Liver diseases are a major health problem worldwide, making it necessary to develop new molecules that help counteract or prevent such diseases. On account of this fact, investigations aiming to obtain natural and/or synthetic compounds possessing hepatoprotective activity have been undertaken. The development of new drugs consists of a variety of steps, ranging from the discovery of the pharmacological effects in cellular and animal models, to finally demonstrate their efficacy and safety in humans. Different models for assessment of the hepatoprotective activity in vitro, ex vivo and in vivo can be found in medical literature. The purpose of this review is to show the features, main advantages and disadvantages of each of the models, the hepatotoxic agents most commonly used (CCl4, acetaminophen, ethanol, d-galactosamine, t-BuOOH, thioacetamide) as well as the biochemical parameters useful to assess liver damage in the different models.
The liver is a key organ. It regulates different functions in the body, such as metabolism, secretion, storage and detoxifying. Liver damage is usually associated with the distortion of some of these functions. The liver is continuously exposed to an elevated amount of toxic agents, because the portal vein supplies blood to this organ after intestinal absorption.1,2
The World Health Organization (WHO) determined that around 2.4 million deaths yearly are linked to some liver disease, and that around 800 thousand of these deaths are attributable to cirrhosis.3 On the other hand, epidemiological studies conducted by the National Institute of Statistics and Geography (INEGI by its Spanish acronym) indicate that in 2013 in Mexico, over 600 thousand deaths were recorded. The main causes were diabetes mellitus (14.25%), followed by ischemic heart diseases (12.63%), cerebrovascular diseases (5.29%) and liver diseases (4.79%). Despite the advances in modern medicine and the development of new hepatoprotective drugs,4–6 the incidence of hepatic diseases has not decreased or stopped; on the contrary, statistics suggest that these continue to increase.7,8
Metabolism or biotransformation of hepatotoxic agents is a detoxifying process where molecules are surgically modified into less toxic shapes by different enzymatic systems. These modifications can generate metabolic products with varying degrees of pharmacological activity or inactive metabolites. There are different types of metabolic reactions: phase 1 reactions are usually oxidations, reductions or hydrolysis (modifying the structure of the reactive group); phase 2 reactions are those in which the drug conjugates with glucuronic acid, sulfates, acetates, methyl groups, glutathione or amino acids, generally to increase its solubility and be excreted. The liver's ability to be able to carry out the different oxidative metabolisms is associated with the high cytochrome P450 cell content.9
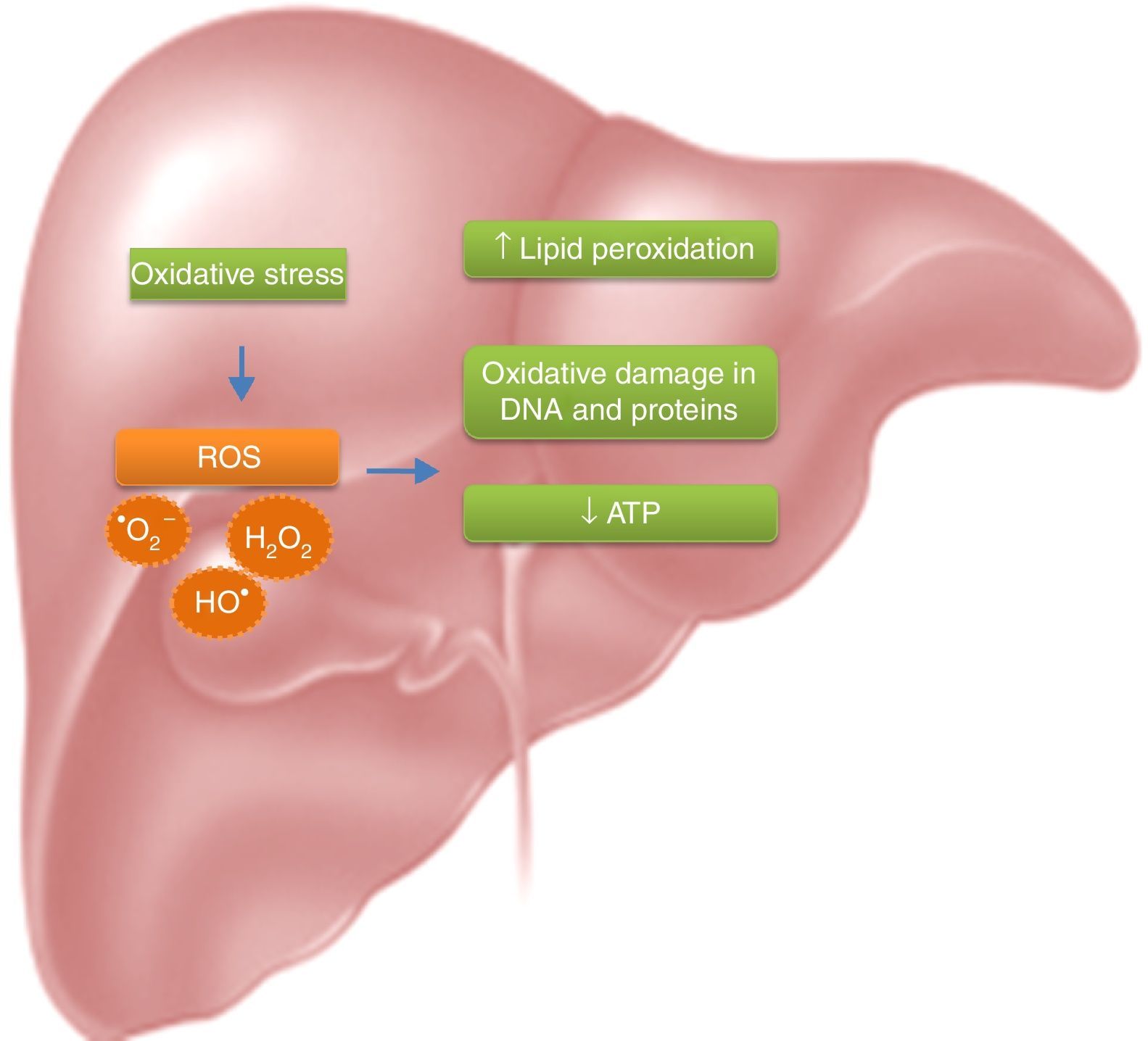
Due to the high metabolite biotransformation rate, free radicals can be generated continuously. Most hepatotoxic substances, mainly damage the liver because of the generated oxidative stress; oxygen reactive species induced a rise in lipid peroxidation, a reduction of ATP and oxidative damage in the DNA and proteins (Fig. 1).10–13
Protecting the liver from the harmful effects of hepatotoxins- which may be ingested- or counteracting the alterations in the antirradical defense mechanisms, is very important; the agents capable of doing this are called hepatoprotective.14
For this reason, researches have been developed in the search of natural and/or synthetic compounds with hepatoprotective activity.8 The development of new pharmaceuticals consists of a variety of steps, going from the discovery of pharmacological side effects in cellular and animal models, to finally prove its efficacy and safety in human beings.1
In vivo and well as ex vivo test models are used to evaluate hepatoprotective activity. These systems measure the ability of the drug to prevent or cure hepatic toxicity (induced by different hepatotoxins) in cellular cultures, organs or in experimental animals (rats, mice, etc.) respectively.1
Evaluation modelsNearly every acute and chronic liver injury can be experimentally induced; necrosis, steatosis, hepatic injuries, cirrhosis and cholestasis. These can all be generated in different models of liver damage.
The objective of hepatoprotective models is for the compounds, fractions or extracts being tested to counteract or avoid the damage generated by hepatotoxins. The magnitude of the hepatoprotective effect can be measured through biochemical makers, survival rate or histology of the liver.
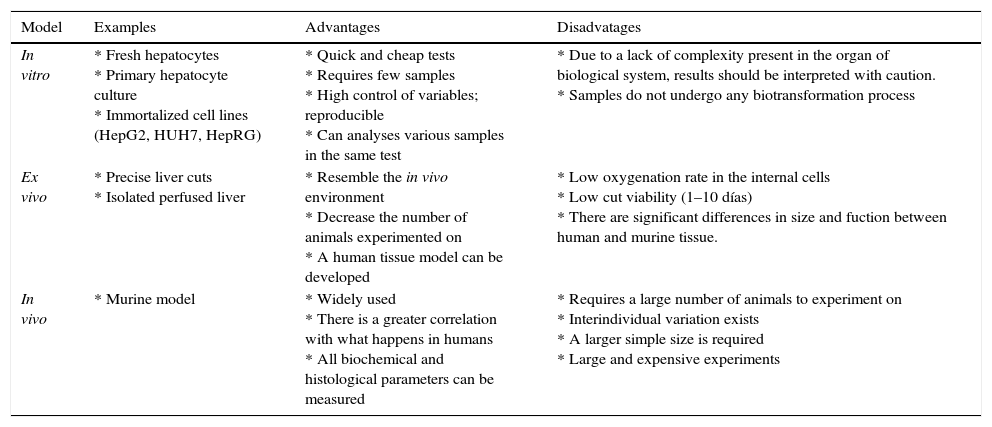
Test methods may be in vitro, ex vivo or in vivo (Table 1); and each one of them can be evaluated to see if the substance is hepatoprotective or hepatocurative, depending on if the hepatoprotective agent is administered before or after the hepatotoxin.
Models of evaluations of hepatoprotective activity.
| Model | Examples | Advantages | Disadvatages |
|---|---|---|---|
| In vitro | * Fresh hepatocytes * Primary hepatocyte culture * Immortalized cell lines (HepG2, HUH7, HepRG) | * Quick and cheap tests * Requires few samples * High control of variables; reproducible * Can analyses various samples in the same test | * Due to a lack of complexity present in the organ of biological system, results should be interpreted with caution. * Samples do not undergo any biotransformation process |
| Ex vivo | * Precise liver cuts * Isolated perfused liver | * Resemble the in vivo environment * Decrease the number of animals experimented on * A human tissue model can be developed | * Low oxygenation rate in the internal cells * Low cut viability (1–10 días) * There are significant differences in size and fuction between human and murine tissue. |
| In vivo | * Murine model | * Widely used * There is a greater correlation with what happens in humans * All biochemical and histological parameters can be measured | * Requires a large number of animals to experiment on * Interindividual variation exists * A larger simple size is required * Large and expensive experiments |
Fresh hepatocytes, primary hepatocyte cultures and immortalized cell lines are used to measure the hepatoprotective effect. It is possible to establish action mechanisms in these models. These models represent the best option for the screening and selection of potential hepatoprotective compounds and it is possible to establish action mechanisms at a cellular and molecular level.15,16
Primary hepatocyte cultures have the characteristic of maintaining normal metabolic liver properties, but it is not possible to maintain them for a long time. On the other hand, cell lines maintain their properties stable for a long time and can be cryopreserved, but immortalized or carcinogenic lines may differ in biochemical and metabolic aspects from normal cells.1
In order to evaluate protection, parameters like transaminase liberation, cell multiplication, morphology, macromolecular synthesis, oxygen consumption, etc., are measured.17,18
Advantages of in vitro models are: They are quick tests (between 2–3 testing days), they require small amounts of the test substances (milligram range) and the experimental conditions may be strictly controlled; different samples may be analyzed in the same test, they are cheap tests and there is little variability; therefore they are considered a reproducible test. In the case of primary cultures or fresh hepatocytes, they require few experimental animals in comparison to in vivo models.
Disadvantages of in vitro models: cells do not function independently in the organism; on the contrary, they form close and complicated nets with each other and with the extracellular matrix; therefore, this should be taken into consideration when interpreting in vitro data and should be verified with in vivo systems. Isolated cells as well as cell lines have an elevated cell differentiation rate due to the loss of natural environment. The substances tested do not go through the absorption and distribution processes, which occurs in the organism. There is little to no cell-to-cell interaction and there is no complexity proper of the organ.19–21
Ex vivo modelsPrecision cut liver slices (PCLS) are an ex vivo tissue culture which imitates multicellular characteristics of in vivo organs. Cellular interaction and spatial disposition remain intact in this model, with the possibility of performing morphological studies. Liver slices have the characteristic of functionally maintaining metabolizing enzymes and biliary canaliculus21; they have proven to be a valid ex vivo system to study metabolism and liver damage and function as a bridge between in vivo systems and cell cultures.22
Isolated perfused livers represent a model combining in vitro characteristics under in vivo circumstances. The first model was developed in porcine livers and later the livers of smaller animals (rats, mice and rabbits). This model preserves the tridimensional structure as well as the cell-to-cell interactions with the possibility of collecting bile in real time. If blood is used as a perfusor liquid, then hemodynamic parameters may be studied.23
Advantages of ex vivo models: they resemble in vivo atmospheres, are low cost, reproducible models. In PCLS the number of experimental animals is reduced, also the model can be developed with human organs.
Disadvantages of ex vivo models: in PCLS the bile flow and functional parameters, such as portal flow, cannot be analyzed.1 There is poor diffusion of oxygen nutrients to the more internal cells, and even with the development of new means of culture, the viability of the slices remains short (8–10 Days).22 In small labs, because of space and budget, the best option is the development of perfused rat liver; however, there are significant differences in the size, function and geometry of the murine liver compared to the human.1
In vivo modelThis model has been widely used; through this model we are able to determine the protection mechanism. The damage produced in experimental animals due to known dosage administration of different hepatotoxins and the magnitude of the damage and/or protection is determined by the different biochemical and metabolic markers, as well as histopathological determinations.
Advantages of in vivo models: is the model with the highest degree of correlation with what occurs in humans and all biochemical and histopathological parameters can be measured. They let us take into account the possible effects of the immune and central nervous systems in the development of hepatic diseases.24
Disadvantages of in vivo models: they require a large number of animals, and usually the studies are developed for long periods of time, increasing ethical and financial aspects. There is an inter-individual variation, and even though models imitating the different hepatic diseases have been developed, there are relevant differences in the molecular pathogenesis between the model and human species. They require a larger sample size to perform the experiment which may be a limiting factor, especially when analyzing natural products.25
Hepatotoxic agents and their action mechanismsThe molecules responsible for liver damage are called hepatotoxins; nowadays it is possible to imitate any form of natural-origin hepatic disease with different chemical substances and pharmaceuticals.
Hepatotoxins may be classified as intrinsic if the agent's behavior is predictable; there is a period of constant latency between exposure and liver damage development, or the injury is dose-dependent (i.e. carbon tetrachloride {CCl4}, thioacetamide, acetaminophen, ethanol). Another classification is idiosyncratic, if the agents are not predictable, but generate liver damage in just a small portion of exposed individuals, the injury is not related to the dosage, it occurs after a variable latent period and it is not reproducible in experimental animals (i.e. halothane, sulfonamides, isoniazid).26,27
Carbon tetrachloride (CCl4)CCl4 toxicity depends on dosage and the duration of exposure. In low dose, effects like loss of Ca2+ homeostasis, lipid peroxidation, and release of cytokines are produced, and apoptotic events may be generated, followed by cellular regeneration. In high doses, or if there is a longer exposure, the effects are more severe and the damage occurs during a longer period of time, the patient may develop fibrosis, cirrhosis, or even cancer.5,28,29
CCl4 is metabolized by the cytochrome P450 dependent of monooxygenases, mainly through the CYP2E1 isoform in the endoplasmic reticulum and mitochondria.16 Hepatotoxicity is produced by the formation of the trichloromethyl radical (CCl3), which is highly reactive. These radicals may saturate the organism's antioxidant defense system, react with proteins, attack unsaturated fatty acids, generating lipid peroxidation, reduce the amount of cytochrome P450, which leads to a functional failure with the consequent lowering of protein and accumulation of triglycerides (fatty liver), and alter water and electrolyte equilibrium with an increase of hepatic enzymes in plasma.30
Lipid peroxidation leads to a cascade of reactions, such as the destruction of membrane lipids, the generation of endogenous toxic substances, which originate more hepatic complications and functional anomalies. For this reason, lipid peroxidation is considered a critical factor in the pathogenesis of liver injuries induced by CCl4.15 The inhibition of the radical CCl3 generation is a key point in the protection against the damage generated. Because of this, this model is widely used for the evaluation of pharmaceuticals and natural products with hepatoprotective and antioxidant activity.31,32
AcetaminophenIt is an analgesic antipyretic analgesic. In high doses, it produces acute liver damage, causing necrosis of the hepatocytes. It is a widely used experimental model of clinical importance as an example of drug-induced liver damage.16
At therapeutic doses, it is mainly metabolized to glucuronic or sulfated and excreted derivatives, the rest metabolizes to intermediate reactives, which are eliminated by conjugation with glutathione. At overdoses, the excess is oxidized by the cytochrome P450 (mainly the CYP2E1 isoform)33 at N-acetyl-p-benzoquinone (NAPQI), which quickly attaches to glutathione. Under excessive conditions of NAPQI and glutathione depletion, a covalent bond of metabolite to proteins, adduct formation, mitochondrial dysfunction and oxidative stress occurs. The result is necrosis or hepatocellular death.19,34
EthanolThe liver is the most susceptible organ to the toxic effects of ethanol. The damage mechanism is due to the metabolism of ethanol by the CYP2E1 isoform of the cytochrome P450 producing oxidative stress with the generation of reactive species of oxygen and the increase of lipid peroxidation, leading to the alteration of the compositions of phospholipids of the cellular membrane.35,36 Membrane lipid peroxidation results in the loss of its structure and integrity, elevating serum levels of glutamyl-transpeptidase, a membrane-bonding enzyme. Ethanol inhibits glutathione peroxidase; it reduces the activity of catalase and dismutase superoxide.16
The decrease in the activity of antioxidant enzymes, dismutase superoxide and peroxidase glutathione is believed to come as a result of the harmful effects of free radicals produced after exposure to ethanol, or alternatively, they could be a direct effect of acetaldehyde, a product of ethanol oxidation.
D-GalactosamineThis hepatotoxin generates a similar damage to viral hepatitis regarding morphologic and functional characteristics. A single dose can cause hepatocellular necrosis and fatty liver.
It induces the exhaustion of the uracil nucleotide, resulting in the inhibition of RNA synthesis and consequently of proteins.37 The toxicity mechanism causes loss of the activity of ion pumps and an increase in cellular membrane permeability, leading to enzyme liberation and an increase in intracellular Ca2+ concentration, which is considered responsible for cellular death.16,36,38
Tert-Butyl hydroperoxide (t-BuOOH)Metabolized to free radicals by cytochrome P450 in hepatocytes generating lipid peroxidation, a decrease of glutathione, it reduces the potential of the mitochondrial membrane and cellular damage; generated damage is similar to oxidative stress, which occurs in cells and tissues.36,39
Alternatively, t-BuOOH can be converted by glutathione peroxidase into tetr-butyl alcohol and glutathione disulfide (GSSG). GSSG is converted into reduced glutathione (GSH) by the GSSG reductase, generating the oxidation of pyridine nucleotides (NAPD). All these events alter the homeostasis of Ca+2 which is considered a critical event to provide openings in the plasmatic membrane, and thus cellular damage.40
ThioacetamideAn organic compound containing sulfur, originally used as a fungicide and currently used for the treatment of leather, in labs and in the textile and paper industries.29 It can induce acute and chronic hepatic injuries and acts over the synthesis of protein, DNA, RNA and over γ-glutamyl transpeptidase (GGT) activity.
Thioacetamide is bio-activated by the CYP450 and/or by the monooxigenase system, which contains flavin, converting the compound into sulfine (a sulfoxide-type compound) and later into sulfone-type compounds. Sulfine is responsible for generating an increase in the nucleus volume, nucleoli enlargement, an increase in intracellular concentration of Ca+2, generating changes in cellular permeability and mitochondrial dysfunction. On the other hand, Sulfone-type compounds are responsible for the liberation of nitric oxide synthase and the nuclear factor kappa B (NF-κB), protein denaturalization and lipid peroxidation.41–43
Liver function markersA decisive step when biological activity models are performed is the analysis of the activity of the tested analyte. Depending on the selected model and its characteristics, the survival rate and the damaged biochemical markers can be determined. Due to the wide variety of functions performed by the liver, there is a wide range of markers through which we are able to determine the functionality or damage generated by this organ or its cells.28 Although there is no biochemical marker specific to liver damage, the combination of several of these, and knowing the correlation they have with the liver, will help to better interpret the results of the hepatoprotective models. Markers can be divided into tests related to the liver's excretory function (bilirubin), tests related to synthetic function (albumin and prothrombin time) and tests related to the integrity of hepatocytes (transaminases, alkaline phosphatase, GGT).
Transaminases or aminotransferasesTransaminases or aminotransferases are enzymes that transfer a group of amino from an amino acid to an acid α-acetate. This process is an important step in the metabolism of amino acids. The aspartate aminotransferase (AST) and the alanine aminotransferase (ALT) are widely used enzymes; the increase in the liberation of these transaminases is linked to liver dysfunction. ALT catalyzes the amino group transference of the L-alanine to α-ketoglutarate to produce pyruvate and L-glutamate; it is elevated in hepatic and renal diseases, i.e. hepatitis, cirrhosis and mononucleosis. AST catalyzes the transference of the amino group of the L-aspartate to α-ketoglutarate to produce oxaloacetate and L-glutamate; the heart, liver and skeletal muscle, are organs rich with this enzyme and the AST liberation is proportional to the damage generated. In a myocardial infarction it starts to increase between 3 and 9h after the event, reaching its peak on the second day; the levels normalize between the fourth and the sixth day. In hepatitis cases, observed elevations are between 7 and 12 times its normal concentrations, with increases of up to 100 times.28
PhosphatasesThese enzymes belong to the hydrolases family and are known for their ability to hydrolyze a wide variety of organophosphate compounds with the formation of phosphate ions and alcohols. Clinically relevant phosphatases are acid phosphatase and alkaline phosphatase. Alkaline phosphatase (ALP) is produced mainly in the liver and bone; when there are no osteogenic diseases, ALP elevation is generally linked to hepatobiliary diseases. It is more specific in obstructive hepatic processes.28,44
Transpeptidase γ-glutamine (GGT)This enzyme is bound to the plasmatic membrane, which catalyzes the transference of the γ-glutamine group of a peptide to itself or other peptides. It is located mainly in hepatocytes; however it can also be found in the proximal renal tubules, intestinal epithelial cells and the prostate. High GGTP levels usually indicate infection in the liver, pancreatic and biliary zones. The specificity of the test is relatively low, but since it is not linked to bone diseases, it is used to link high ALP levels to liver damage.44
BilirubinBilirubin is the most important metabolite of the heme group, found in hemoglobin, myoglobin and cytochromes. It is highly insoluble in water in its most common isomeric form, and most of it is transported by albumin. The liver is responsible for eliminating bilirubin by turning it to a more hydrosolube compound, thus allowing its elimination of plasma for its eventual excretion. It is the most important test of the hepatic metabolic function; however, it is only possible to determine it in in vivo models.44
Total proteinsThe liver synthetizes most plasmatic proteins, and in most hepatic diseases the levels are reduced. Albumin, α-1 antitrypsin, ceruloplasmin, and α-fetoprotein are proteins linked to acute liver damage.
Lactato deshydrogenase (LDH)Lactato deshydrogenase is an enzyme located in the cellular cytoplasm. It catalyzes the interconversion of the lactate and pyruvate; LDH liberation may be interpreted as the opening of the cellular membrane or cellular death. This enzyme is not specific to the liver and it is widely used in in vitro models because it is expressed in most cellular lines.45
AST, ALT and ALP are most commonly analyzed in all hepatoprotective models, while the quantification of total proteins and LDH are generally used as parameters of in vitro cytotoxicity.46
ConclusionsLiver diseases are a major health problem, domestically and around the world; thus, it is necessary to develop new molecules which help counteract or prevent them. The discovery and development of new drugs begins with the demonstration of the pharmacological effects, to later conduct safety and efficacy studies in human beings. In vitro models are widely used; they are fast, cheap, reproducible techniques and require a lower sample. Nevertheless, the results ought to be reevaluated by other models. Ex vivo models are an intermediate point between in vivo and in vitro models, but are less utilized. Unlike the other two, in vivo models provide a wide range of information. They are widely used to verify the activity of new compounds, although they are more expensive and go through many experimental animals. Hence, they are generally used after an in vitro or ex vivo evaluation, as a step previous to clinical trials.
Conflict of interestThe authors have no conflicts of interest to declare.
FundingNo financial support was provided.
Special thanks go to the Mexican National Council of Science and Technology (abbreviated CONACYT) for their support for the national scholarship no. 359832 and project no. 180997 of the Basic Scientific Research Grant 2012.