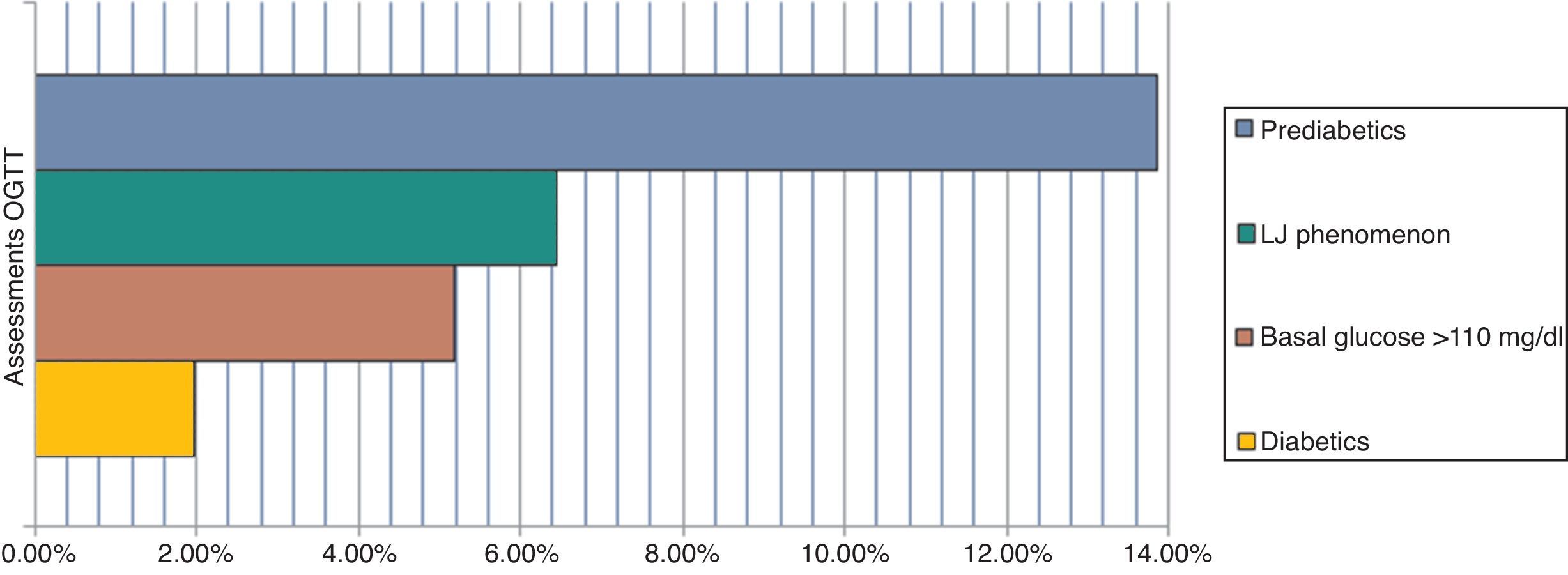
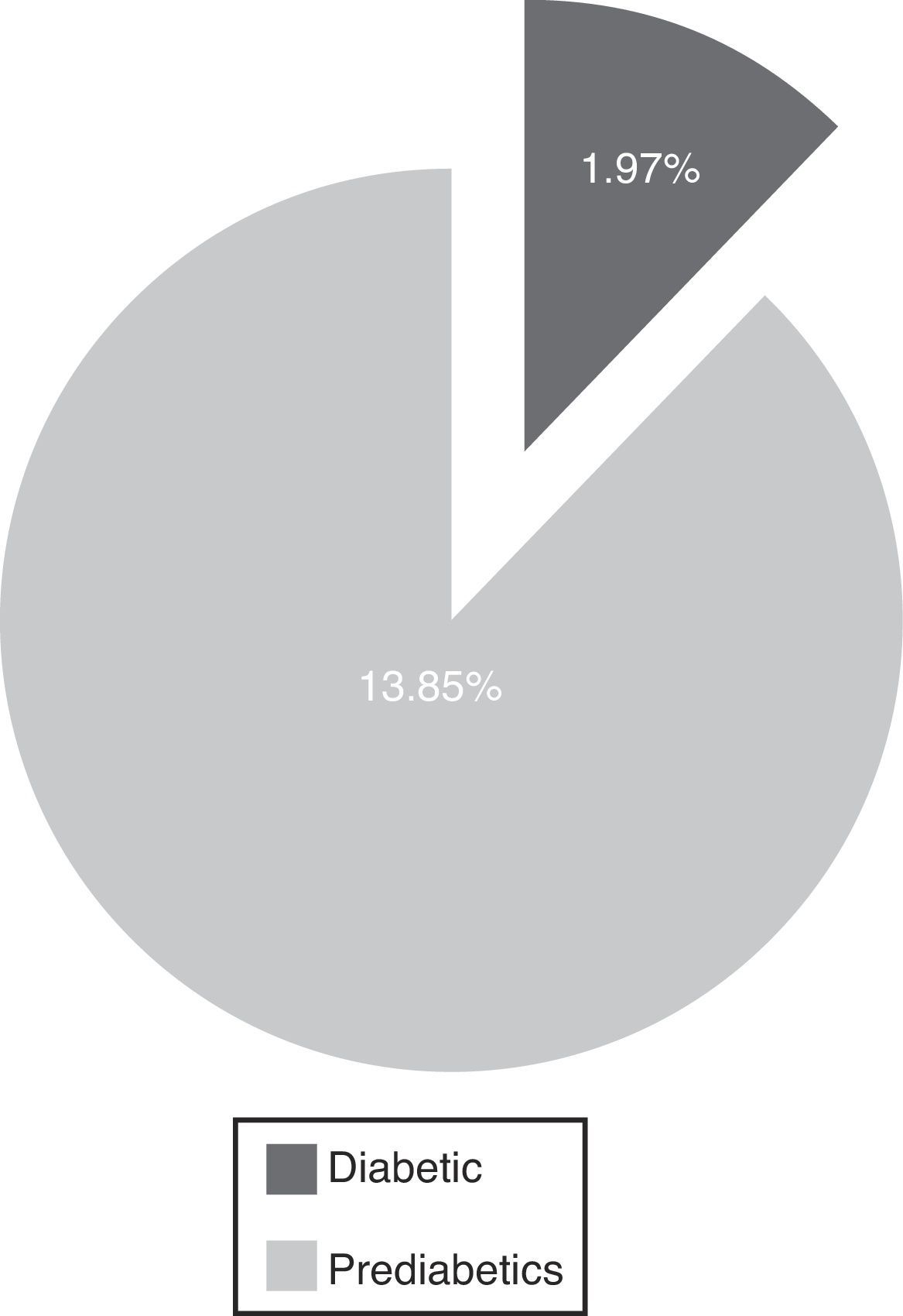
The main results are 1.97% diabetic and 13.85% pre-diabetic. The LJ phenomenon occurred with 6.45% (alteration in glucose curve after oral load decreases plasma glucose concentration determination after 60min and returns at relatively higher than the basal concentration levels at 180min) and 5.19% were poorly studied, including a >110mg/dl baseline (p=<0.05). We determined a high rate of pre-diabetic patients and a reduced rate of diabetes coincident with Qiao et al. We highlight the usefulness of the OGTT, which must be rigorously evaluated, and reaffirm the importance of the LJ phenomenon as it may interfere with the final results of unsolved events, and evidence the importance of interindividual biological variability.
Diabetes mellitus (DM) is a metabolic disorder that is a consequence of the deficiency in the secretion of insulin, in the effectiveness of its actions, or both. It is one of the most prevalent chronic diseases in the world. It constitutes a public health problem due to the progressive increase of its incidence, to the point where it is considered an epidemic.1 Chronic hyperglycemia with disturbances in the metabolism of carbohydrates, fats and proteins, and alterations in microcirculation are all consequences of this disorder. Current DM classification according to the American Diabetes Association – ADA – is shown in Table 1.
Classification of DM.9
| I. Type 1 diabetes |
| A. Immune-mediated |
| B. Idiopathic |
| II. Type 2 diabetes |
| III. Other specific types |
| A. Genetic defects of β-cell function |
| B. Genetic defects in insulin action |
| C. Diseases of the exocrine pancreas |
| D. Endocrinopathies |
| E. Drug- or chemical-induced |
| F. Infections |
| G. Uncommon forms of immune-mediated diabetes |
| H. Other genetic syndromes sometimes associated with diabetes |
| IV. GDM |
About 63% of the 57 million deaths in the world in 2008 were caused by a non-transmissible chronic disease, including diabetes mellitus; thus, 80% of these deaths occurred in low and medium-income countries.2 By the year 2000, about 171 million people suffered from DM around the world, and this number is estimated to go up to 336 million by the year 2030.3,4 On the other hand, approximately 197 million people worldwide suffer from glucose intolerance – pre-diabetic patients, which usually leads to obesity. Around 90% of type II diabetes is attributable to overweightness and to the metabolic syndrome. This number is also expected to increase to 420 million by the year 2025.5 In addition, the human and financial costs of DM are also on the rise.6
A similar situation occurs in Peru, where TIIDM prevalence (TIIDM) ranges from 1 to 8%, Lima and Piura being the most affected regions.7 In conclusion, DM is a health problem around the world, one that is threatening to reach pandemic levels by 2030, with alarming increases of TIIDM among children and with potentially devastating consequences.5
DM is associated with an increase in risk of premature death; thus, every year almost 4 million deaths are caused directly by this disease. 80% of these occur in underdeveloped countries, constituting 6.8% of overall mortality.8
DM diagnostic methods are: random glucose test >200mg/dl over clinical symptoms, fasting plasma glucose test >126mg/dl, oral glucose tolerance test (OGTT) after a fast, and glycosylated hemoglobin test A1c≥6.5% (Table 2).9 The test most frequently used is the OGTT, used in clinical practice for glycemic and insulin diagnoses.10–12 Moreover, it is the most sensitive method for the diagnosis of DM within the fasting glycemic test, although it requires characteristics for its implementation, such as fasting basal glycemia<110mg/dl, doses of 75g anhydrous glucose in adults or 1.75g/kg for children, performance in the morning after a 10–16h fast, the duration of the test is 180min, etc.13 One of the main disadvantages of OGTT is its reproducibility, thus it is recommended to have at least 2 pathological OGTTs when basal glycemia is under 110mg/dl.14
Diagnostic criteria for DM.9
| 1. AlC≥6.5%. The test should be performed in a laboratory using a method that is NGSP certified and standardized to the DCCT assay.a |
| OR |
| 2. FPG≥126mg/dl (7.0mmol/l). Fasting is defined as no caloric intake for at least 8h.a |
| OR |
| 3. 2-h plasma glucose≥200mg/dl (11.1mmol/l) during an OGTT. The test should be performed as described by the World Health Organization, using a glucose load containing the equivalent of 75g anhydrous glucose dissolved in water.a |
| OR |
| 4. In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose≥200mg/dl (11.1mmol/l). |
Moreover, inter-individual variabilities have been described according to glucose metabolism, which generate slants in the interpretation of clinical criteria that ought to be estimated. According to Clinical Laboratory Standards Institute (CLSI), the resultant parameters of OGTT should be assessed as the area under the curve of glucose (AUCG) and insulin (AUCI) basal indexes, the maximum allowable error, among others, mainly to determine the sensitivity to peripheral insulin.9,15–18 After an oral glycemia overload, the increase in glycemia does not depend solely on glucose. There are also intestinal hormones involved, the speed of gastric emptying and the composition of the intake. Thus, tolerance to glucose and sensitivity to insulin are different concepts, making the indexes of correction and evaluation of glycemic sensitivity necessary to guarantee the quality of the results and explain the recurrence of these phenomena.18
Having said that, the objective of this study was to evaluate the criteria for interpretation of OGTT in the Hospital Nacional Docente Madre-Niño “San Bartolomé” (HONADOMANI SB) in Lima, Peru, and determine the percentages of pre-diabetic patients, those who should not have been included within the test (basal glucose>110mg/dl) and those who presented alterations in the curve of glucose during biochemical determinations.
Materials and methodA non-experimental, prospective cross-sectional analytic study was conducted.
Population and samplePopulationThe population consisted of all ambulatory patients referred to us by agreement, at HONADOMANI SB.
SampleBlood samples referred from outpatient clinics independently from the Department of Help to diagnosis at the Biochemistry Department for glycemia determination though OGTT, which complies with the quality criteria according to CLSI Guide POCT12-A3, the ADA's guidelines and the recommendations for the laboratory analysis of DM diagnosis and management and the standardized operational procedures – SOP – of the health center.19,20 These are selected respecting the following previously established criteria of inclusion and exclusion.
Inclusion criteriaPatients (male and female) in a fasting state of at least 10h, ages ranging from 18 to 65 years of age, stable, without a stress condition, pharmaceutical consumption, or debilitating situations like surgery. Also, patients were required to complete the test (180min, 3 blood collections) post-glycemic overload. The collection of samples must comply with the quality guidelines previously mentioned.
Exclusion criteriaPatients who did not comply with the fasting state of at least 10h. Patients outside the age range (19–65 years old). Patients who were suffering from a debilitating or oncological situation, or undergoing pharmacological therapies. Those patients who left the test or complicated blood collections. Those samples which were collected without following the quality and normativity criteria of CLSI or ADA, in addition to samples which were clearly contaminated.
Data collection techniques and sample processingPre-analytic stageThe collection was conducted in the Phlebotomy area of the HONADOMANI SB between 6am and 9am, using BD Vacutainer® (Franklin Lakes, New Jersey, USA) of 3ml, with a red cap which was mixed by inversion of 8–10 times, according to the CLSI H03-A6 guide. In order to achieve this, patients must have remained fasting for at least 10h prior to the collection, with a payment slip from the previous day of the test, and with a lemon and a disposable cup.21
The first sample (basal) was used to evaluate the admission of patients to the test, excluding those who were above 100mg/dl. Subsequently, they were overloaded with oral glucose (anhydride preparation: “lemonade”) using the following formula; 75g of anhydride glucose for adults or 1.75g/kg for children. The patient was monitored for the whole duration of the test (180min). The samples were collected at 60 and 180min after the glycemic overload. Conventional cohort values were used at >200mg/dl for diabetics and those indicated by the World Health Organization (WHO) for the diagnosis of pre-diabetic patients of 100–199mg/dl (impaired glucose tolerance).22–24
Analytic stageThe processing in the clinical lab was conducted following the algorithm established by the hospital for OGTT. This includes the reception and registration of the sample through the standardized code and its processing within 120min after the sample was drawn. The biochemical processing was conducted through the Biochemical Biosystems A25 auto-analyzer (Pennsylvania, USA), which has a daily and historic registry of biochemical analyses. The method employed for the determination of glucose was glucose oxidase-peroxidase through the glucose activity test Trinder.25
Post-analytic stageUndetermined results or those which were outside the linearity of the trial were repeated and/or diluted. The results validated by Medical Technologists were inputted into the integrated health system – SIGOS – to inform patients within the stipulated times.
Data analysis techniqueData analysis was performed in three basic processes: codification, from the Biochemical Biosystems A25 auto-analyzer's historical registries system; tabulation, statistical verification and the creation of charts and tables using the statistical analyzer SPSS 20.0 and Microsoft Office Excel 2010 for Windows. The evaluation of the distribution of variables was conducted using KMO and Bartlett's test of sphericity, resulting in a matrix of correlations between adequate variables (p=<0.05).
EthicsWithin the diabetic ethics framework, the safeguarding, reliability and irreplaceable value of the obtained information will only be used for the purposes of this study. This research has the approval of the Department of Teaching and Aid for Research and the Ethics and Research Commission of the HONADOMANI SB (Reg. 78-2015).
LimitationsSeveral limitations should be taking into account before interpreting the results.
First, the biochemical auto-analyzer informatics registry does not have detailed registries or information of the selected patients, such as age, gender, attributable risk factors, physiological condition, diseases, demographic characteristics and family history, etc. Second, the determination of glycemia varies considerably between demographics and age groups. Thus, the pre-diabetic and diabetic prevalence rates encountered are not transposed toward the general population of Peru. Third, the OGTT results could not be compared to the standard reference tests like Hb1Ac, to determine its variability and inaccuracy.26 Lastly, the biochemical processing was performed under an internal and external quality control; however, without a quality responsible planning.
Despite these limitations, our research is the first to evaluate the resultant parameters of the OGTT.
ResultsFrom the conducted research, 1.97% of patients were diabetic, 13.85% were pre-diabetic, 6.45% produced the “LowJump” (LJ) glucose reduction-rise phenomenon (alteration of the glucose curve post-oral overload which tends to reduce the concentration of plasma glucose in its determination at 60min and returns to relatively superior levels to the basal concentration at 180min), and 5.19% of the patients should not have been included in the study due to the fact that they did not have over 110mg/dl of basal glucose (p=<0.05) (Fig. 1).11,27
DiscussionThe evaluation of OGTT interpretation criteria exposes an elevated rate of pre-diabetic patients and a reduced rate of diabetics, which in conjunction constitute 15.8% of the prevalence (Fig. 2).
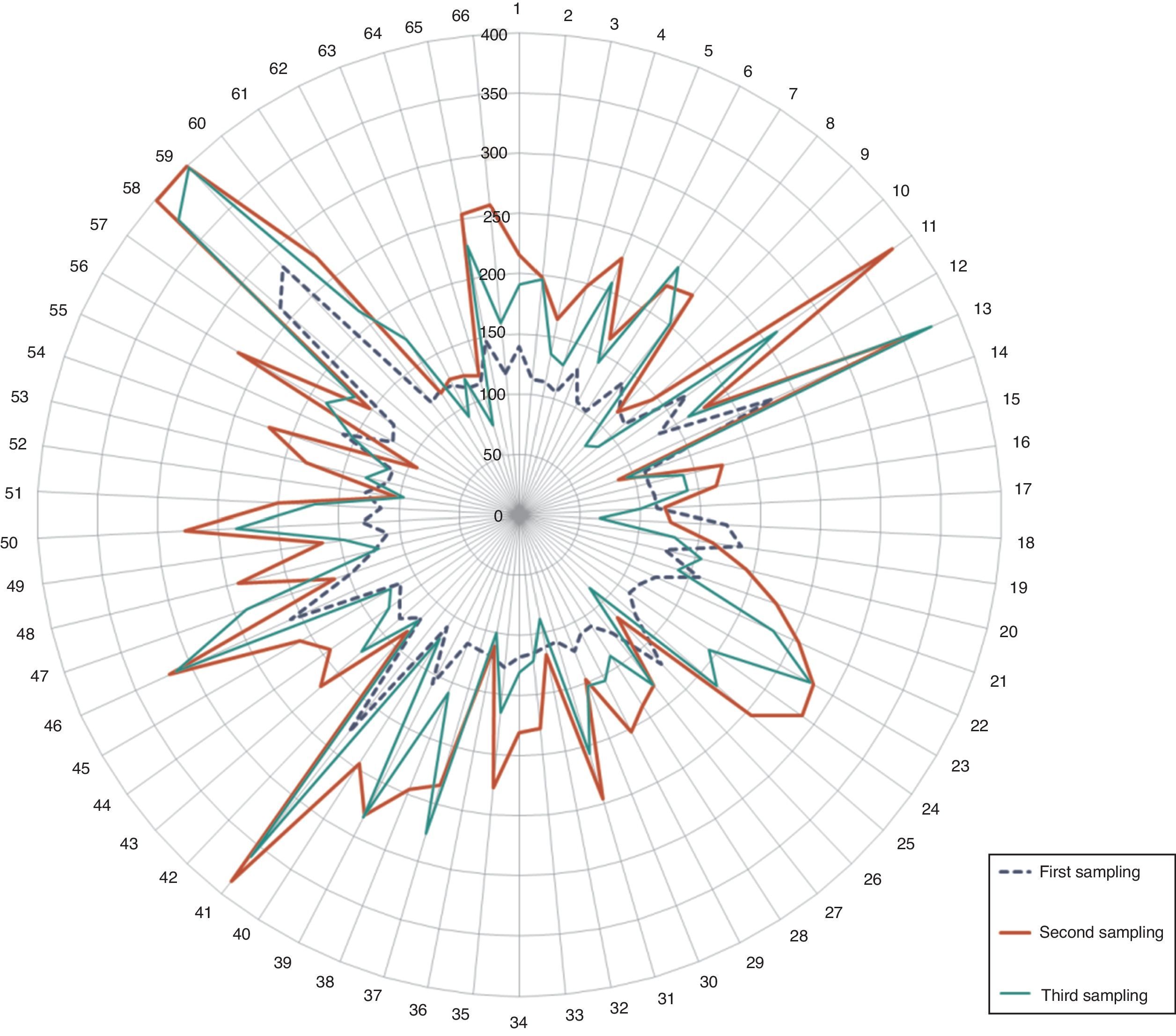
Percentages of pre-diabetic and diabetic patients. The radial distribution of patients wrongly included in the test is shown in Fig. 3, highlighting the values of the second glycemia sampling in comparison to the first and the third.
The prevalence of diabetic patients determined in this research was half of that communicated in the last report of DM prevalence in Lima and Callao, Peru (3.9%).28 The prevalence rate discovered is somewhat similar to urban averages in populations over 3000asl. (Huaraz – 1.3%).23 DM is a chronic, degenerative, progressive but manageable disease. It has a great impact on the economy of the healthcare system. It requires serious control and a reasonable stratification of its systemic complications.
In the same way, the proportion of patients who were pre-diabetic or had a high risk of diabetes or glucose intolerance was 13.85%; prevalence within the regional average is between 5 and 15%.29 Glucose intolerance is a risk factor for the development of TIIDM and implies a high cardiovascular risk.30,31 In our country, the reported prevalence is up to 90.8% in patients who are 50 years and older, a highly elevated prevalence, suggesting the immediate prioritization of health care attention in order to avoid future complications.32
The progression from normoglycemia to diabetes may take several years, which involves intermediate stages of dysglycemia. This atero-thrombogenic alteration becomes evident with the alteration of glucose when fasting, emergence of glycated proteins and progressively cellular hypofunction, increasing the risk of morbidity in patients.33,34 Thus, diabetes indicates a decrease in the pancreatic reserve (up to 50% when there is no diabetic manifestation), this previous metabolic stage is evident by glycemia between 100 and 199mg/dl by the OGTT, which serves as a red flag to avoid its progression to DM.24 Intervention in pre-diabetic patients is an efficient strategy, since it avoids or slows down the progressive deterioration of the pancreas. This strategy includes an efficient diagnosis, modification in lifestyles, body weight management, ranges from 45 to 65% of daily energetic intake, physical exercise, pharmacological treatment, detection algorithms, etc.18 In this manner, it is demonstrated and confirmed that the control of glycemia is an effective measure to reduce the load of microvascular and cardiovascular complications, such as retinopathy, nephropathy and neuropathy in patients with TIDM as well as TIIDM.35–37
The main complication in the misguided selection of patients for OGTT is the dizzy feeling and loss of consciousness during the glycemic overload. We found that 5.19% of patients should not have been included in the test (Fig. 3). It may be a minimum finding, however, it is of relevance given the fact that the lab procedures are standardized, and follow internationally established flowcharts which rule under a quality control system. Moreover, the diagnosis of over half of these patients was dysglycemia (3.2%), 1.5% were diabetics and only 0.5% were healthy patients.9,11,12,15,16 Considering that, these mistakes in patient selection are a result of the generally poor knowledge of the personnel on these investigations, work overload, or the fact that there is no other diagnostic method. Thus, the modification of inclusion criteria of patients in the OGTT, POE, should be considered and rely on the CLSI POCT12-A, Clinical Laboratory Improvement Amendments, (CLIA), ADA guidelines, etc. Also, the use of other diagnostic methods (i.e. the A1c Glycosylated Hemoglobin test), evaluating their sensitivity, disadvantages and costs.
Radial distribution of patients wrongly included in the OGTT. Observe the elevated values in the second sampling (red line/thicker line). Glycemic concentration is expressed in mg/dl. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Lastly, we reaffirm the importance of the LJ phenomenon in the interpretation of the OGTT, because it could interfere in the final results. From the final reports of this phenomenon 1.2% were dysglycemic in ranges of 100–175mg/dl (Fig. 1), which leads us to consider its involvement in the development of the trial and interferences with glycemia – increasing it or reducing it – generating undetermined results, or even worse, false results.24
This finding proves the importance of inter-individual biological variability, evident by the events through which it may occur, like different physiological conditions, metabolism of the saturation of glycemia, type of anhydride glucose, population group, diabetics, physiological conditions where typologies of the individuals, pre-diabetics, or other non-clear situations occur. Thus, it is necessary to evaluate its reach in interference, to establish its total allowable error and understand the biochemistry and cellular behavior during the development of the test among individuals.
Furthermore, a judicious monitoring is required in order to understand and know its physiology and the events through which it occurs, corresponding to the different metabolisms during the test and their diagnostic implications. We definitely suggest that other glycemia tests should be conducted in a patient where this phenomenon is discovered, (Hb1A1c, etc.) or repeat the OGTT twice.11
Diabetes mellitus is a metabolic disease which imposes a high economic and social cost around the world, hence its prevention and treatment should be considered imperative in health and a priority worldwide. The diagnosis should be thoroughly examined, from the selection of patients to the determined ranges of glucose in each collection, with the purpose of avoiding undetermined results or false negatives.
FundingThis study was self-financed by the authors.
Conflict of interestThe authors state that there are no conflicts of interests.