Stereotactic surgery is used to place electrodes or cannulas in the brain in order to study the function of several brain structures in preclinical research. The hippocampus has been extensively studied with this methodology due to its involvement in a wide range of neurological, cognitive, emotional, and affective disorders. However, the effects of stereotactic surgery on coordination and motor activity should be evaluated in order to determine whether this surgical procedure causes any neurological alterations that may bias the results of studies incorporating this technique.
MethodsWe evaluated the effects of stereotactic surgery and implantation of a cannula into the hippocampus of female Wistar rats on the motor activity, forced swim, and rotarod tests. The stage of the oestrous cycle was included in the statistical analysis.
ResultsStereotactic surgery had no impact on any of the motor activity variables assessed in the open field (squares crossed, time spent in grooming, and rearing), forced swim (turning behaviour, lateral swimming, latency to first immobility, and time spent immobile), and rotarod (latency to fall) tests, compared with intact rats. Regardless of surgical manipulation, rats in the metestrus and diestrus stages crossed a greater number of squares and displayed longer immobility times than those in the proestrus and estrus stages.
ConclusionStereotactic surgery for cannula placement in the dorsal hippocampus does not affect coordination and motor activity in rats. We can therefore conclude that this procedure has no neurological complications that may interfere in the interpretation of results of studies applying this technique.
La cirugía estereotáxica permite el implante de electrodos o cánulas para estudiar el funcionamiento de diversas estructuras cerebrales a nivel preclínico. El hipocampo ha sido ampliamente estudiado con esta metodología, debido a su participación en desórdenes neurológicos, cognitivos, emocionales y afectivos. Sin embargo, el efecto per se de esta metodología sobre la coordinación y la actividad motora, para identificar o descartar alteraciones neurológicas que pudieran influir en los resultados de protocolos que la utilizan, requiere ser explorado.
MétodosSe evaluó el efecto de la cirugía estereotáxica y el implante de cánula en el hipocampo de ratas hembra Wistar en las pruebas de actividad locomotora, nado y Rota-rod. El análisis estadístico consideró la fase del ciclo estral de las ratas.
ResultadosNinguna de las variables evaluadas en las pruebas de actividad locomotora (cuadros cruzados, tiempo de acicalamiento y conducta vertical), nado (giros, nado lateral, latencia a la primera inmovilidad y tiempo de inmovilidad) o Rota-rod (latencia a la caída), fueron modificadas por la manipulación quirúrgica, en relación con ratas intactas. Independientemente de la manipulación quirúrgica, las ratas en metaestro-diestro cruzaron más cuadros y tuvieron mayor tiempo de inmovilidad, que las ratas en proestro-estro.
ConclusiónLa cirugía estereotáxica y el implante de cánula en el hipocampo dorsal carecen de efectos sobre la coordinación y la actividad locomotora de la rata, por lo que se descarta algún daño neurológico que pudiera interferir en la interpretación de resultados en protocolos que incluyen esta manipulación experimental.
Stereotactic surgery is a technique enabling brain structures to be located for electrode or cannula implantation, with the ultimate aim of exploring brain function.1 These procedures have enabled the identification of neuroanatomical and neurochemical circuits involved in brain function and certain neurological disorders (epilepsy, movement disorders, and neurodegenerative diseases),2–5 learning and memory impairment,6,7 and emotional8,9 and affective disorders.10 Furthermore, stereotactic surgery is used to explore the therapeutic potential and action mechanisms of potentially effective substances for the treatment of the previously mentioned disorders in humans.9,11–14
The hippocampus is one of the most frequently studied brain structures due to its involvement in the neurobiology of multiple neurological, cognitive, emotional, and affective disorders. Intrahippocampal microinjection of such substances as kainic acid or pilocarpine3,4 has been used to experimentally reproduce temporal lobe epilepsy, which is associated with a reduction in the number of neurons in the hippocampal CA1 region.15 Under these experimental conditions, status epilepticus leads to memory and learning impairment,16,17 which may be minimised by administering anticonvulsants.14
Other studies have explored the role of the hippocampus in neurological disorders associated with consumption of certain foods. Intrahippocampal microinjection of such neurotoxins as methylazoxymethanol, present in cycad seeds (Dioon spinulosum),2 or linamarin, found in cassava root (Manihot esculenta Crantz),18 promotes the loss of motor coordination and reduces the number of neurons in the hippocampal CA1 in rats.18 These experimentally-induced alterations seem to be analogous to some of the neurological symptoms associated with consumption of cycad seeds or cassava root in humans (amyotrophic lateral sclerosis-parkinsonian dementia, tropical ataxic neuropathy, and konzo).19–21
Experimental models of locomotor impairment are used to evaluate the effects of intrahippocampal microinjection of neurotoxic compounds and substances that are potentially beneficial in humans (for example, as treatments for some neurological or psychiatric disorders).2,11,14,15 However, very few studies have used specific tests to evaluate the intrinsic locomotory impact of stereotactic surgery. This study aimed to evaluate the effects of stereotactic surgery on locomotor activity and coordination in female rats in order to confirm or rule out any associated neurological alterations that may interfere with the interpretation of results in studies employing this type of experimental manipulation.
MethodsSubjectsWe used 56 3-month-old female Wistar rats weighing between 250 and 300g at the beginning of the study. Rats were housed in acrylic cages (4-5 rats per cage) in a vivarium. They were kept at room temperature (25±1°C) with a 12:12 light–dark cycle (lights on at 7:00am) and had ad libitum access to food and water. Rats were managed following the international ethical standards established in the Guide for care and use of laboratory animals,22 and the Mexican official guidelines for the care and use of laboratory animals.23
Vaginal smearsPrior to behavioural tests, vaginal smears were obtained once daily from each rat. Only the rats with 3 consecutive regular cycles (4-5 days) were included in our study. After behavioural tests, we obtained vaginal smears with cotton buds dampened in saline solution to determine the phase of the oestrus cycle. Vaginal discharge was examined under a light microscope (40×). Rats were divided into 2 groups based on vaginal smear results24: pro-oestrus/oestrus phase, characterised by high levels of ovarian hormones, and metoestrus/dioestrus phase, with low levels of these hormones.25
Experimental groupsTwo experiments were conducted. In the first, rats were randomly allocated to 2 groups: 1) an intact group (n=14), where rats did not undergo stereotactic surgery, and 2) a surgery group (n=14), with rats undergoing stereotactic surgery plus dorsal hippocampal microinjection of a vehicle used in various experimental protocols (0.3μL of cyclodextrin solution 35%). Rats in both groups were evaluated using the forced swim and locomotor activity tests. The second experiment included 2 groups: 1) an intact group (n=14) and 2) a surgery group (n=14), with rats undergoing surgery and vehicle microinjection. Both groups were assessed using the rotarod test.
After all behavioural tests were completed, vaginal smears were obtained to identify the phase of the oestrus cycle. Rats were then classified in 2 groups according to the level of ovarian hormones (high in pro-oestrus and oestrus phases and low in metoestrus and dioestrus phases) to minimise the influence of hormonal differences on the analysis of the results.
Stereotactic surgeryRats were administered 0.05mg/kg atropine sulfate i.p. (Sigma–Aldrich Co.; St. Louis, MO, USA) and subsequently anaesthetised26 with pentobarbital sodium (50mg/kgi.p.; Anestesal®, Pfizer; Mexico City, Mexico). During deep anaesthesia, the rats’ heads were placed in a stereotactic apparatus (Stoelting; Wood Dale, IL, USA). The cranium was exposed and a dental lab drill (35000rpm; Saeshin Dental Lab, South Korea) was used to drill a hole to implant a cannula into the dorsal hippocampus (AP=−3.8mm; ML=−2mm; DV=−2mm) according to the stereotactic coordinates of Paxinos and Watson's1 rat brain atlas (1998 edition). A stainless steel guide cannula (8mm long, 0.7mm in diameter) was subsequently implanted and secured to the skull with dental acrylic (Arias Distribuidora Dental; Tlalnepantla, Mexico). After surgery, lidocaine (Laboratorios PISA; Mexico City, Mexico) was applied topically to the area surrounding the cannula implant. For the first 3 days after surgery, an analgesic agent (dipyrone 50, Virbac; Guadalajara, Mexico) was administered intraperitoneally to reduce pain. Rats were allowed to recover from surgery for 4 days before behavioural tests were conducted.
MicroinjectionA vehicle was injected through the stainless steel cannula, which was connected to a 10μL Hamilton syringe by means of a polyethylene tube. An automatic infusion pump (KD Scientific; Holliston, MA, USA) was used to microinject 0.3μL of vehicle over 5 minutes (speed: 0.06μL/min). Rats were able to move freely during this procedure. Rats were left to rest for 5 minutes after microinjection to prevent the vehicle from returning by capillarity. The rats were then evaluated with a series of behavioural tests.
Locomotor activity testEach rat was placed for 5 minutes inside an acrylic cage (44×33×20cm) whose base was divided into squares measuring 11×11cm. We assessed the following: 1) number of squares crossed (the rat was considered to have crossed a square when at least three-fourths of its body passed from one square to another); 2) vertical time in seconds (total time spent in a rearing position), and 3) grooming time in seconds (total time spent grooming, including elliptical movements of both front legs over the nose, ears, body, and genital area following a cephalocaudal direction).27
Forced swim testEach rat was placed for 5 minutes inside a glass tank (26×29×50cm) filled with water at a temperature of 25±1°C (water level: 30cm). This test evaluated the following variables: 1) latency to first episode of immobility, in seconds (time elapsed from the moment when the rat was placed inside the container to the first episode of immobility); 2) total immobility time (sum of all immobility episodes; rats were considered to be immobile when they made only the necessary movements to keep the head above water); 3) total lateral swimming time, in seconds (sum of all episodes of uncoordinated swimming on one side); and 4) total number of spins (total number of episodes of rats turning on their own axes while swimming).2,28
We recorded video footage of all sessions. Variables were quantified by 2 independent observers using software specifically designed for recording rat behaviour.
Rotarod testRats completed 4 training sessions (one session daily) on an accelerating rotarod (speed 4 to 20rpm; LE 8300, LSI Letica, Panlab Scientific Instruments; Barcelona, Spain); each session lasted 5 minutes. Stereotactic surgery was performed after training was complete. Four days later, rats were placed on an accelerating rotarod (speed 4 to 20rpm), completing 5 sessions with 3-minute resting periods between sessions. We evaluated latency to fall, that is, the time it took for the rat to fall from the rod. This variable is used to identify any alterations in motor coordination and balance.29
Verification of the site of cannula implantation and microinjectionAfter the completion of behavioural tests, vaginal smears were obtained and animals euthanised with pentobarbital overdose (PISA-Agropecuaria; Guadalajara, Mexico). The cannula was used to mark the injection site with Evans blue (Sigma–Aldrich Co.; St. Louis, MO, USA). Rats’ brains were perfusion fixed, extracted, and sliced. The site of cannula implantation was checked with a light microscope; Paxinos and Watson's1 rat brain atlas was used for reference. The statistical analysis included only data from those rats in which cannulas were found to have been implanted correctly in the hippocampal CA1 region.
Statistical analysisData were analysed using 2-way ANOVA for independent samples; factors were surgical condition (intact or subjected to stereotactic surgery) and phase of oestrus cycle (pro-oestrus/oestrus and metoestrus/dioestrus). Given P-values ≤.05, we applied the post hoc Student–Newman–Keuls test. Results were expressed as means±SD.
ResultsPhase of the oestrus cycleAccording to the analysis of vaginal smears, 7 rats were in the pro-oestrus/oestrus phase and 7 in the metoestrus/dioestrus phase in each of the experimental groups.
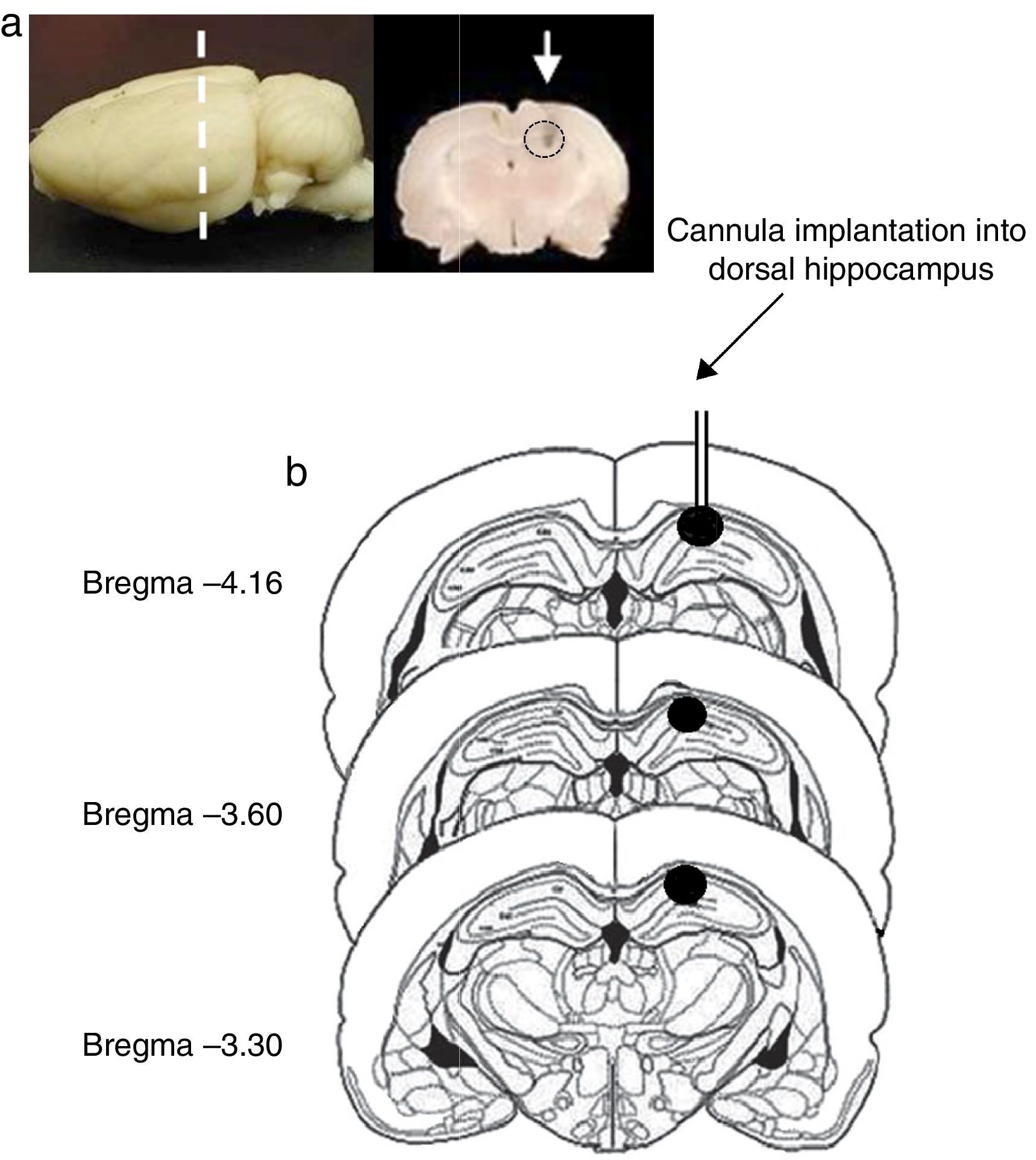
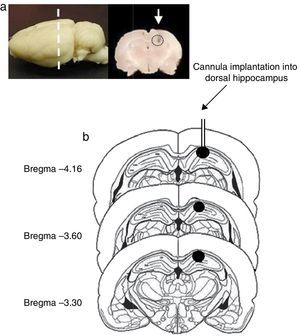
Site of cannula implantationOf all rats included in the study, 2 were excluded from statistical analysis (one rat in the pro-oestrus/oestrus phase in experiment 1 and another one in the metoestrus/dioestrus phase in experiment 2), due to the presence of oedema in the area surrounding the implantation site and extending from the dorsal hippocampus to the cerebral cortex. In the remaining rats, the histological study confirmed correct cannula implantation into the dorsal hippocampus (CA1) between coordinates AP=−3.3 and AP=−4.6mm from bregma (Fig. 1).
(a) Image of the rat brain showing the site of cannula implantation at the anteroposterior level (dashed line) and a coronal section of the brain showing the site of cannula injection (white arrow). (b) Diagram of brain coronal sections, displaying the site of cannula implantation into hippocampal CA1 (black circles). Based on Paxinos and Watson's rat brain atlas (1998 edition).
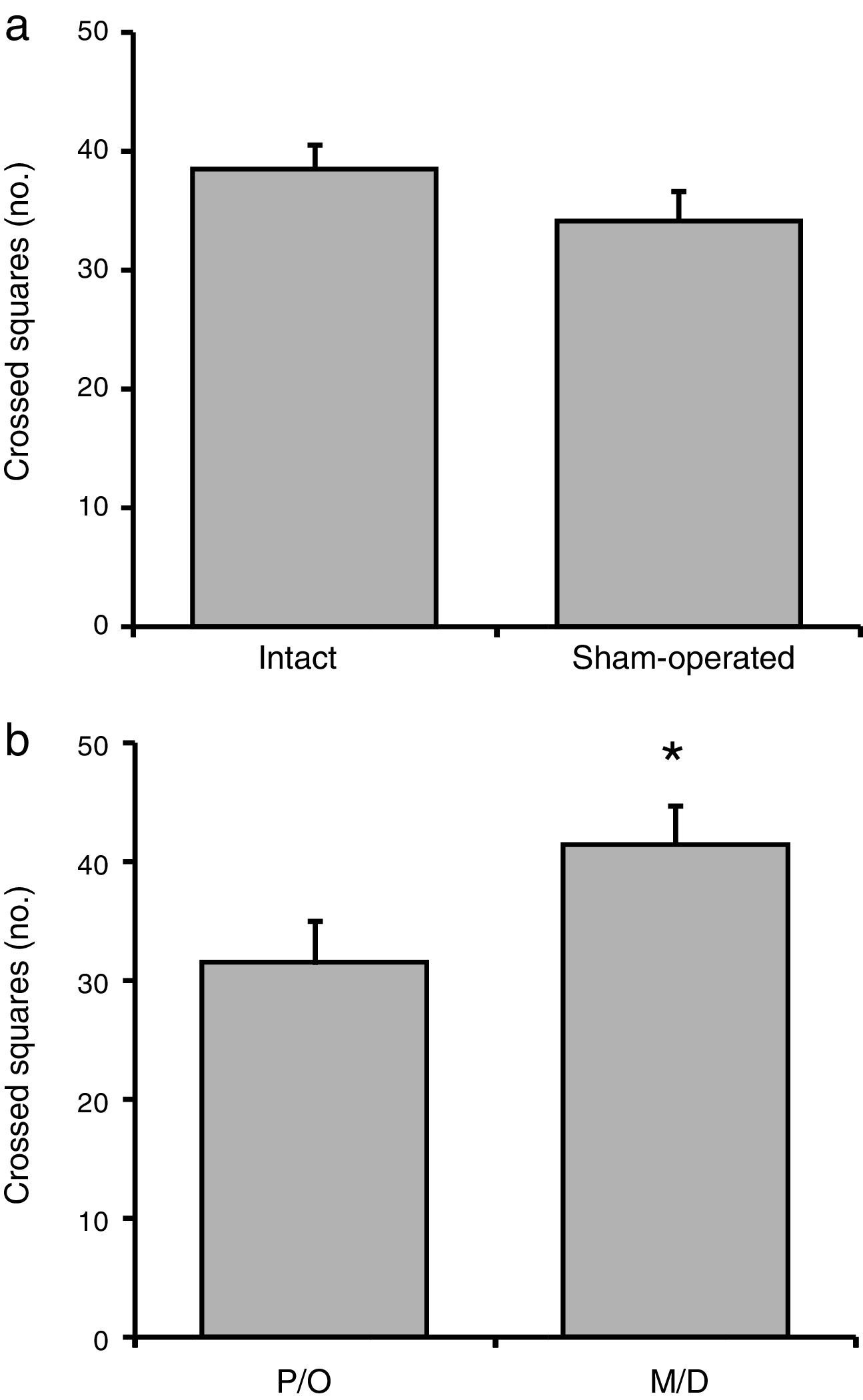
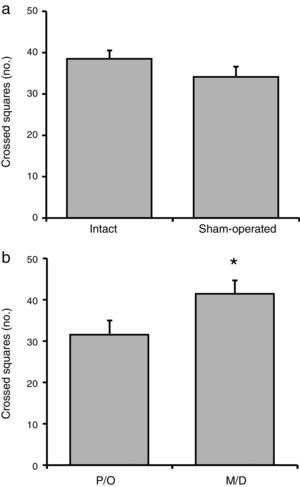
No significant difference in the number of crossed squares was observed between the intact and surgery groups (F[1, 23]=1.38; P=.25) (Fig. 2a). We did, however, find statistically significant differences between rats in the pro-oestrus/oestrus and in the metoestrus/dioestrus phases (F[1, 23]=6.58; P<.02). According to the post hoc test, rats in the metoestrus/dioestrus phase crossed a higher number of squares than those in the pro-oestrus/oestrus phase (Fig. 2b). Factor interaction revealed no significant differences (F[1, 23]=1.42; P=.25) (intact rats in pro-oestrus/oestrus phase [n=7]: 33.28±2.19; sham-operated rats in pro-oestrus/oestrus phase [n=6]: 33.34±3.07; intact rats in metoestrus/dioestrus phase [n=7]: 43.71±2.23; sham-operated rats in metoestrus/dioestrus phase [n=7]: 37.14±3.62).
Number of crossed squares in the locomotor activity test. (a) Surgery caused no significant changes in the number of crossed squares compared to intact rats. (b) Rats in the metoestrus/dioestrus (M/D) phase crossed a significantly higher number of squares than rats in the pro-oestrus/oestrus phase (P/O).
*P<.05 vs P/O.
No significant differences in grooming time were observed between intact and sham-operated rats (F[1,23]=0.63; P=.43; intact: 40.06±1.47s vs surgery: 41.51±1.23s) or related to phase of the oestrus cycle (F[1, 23]=2.89; P=.10; pro-oestrus/oestrus: 39.23±1.37s vs metoestrus/dioestrus: 42.34±1.24s). Likewise, interaction between factors revealed no significant differences (F[1, 23]=1.42; P=.25) (intact rats in pro-oestrus/oestrus phase [n=7]: 37.17±2.09s; sham-operated rats in pro-oestrus/oestrus phase [n=6]: 41.29±1.35s; intact rats in metoestrus/dioestrus phase [n=7]: 42.94±1.51s; sham-operated rats in metoestrus/dioestrus phase [n=7]: 42.74±2.06s).
Vertical behaviourNo significant differences in vertical time were observed between intact and sham-operated rats (F[1, 23]=1.43; P=.24; intact: 65.66±1.79s vs surgery: 68.47±1.49s) or in terms of phase of the oestrus cycle (F[1, 23]=2.18; P=.15; pro-oestrus/oestrus: 65.33±2.07s vs metoestrus/dioestrus: 68.79±1.14s). Factor interaction revealed no significant differences (F[1, 23]=0.14; P=.70) (intact rats in pro-oestrus/oestrus phase [n=7]: 63.47±3.03s; sham-operated rats in pro-oestrus/oestrus phase [n=6]: 67.17±2.83s; intact rats in metoestrus/dioestrus phase [n=7]: 67.84±1.79s; sham-operated rats in metoestrus/dioestrus phase [n=7]: 69.75±1.43s).
Forced swim testLateral swimming time and number of spinsAll animals, whether intact or sham-operated, showed a normal swimming pattern in the forced swim test; none displayed lateral swimming or spinning behaviour.
Latency to first immobility episodeWe observed no significant differences in latency to the first episode of immobility between intact and sham-operated rats (F[1, 23]=0.04; P=.83; intact: 26.84±2.25s vs surgery: 27.53±2.42s) or in terms of phase of the oestrus cycle (F[1, 23]=2.07; P=.16; pro-oestrus/oestrus: 29.54±1.84s vs metoestrus/dioestrus: 24.84±2.52s). Factor interaction revealed no significant differences (F[1, 23]=0.39; P=.53) (intact rats in pro-oestrus/oestrus phase [n=7]: 30.22±2.68s; sham-operated rats in pro-oestrus/oestrus phase [n=6]: 28.86±2.73s; intact rats in metoestrus/dioestrus phase [n=7]: 23.47±3.32s; sham-operated rats in metoestrus/dioestrus phase [n=7]: 26.21±3.96s).
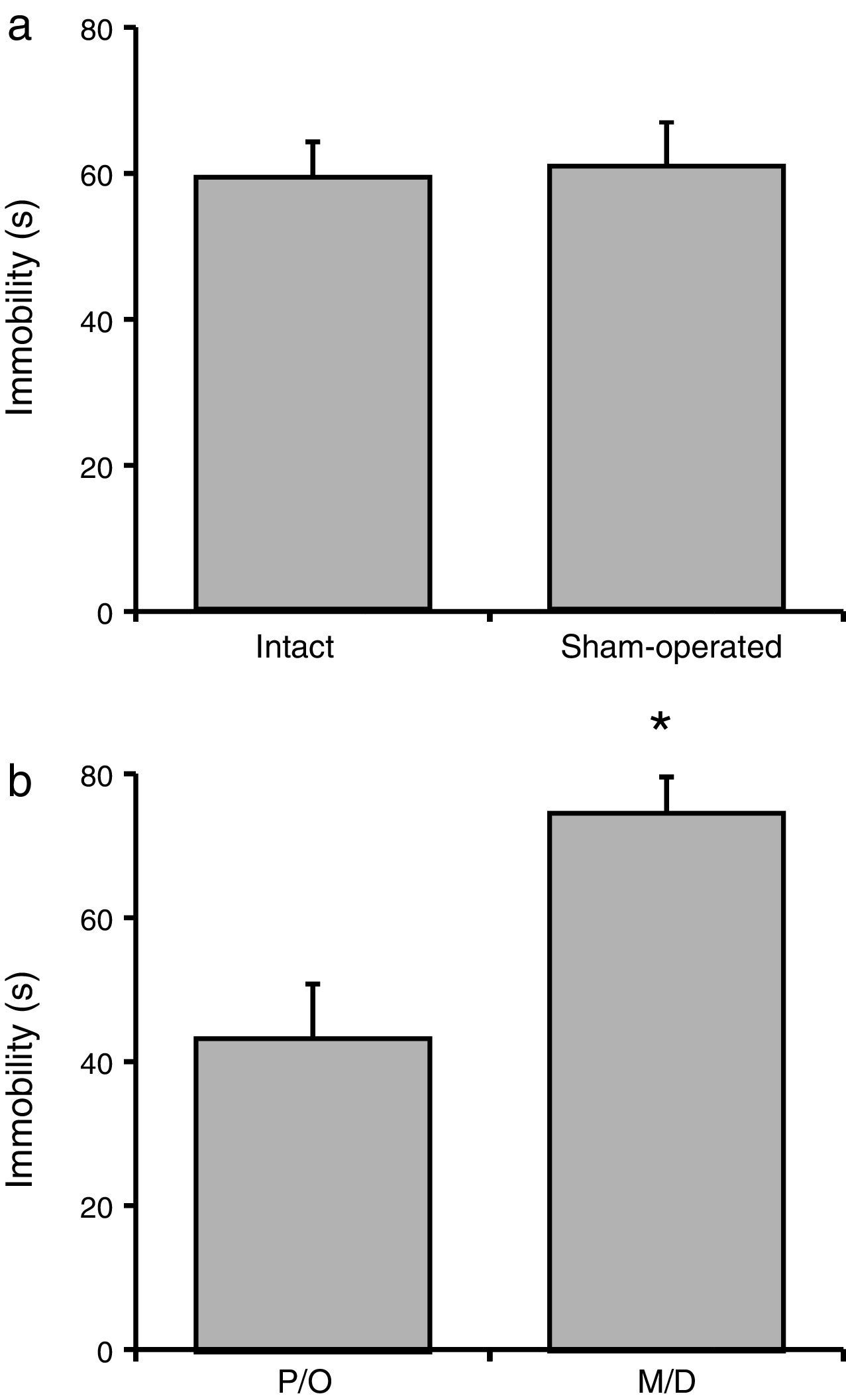
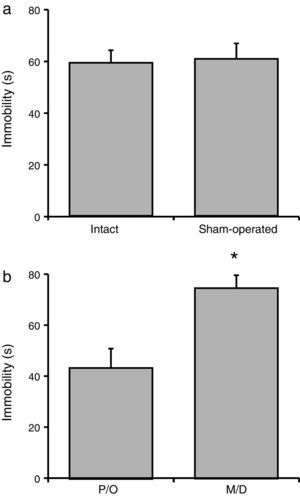
Total immobility timeNo significant difference in total immobility time was observed between the intact and sham-operated groups (F[1, 23]=21.06; P=.62) (Fig. 3a). However, there were statistically significant differences between rats in the pro-oestrus/oestrus phase and the metoestrus/dioestrus phase (F[1, 23]=90.95; P<.001). According to the post hoc test, rats in the pro-oestrus/oestrus phase were immobile for less time than those in the metoestrus/dioestrus phase (Fig. 3b). Nevertheless, factor interaction revealed no significant differences (F[1, 23]=77.21; P=.35) (intact rats in pro-oestrus/oestrus phase [n=7]: 44.00±6.61s; sham-operated rats in pro-oestrus/oestrus phase [n=6]: 42.38±7.75s; intact rats in metoestrus/dioestrus phase [n=7]: 74.91±9.07s; sham-operated rats in metoestrus/dioestrus phase [n=7]: 80.07±10.68s).
Rotarod testLatency to fallWe observed no significant difference in latency to fall between the intact and sham-operated groups (F[1, 23]=0.03; P=.85; intact: 151.47±5.36s vs surgery: 149.92±6.64s) or in terms of the phase of the oestrus cycle (F[1, 23]=3.40; P=.07; pro-oestrus/oestrus: 142.10±2.94s vs metoestrus/dioestrus: 158.29±7.11s). Factor interaction revealed no significant differences (F[1, 23]=0.14; P=.70) (intact rats in pro-oestrus/oestrus phase [n=7]: 142.32±3.78s; sham-operated rats in pro-oestrus/oestrus phase [n=7]: 143.10±4.96s; intact rats in metoestrus/dioestrus phase [n=7]: 160.62±9.08s; sham-operated rats in metoestrus/dioestrus phase [n=6]: 155.29±11.61s).
DiscussionIn our study, stereotactic surgery for cannula implantation plus vehicle microinjection into the hippocampal CA1 region had no negative impact on activity and motor coordination. All Wistar rats displayed the behavioural patterns typical of their oestrus cycle phase regardless of whether they had undergone stereotactic surgery.
The locomotor activity test is frequently used to quantify variables of displacement, exploration, and emotional status under experimental circumstances.30,31 In our study, the locomotor activity test was used to confirm that the experimental manipulation (stereotactic surgery for cannula implantation through the motor cortex plus vehicle microinjection into the dorsal hippocampus) caused no motor hyper- or hypoactivity (larger or smaller numbers of crossed squares), changes in exploration behaviour (vertical time), or changes in motivation (grooming time). Our results support the hypothesis that surgical manipulation and microinjection do not cause neurological damage. In line with this idea, neurotoxic lesions to the dorsal hippocampus have been reported to cause motor hyperactivity.11,18 Similarly, large quantities of cassava or cycad seeds have been observed to cause neurological alterations in humans.5,20,21,32 Our findings suggest that stereotactic surgery, cannula implantation, and microinjection in rats do not cause brain damage with potential to impair locomotor activity. Changes in motor activity were found to be associated with the phase of the oestrus cycle, but not with surgical manipulation. Rats in the metoestrus/dioestrus phase crossed a higher number of squares than rats in the pro-oestrus/oestrus phase. This increase in motor activity may be associated with higher levels of anxiety during the metoestrus/dioestrus phase, which is characterised by low concentrations of ovarian hormones.25,33
Grooming is regarded as a marker of emotional and motivational status in rats. Grooming time increases in rats experiencing mild stress34,35 and decreases under severe stress36,37 compared to intact rats. Our study found no significant differences in grooming time between intact and sham-operated rats, which suggests that stereotactic surgery and cannula implantation into the dorsal hippocampus have no impact on emotionality and motivation in rats. Likewise, our study revealed no association between vertical time and surgery or the phase of the oestrus cycle. Vertical behaviour is considered to be an indicator of exploration,38,39 which requires a high level of motor coordination. Vertical behaviour increases in rats with neuronal damage to the dorsal hippocampus, which is probably associated with hyperactivity and poor motor coordination.18 Rats that underwent surgery displayed no behavioural changes, suggesting that the surgical procedures described in this study cause no brain damage.
Rats with vestibular lesions40 or neurological impairment associated with the consumption of foods containing neurotoxic compounds18,19 display lateral swimming and spinning behaviour due to poor motor coordination. In our study, however, none of the rats (whether intact or sham-operated) displayed an abnormal swimming pattern, which rules out the possibility of surgery-related neurological damage. Latency to first immobility period and total immobility time in the forced swim test were similar in both groups (intact and sham-operated). Differences in total immobility time were linked only to the phase of the oestrus cycle. Rats in the metoestrus/dioestrus phase were immobile for a longer total time than rats in the pro-oestrus/oestrus phase. Our findings are consistent with results reported in the literature: rats in the metoestrus/dioestrus phase are more vulnerable to stress and are immobile for a longer time during the forced swim test, whereas rats in the pro-oestrus/oestrus phase, with high concentrations of ovarian hormones, are immobile for a shorter time, as occurs after administration of such hormones as progesterone and allopregnanolone.41,42
The rotarod test assesses motor coordination and balance.43 Intact (healthy) animals will be able to remain on the rotating rod for longer periods due to preserved limb coordination and balance.44 In contrast, animals with neurological alterations or receiving neurotoxic compounds or sedatives usually display shorter latencies to fall.45,46 In our study, rats that underwent stereotactic surgery for cannula implantation into the dorsal hippocampus displayed no significant difference in latency to fall compared to intact rats. Likewise, no differences were found between rats in the pro-oestrus/oestrus phase and those in the metoestrus/dioestrus phase. This suggests that experimental manipulations caused no neurological alterations potentially affecting motor coordination and activity.
Our results show that stereotactic surgery and cannula implantation into the dorsal hippocampus cause no alterations in locomotor activity and coordination in rats, ruling out the possibility that this type of manipulation may cause neurological impairment interfering with the interpretation of results from experimental studies.
FundingThis study was funded by grants I010/458/2013, C-703/2013 and I010/152/2014, C-133/2014.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank the Mexican National Council of Science and Technology (CONACyT) for financial support for postgraduate research in neuroethology, granted to the first, third, and fourth authors (scholarships 272294, 297410, and 297560, respectively).
Please cite this article as: Hernández-López F, Rodríguez-Landa JF, Puga-Olguín A, Germán-Ponciano LJ, Rivadeneyra-Domínguez E, Bernal-Morales B. Análisis de la actividad y coordinación motora en ratas con cirugía estereotáxica e implante de cánula en el hipocampo dorsal. Neurología. 2017;32:579–586.