Background: Nowadays, there are very few studies about massive transfusion in our country. This situation generates the necessity to the elevation of possible new strategies to diminish mortality and its adverse effects.
Material and methods: All massive transfusions were evaluated in a retrospective way from October 2010 to October 2012. All diagnosis groups were recorded and the patients were divided into three groups depending on the ratio between packed red blood cells (PRBC) and fresh frozen plasma (FFP) units (ratios ≤2, >2, and without FFP). Their mortality and/or survival were evaluated 30 days after as well as all the factors associated with the event.
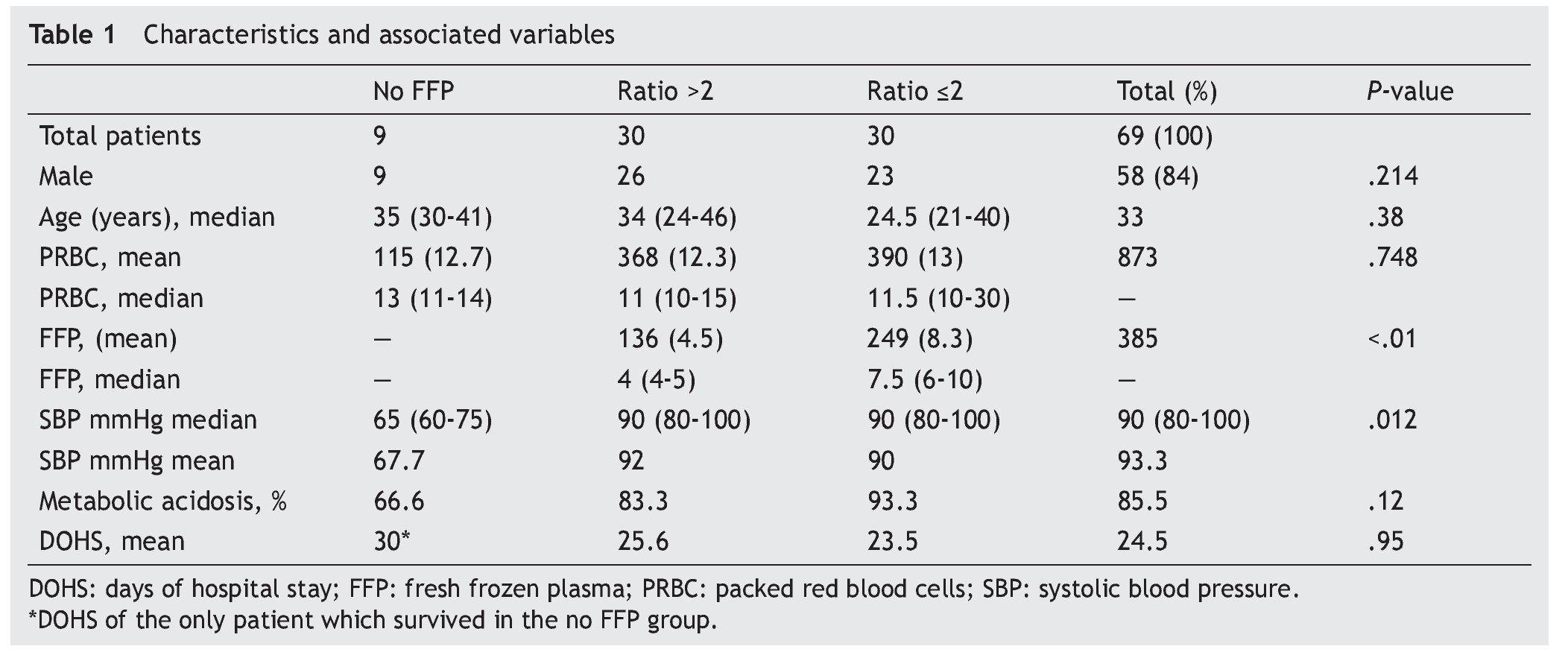
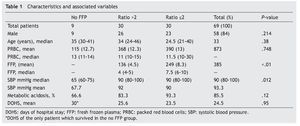
Results: A total of 69 patients were included (37 trauma patients, 28 gunshot wounds and 4 with lacerated wounds); the groups (ratios ≤2, >2, and no plasma at all) were distributed as follows: 30, 30 and 9 patients each, with an overall mortality rate of 60.8% within 30 days. A lower survival rate (12%) in the no plasma group (P=.015) was found and systolic blood pressure during transfusion had a mean of 67.7 mmHg (P=.012) in this group. Fresh frozen plasma units were 136 and 249 for >2 and ≤2 ratios respectively (P<.01); 85.5% of all patients developed metabolic acidosis during the transfusion, and the number of days in the hospital after the event had a mean of 24.5 days in all patients.
Conclusions: High rates of massive transfusion mortality are still being reported in our field. The use of transfusion strategies contribute to elevate the survival rate in patients with massive transfusion treatment.
Introduction
The need for massive transfusion in an acute hemorrhage is catalogued as one of the most important risk factors for death among multiple trauma patients.1 Massive transfusion is defined as the reposition of the blood volume in 24 h or 7% of the ideal weight in adults or 9% in children; however, there are different definitions like a replacement higher than 50% of the blood volume in 3 h, or more practical definitions like the transfusion of over 4 PRBC in 1 h, or over 10 units of PRBC in a period of 24 h.2
In the first studies in which massive transfusion was linked to mortality the numbers were close to 90%,3 even nowadays rates between 30% and 70% continue to be reported.4,5 Therefore, massive hemorrhage morbi-mortality remains unacceptably high, and thus new therapeutic schemes have been suggested in the last decade with the purpose of increasing survival.6 The institution of these massive transfusion protocols has brought benefits and they have been attributed mainly to keeping a close or equivalent ratio to fresh frozen plasma (FFP) in relation to red blood cells (RBC) transfused.7
Even though each hemoderivate has precise indications, the circumstances of the bleeding (with or without tissue damage) determine the different transfusion thresholds.8 One of the main challenges in the timely reinstitution with hemocomponents is also the identification of the patient who will have massive transfusion and the prediction of transfusion requirements.9
Nowadays the frequency of massive hemorrhages in our country varies greatly and there are few studies in our field, which confirms the need to institute these protocols. Few institutions have standardized massive transfusion protocols. The objective of the present study is to describe mortality and the use of RBC:FFP ratio in trauma patients subject to massive transfusion, as well as to detect factors associated with morbidity and mortality.
Materials and methods
A retrospective analysis of acute hemorrhage patients within the shock-trauma area was performed. Patients who had transfusions of 10 or more PRBC in a period of less than 24 h from october 1st 2010 to october 30th 2012 were evaluated. Acute hemorrhage and massive transfusion patients were recruited through the blood bank’s CiBank V1.0® (TDI) operative system, excluding patients who were under 15 years old, patients with neoplastic diseases, and patients with previous chronic diseases. RBC:FFP ratio was obtained for each case (i.e. 10 RBC + 5 FFP = 10 / 5 = 2 ratio) and the population was divided into three different groups, those with an RBC:FFP rate ≤2, another group where the RBC:FFP ratio was >2 and a third group which did not receive FFP during massive transfusion. Patients’ characteristics were described according to age, gender and diagnosis (admission and injury), both diagnoses were evaluated in percentage and divided according the type of injury: vital organ injury grades 3 to 5 according to the American Association for the Surgery of Trauma Organ Injury Scale,10 severe head injury (SHI), larger-caliber vessel and other injuries. This last group was formed by patients with multiple fractures and limb amputations. Mortality and/or survival rates after 30 days and the amount of in-patient days after the event were evaluated. Factors associated with the hemorrhagic event, like systolic blood pressure (SBP) during transfusion and the presence of metabolic acidosis, as well as the number of PRBC and fresh frozen plasmas transfused, were analyzed. A chart representing survival rates up to 30 days after the event was created using the Kaplan-Meier curve, and an evaluation of the difference between groups using the log-rank test was performed, using mean and median as central tendency measurements and standard deviation and inter-quartile ranges as dispersion measurements. The x2 test was used for qualitative variables, and one-way ANOVA was used for differences between groups in the case of three groups. The Mann-Whitney U test was used as well in the case of two groups with a non-parametric distribution. The SPSS v.20 statistical program was used.
Results
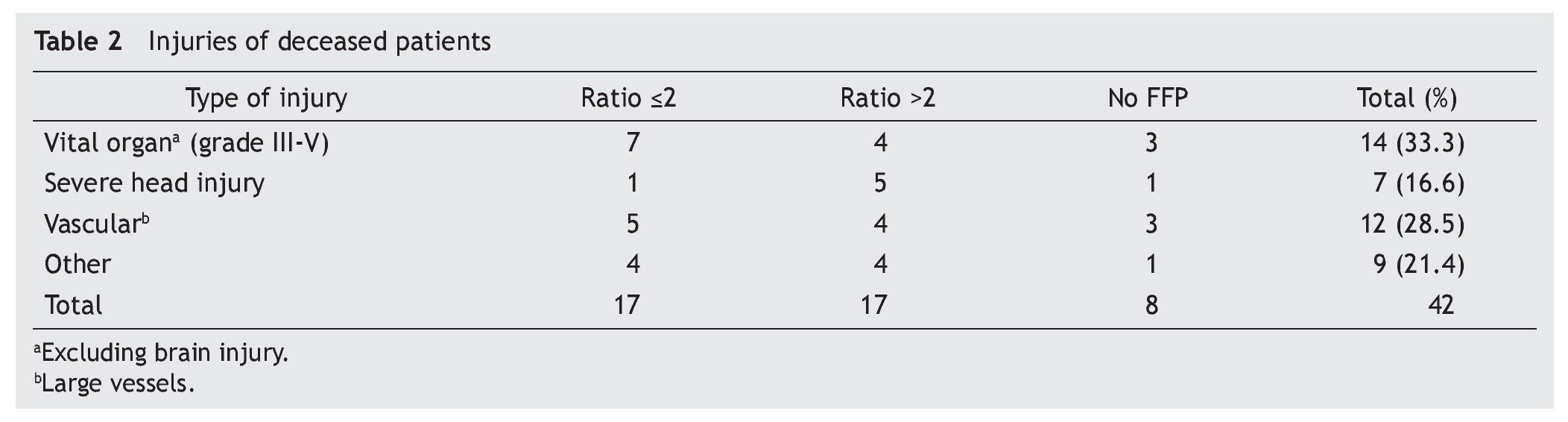
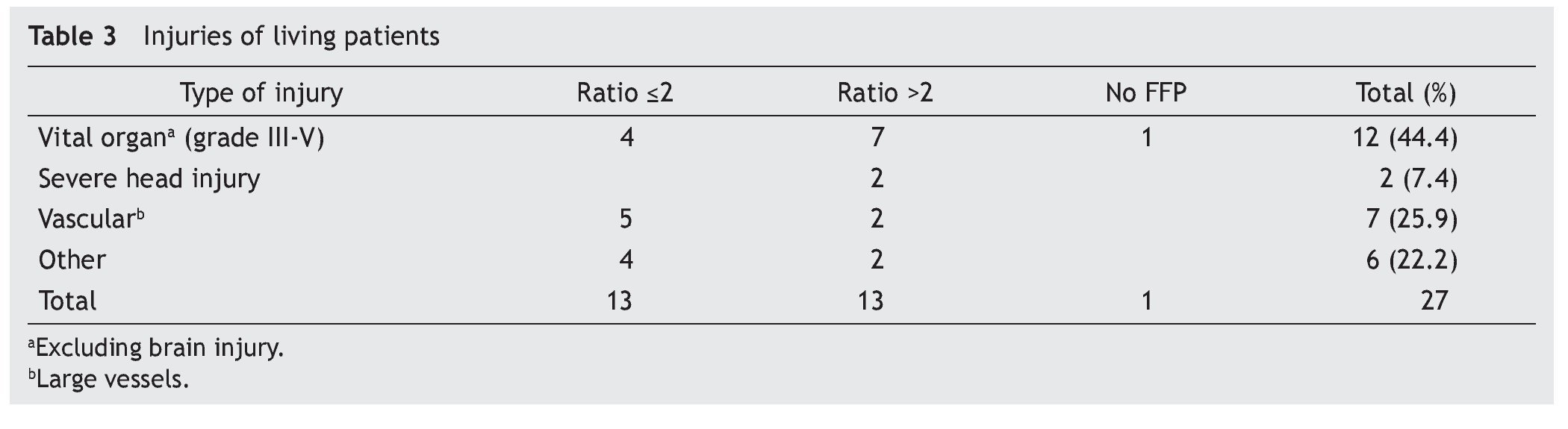
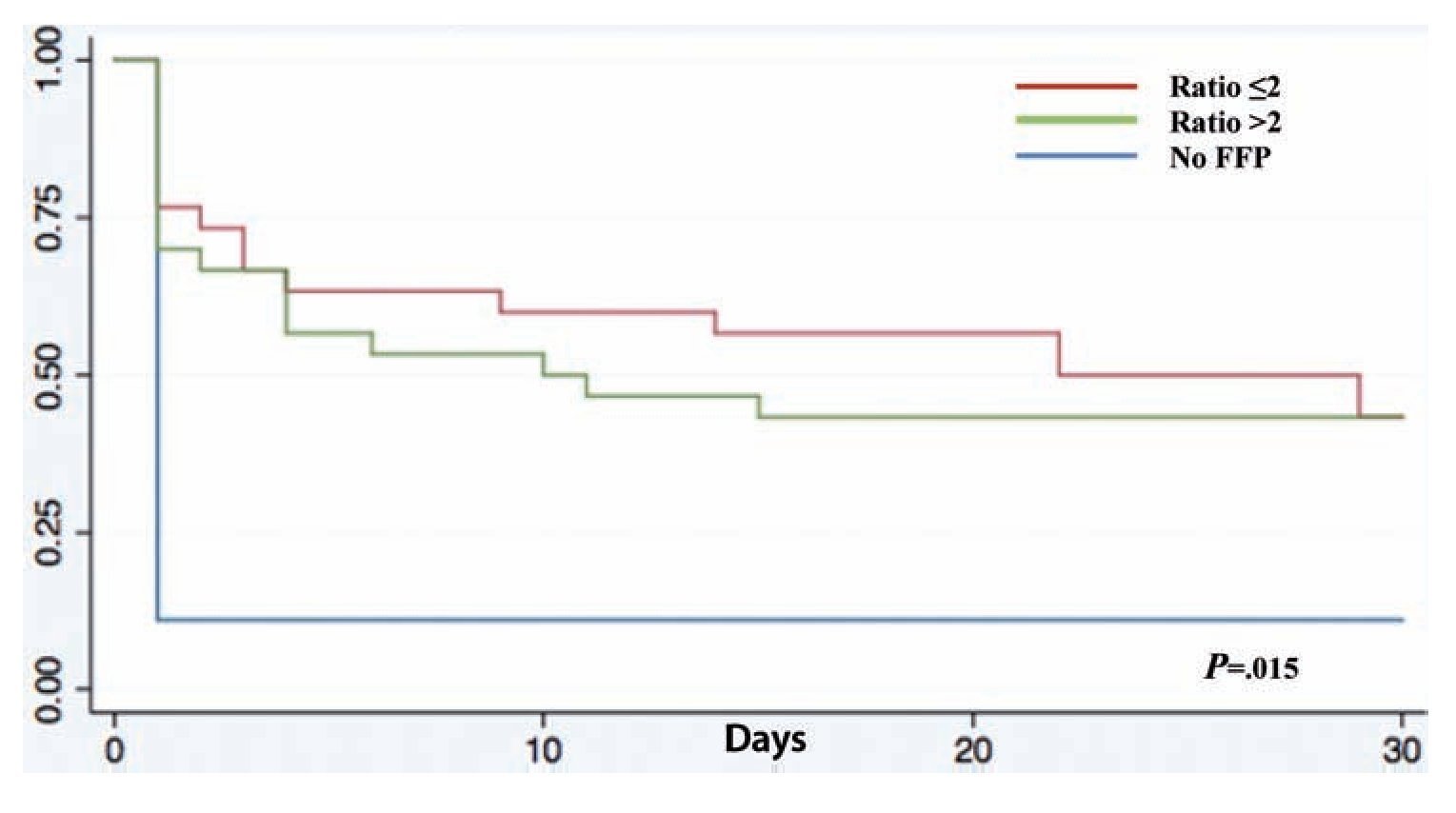
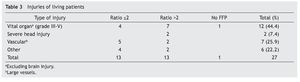
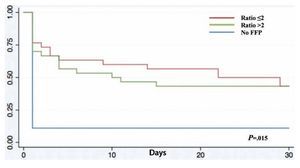
A total of 83 registries of patients with massive transfusions were obtained, excluding 9 patients with neoplastic diseases, 4 with previous chronic disease, and 1 younger than 15 years old. A total of 69 patients were included in the study with admission diagnoses distributed as follows: 37 trauma patients, 28 gunshot wounds and 4 with lacerated wounds. The average age was 33 years old, the majority of whom were male (84%). The groups were divided as follows: groups’ ratios ≤2 with 30 patients, ratios >2 with 30 patients and the no-plasma group with 9 patients (table 1). Diagnoses according to the type of injury and their comparison between groups are shown in tables 2 and 3. Mortality at 30 days resulted in 56.6% for the ratio ≤2 group just as in the ratio >2 group with 56.6% and 88% for the group without FFP (figure 1); obtaining an overall mortality of 60.8%. A distinctly lower survival rate of 12% was observed during the first 24 h in the group of patients who did not receive FFP (P=.015) showing a significant difference with respect to the other groups. The other two groups (>2 and ≤2) showed no differences (figure 1). Systolic blood pressure during transfusion within the no-plasma group had a mean of 67.7 mmHg proving a difference from the other two groups (P=.012) which presented means of 90 and 92 mmHg (table 1). 85.5% of the patients presented metabolic acidosis during the transfusion. The mean for the amount of in-hospital days was 24.5, without a significant difference among the groups, keeping in mind that in the no-plasma group only 1 patient survived longer than 24 h thus he was not included in the Days of hospital Stay (DOHS) analysis.
Figure 1 Kaplan-Meier survival curve. FFP: fresh frozen plasma.
Regarding transfusions, 136 units were transfused for the >2 group and 249 units for the ≤2 group (P<.01) with means of 4.5 and 8.3, and medians of 4 and 7.5 respectively (P<.01). RBC transfusion did not show significant difference between the groups (P=.748) (table 1).
Discussion
Nowadays high mortality rates related to massive transfusions are still reported in our field. Therefore, we suggest through this study the use of FFP in massive transfusions, the above as a consequence of obtained results of different RBC:FFP ratios which suggest that FFP transfusion in similar amounts to RBC transfused could improve survival rates at 24 h and at 30 days after the hemorrhagic event. In our survival chart at the end of follow-up there wasn’t a statistically significant difference between the >2 group and the ≤2 group, we can see a slight tendency favoring survival rates in the ≤2 RBC:FFP ratio, but this could be a result of 5 of the patients in the ratio >2 group having a severe head injury diagnosis (table 2) which may cause a bias in this tendency.
It is difficult to affirm the participation of FFP in the hemodynamic state of these types of patients, however looking at our results and the differences regarding SBP between the no-plasma group and the other two groups, and knowing the role FFP plays in reducing endothelial vascular permeability in patients with hemorrhagic shock,11 suggests its implication as a helper in hemodynamic stabilization of massive hemorrhage patients.
Additionally we stress the importance of the identification of metabolic deterioration, such as acidosis, present in most patients who undergo massive transfusion, knowing that patients at the beginning of crystalloid reanimation are administered large volumes of isotonic saline solution (Na+Cl– 0.9%) with the purpose of expanding intravascular volume in massive bleeding, conditioning us to a hyperchloremic metabolic acidosis.12
Currently in Mexico the guidelines for massive transfusions suggest the administration of the blood components in deficit according to the patient’s clinical evolution and the results of the coagulation tests,13 thus through this study we promote an update of the guidelines with a reference to massive transfusions. On the other hand, international studies suggest sticking as closely as possible to a 1:1 ratio (RBC:FFP) in order to reduce mortality at least in the first 24 h.14
Therefore, after having identified in this document the main risks of the patients undergoing massive transfusions in our hospital, we also suggest the institution of standardized protocols which closely stick to international transfusion guidelines. It is important to note that the use of FFP is relevant for the attention of these patients and acknowledging the challenge that the prediction of the use of timely restitution with hemoderivatives implies, we recommend the use of prediction scores like the TASH as part of the algorithm for massive hemorrhage diagnosis,15 as well as the use of thawed plasma with the purpose of shortening the availability times of said component,16 moreover it is necessary to insist on the need of prospective studies with variable adjustment like the severity of the injury in trauma patients. Massive transfusion is in many cases inevitable; recognizing the risk that comes along with this practice allows us to establish a treatment preventive protocol in which fresh plasma seems to play a very important role.
Received: January 2014;
Accepted: July 2014
*Corresponding author:
Departamento de Patología Clínica,
Hospital Universitario “Dr. José Eleuterio González” de la UANL.
Ave. Francisco I. Madero s/n y Ave. Gonzalitos,
Col. Mitras Centro, Monterrey,
Nuevo León, México C. P. 64460.
E-mail addresses: felipe_1411@msn.com; felipe.mercadodelangel@gmail.com (F. Mercado del Angel).