Periodontal disease and dementia are very prevalent, especially in elderly populations. Multiple studies have shown a link between these diseases; however, the conditions are highly heterogeneous and so is the diagnostic methodology, which may hinder interpretation and comparison of the results. The aim of this article is to provide a critical review of the literature linking these 2 processes.
DevelopmentWe retrieved 22 studies, most of which were retrospective, and analysed various methodological variables including study population, diagnosis of periodontitis, definition of dementia, adjusted variables, and results. The different aetiopathogenic mechanisms that may affect the progression and interaction of these 2 conditions were also analysed.
ConclusionsAlthough available evidence indicates a positive association between periodontitis and dementia, both the strength of that association and the presence of a causal relationship have yet to be determined.
La enfermedad periodontal y la demencia son enfermedades muy prevalentes, especialmente en poblaciones envejecidas. Numerosos estudios han demostrado una relación entre ambas afecciones, pero la alta heterogeneidad en el diagnóstico, así como la metodología empleada, pueden dificultar la interpretación y la comparación de los resultados obtenidos. El objetivo de este artículo es realizar una revisión crítica de las publicaciones que asocian ambos procesos.
DesarrolloSe incluyen 22 artículos, mayoritariamente estudios retrospectivos, y se analizan diversas variables metodológicas, como población de estudio, diagnóstico de periodontitis, definición de demencia, variables ajustadas y resultados. Además, se analizan los diferentes mecanismos etiopatogénicos que pueden influir en la progresión de ambas enfermedades y en la interacción entre ellas.
ConclusionesAunque la evidencia disponible indica una tendencia positiva a la asociación entre periodontitis y demencia, se desconoce el grado de esta asociación y la existencia de una relación causal entre ambas enfermedades.
Periodontitis is a chronic inflammatory disease of infectious origin which manifests as destruction of the tissue supporting the teeth. This irreversible loss of support, caused by the presence of deep periodontal pockets and advanced attachment loss, may lead to tooth loss.1 The different types of periodontitis (chronic, aggressive, as a manifestation of systemic disease, or necrotising) are included in the group of periodontal diseases according to the last international consensus of 1999.2
Dementia is defined as a clinical syndrome characterised by persistent, progressive impairment of such higher brain functions as memory, language, orientation, calculation, and spatial perception. Prevalence increases exponentially with age, with the disease representing one of the main causes of dependence and disability among the elderly population.3 The disorder includes such conditions as Alzheimer disease (AD), vascular dementia (VD), and other less frequent types of dementia, such as Lewy body dementia, frontotemporal lobar degeneration, or dementia associated with Parkinson's disease. Although some diagnostic criteria for dementia include the presence of memory impairment, some types of dementia, such as frontotemporal lobar degeneration, Lewy body dementia, dementia associated with Parkinson's disease, and VD, may not present severe memory impairment until advanced stages.4
Periodontitis and dementia are highly prevalent in elderly patients. According to the 2010 Spanish survey on oral health,5 10.8% of the adult population aged between 65-74 have deep periodontal pockets, and 17.7% of this population cohort have a clinical attachment loss of at least 6mm. In Spain, dementia prevalence rates range from 5.2% to 16.3% among patients older than 65 years, reaching 22%-30% in patients aged 85 or older.3,4
Numerous recent studies have linked these 2 conditions, based on the study population involved and a possible common pathophysiological mechanism.
The aim of the present article is to review the results published up to November 2015 on the association between periodontal disease and dementia and on common pathophysiological mechanisms which may explain the possible relationship between both entities.
DevelopmentThe literature search was performed independently by 2 reviewers (PP and YL) in November 2015, using the PubMed, EMBASE, and Web of Science electronic databases. Furthermore, we also conducted a manual search on the most relevant periodontics and dementia journals. The search strategy used was as follows: (periodontitis OR periodontal disease) AND (cognition OR memory OR poor cognitive function OR dementia OR cognitive impairment OR cognitive decline OR cognitive function OR Alzheimer's disease OR cognitive disability OR memory impairment OR cognitive loss), using both MeSH terms and free-text terms.
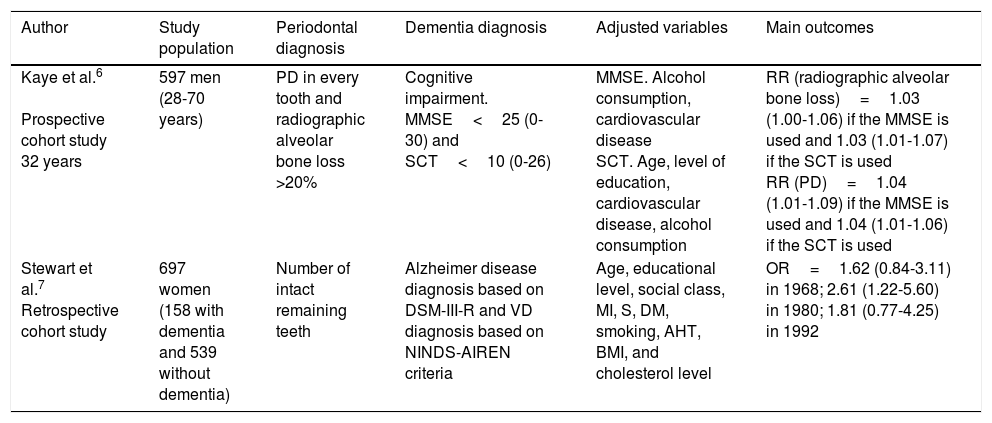
Studies on the association between periodontitis and dementiaTables 1–3 show the most significant cohort studies,6,7 case–control studies,8–10 and cross-sectional studies11–27 on the association between periodontitis and dementia. The interpretation of results is limited due to the lack of consensus on the definition of periodontal disease and the classification of neurological diseases; results are also limited due to patient selection.
Cohort studies.
| Author | Study population | Periodontal diagnosis | Dementia diagnosis | Adjusted variables | Main outcomes |
|---|---|---|---|---|---|
| Kaye et al.6 Prospective cohort study 32 years | 597 men (28-70 years) | PD in every tooth and radiographic alveolar bone loss >20% | Cognitive impairment. MMSE<25 (0-30) and SCT<10 (0-26) | MMSE. Alcohol consumption, cardiovascular disease SCT. Age, level of education, cardiovascular disease, alcohol consumption | RR (radiographic alveolar bone loss)=1.03 (1.00-1.06) if the MMSE is used and 1.03 (1.01-1.07) if the SCT is used RR (PD)=1.04 (1.01-1.09) if the MMSE is used and 1.04 (1.01-1.06) if the SCT is used |
| Stewart et al.7 Retrospective cohort study | 697 women (158 with dementia and 539 without dementia) | Number of intact remaining teeth | Alzheimer disease diagnosis based on DSM-III-R and VD diagnosis based on NINDS-AIREN criteria | Age, educational level, social class, MI, S, DM, smoking, AHT, BMI, and cholesterol level | OR=1.62 (0.84-3.11) in 1968; 2.61 (1.22-5.60) in 1980; 1.81 (0.77-4.25) in 1992 |
AHT: arterial hypertension; BMI: body mass index; DM: diabetes mellitus; DSM-III-R: Diagnostic and Statistical Manual of Mental Disorders III; MI: myocardial infarction; MMSE: Mini–Mental State Examination; NINDS-AIREN: National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences; OR: odds ratio; PD: probing depth; RR: relative risk; S: stroke; SCT: Spatial Copying Task; VD: vascular dementia.
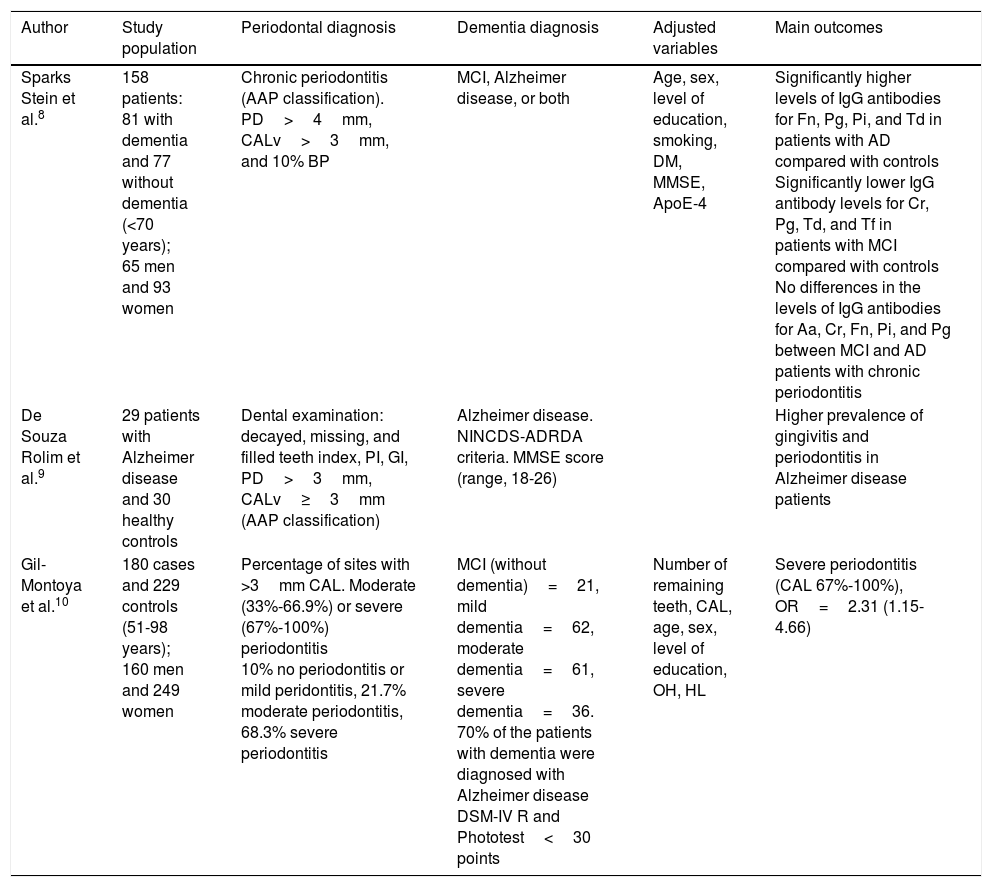
Case–control studies.
| Author | Study population | Periodontal diagnosis | Dementia diagnosis | Adjusted variables | Main outcomes |
|---|---|---|---|---|---|
| Sparks Stein et al.8 | 158 patients: 81 with dementia and 77 without dementia (<70 years); 65 men and 93 women | Chronic periodontitis (AAP classification). PD>4mm, CALv>3mm, and 10% BP | MCI, Alzheimer disease, or both | Age, sex, level of education, smoking, DM, MMSE, ApoE-4 | Significantly higher levels of IgG antibodies for Fn, Pg, Pi, and Td in patients with AD compared with controls Significantly lower IgG antibody levels for Cr, Pg, Td, and Tf in patients with MCI compared with controls No differences in the levels of IgG antibodies for Aa, Cr, Fn, Pi, and Pg between MCI and AD patients with chronic periodontitis |
| De Souza Rolim et al.9 | 29 patients with Alzheimer disease and 30 healthy controls | Dental examination: decayed, missing, and filled teeth index, PI, GI, PD>3mm, CALv≥3mm (AAP classification) | Alzheimer disease. NINCDS-ADRDA criteria. MMSE score (range, 18-26) | Higher prevalence of gingivitis and periodontitis in Alzheimer disease patients | |
| Gil-Montoya et al.10 | 180 cases and 229 controls (51-98 years); 160 men and 249 women | Percentage of sites with >3mm CAL. Moderate (33%-66.9%) or severe (67%-100%) periodontitis 10% no periodontitis or mild peridontitis, 21.7% moderate periodontitis, 68.3% severe periodontitis | MCI (without dementia)=21, mild dementia=62, moderate dementia=61, severe dementia=36. 70% of the patients with dementia were diagnosed with Alzheimer disease DSM-IV R and Phototest<30 points | Number of remaining teeth, CAL, age, sex, level of education, OH, HL | Severe periodontitis (CAL 67%-100%), OR=2.31 (1.15-4.66) |
Aa: Aggregatibacter actinomycetemcomitans; AAP: American Association of Periodontics; ApoE-4: ApoE-4 genotype; BP: bleeding on probing; CAL: clinical attachment loss; CALv: clinical attachment level; Cr: Campylobacter rectus; DM: diabetes mellitus; DSM-IV-R: Diagnostic and Statistical Manual of Mental Disorders IV; Fn: Fusobacterium nucleatum; GI: gingival index; HL: hyperlipidaemia; IgG: immunoglobulin G; MCI: mild cognitive impairment; MMSE: Mini–Mental State Examination; NINCDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association; OH: oral hygiene; OR: odds ratio; PD: probing depth; PI: plaque index; Pi: Prevotella intermedia; Pg: Porphyromonas gingivalis; Td: Treponema denticola; Tf: Tannerella forsythia.
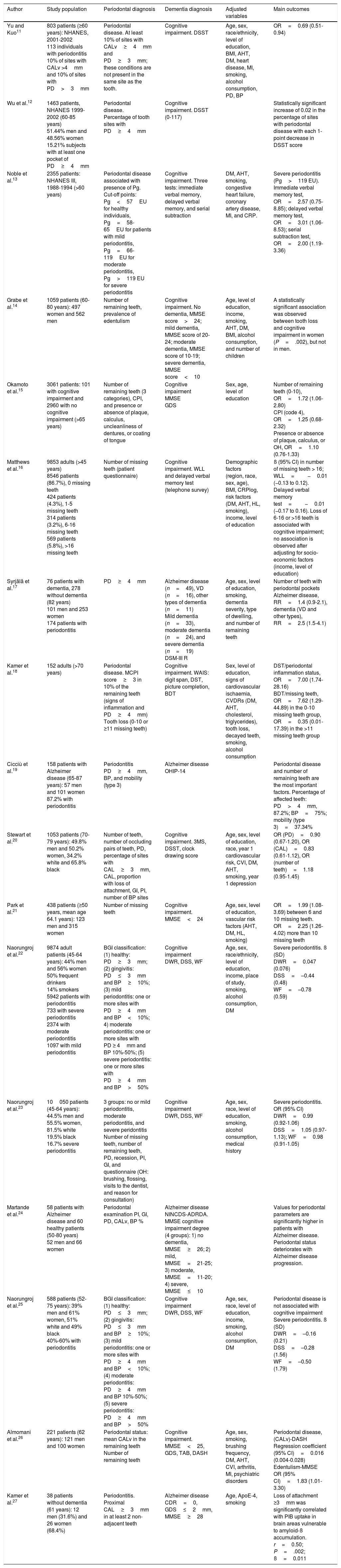
Cross-sectional studies.
| Author | Study population | Periodontal diagnosis | Dementia diagnosis | Adjusted variables | Main outcomes |
|---|---|---|---|---|---|
| Yu and Kuo11 | 803 patients (≥60 years): NHANES, 2001-2002 113 individuals with periodontitis 10% of sites with CALv >4mm and 10% of sites with PD>3mm | Periodontal disease. At least 10% of sites with CALv≥4mm and PD≥3mm; these conditions are not present in the same site as the tooth. | Cognitive impairment. DSST | Age, sex, race/ethnicity, level of education, BMI, AHT, DM, heart disease, MI, smoking, alcohol consumption, PD, BP | OR=0.69 (0.51-0.94) |
| Wu et al.12 | 1463 patients, NHANES 1999-2002 (60-85 years) 51.44% men and 48.56% women 15.21% subjects with at least one pocket of PD≥4mm | Periodontal disease. Percentage of tooth sites with PD≥4mm | Cognitive impairment. DSST (0-117) | Statistically significant increase of 0.02 in the percentage of sites with periodontal disease with each 1-point decrease in DSST score | |
| Noble et al.13 | 2355 patients: NHANES III, 1988-1994 (>60 years) | Periodontal disease associated with presence of Pg. Cut-off points: Pg<57EU for healthy individuals, Pg=58-65EU for patients with mild periodontitis, Pg=66-119EU for moderate periodontitis, Pg>119 EU for severe periodontitis | Cognitive impairment. Three tests: immediate verbal memory, delayed verbal memory, and serial subtraction | DM, AHT, smoking, congestive heart failure, coronary artery disease, MI, and CRP. | Severe periodontitis (Pg>119 EU). Immediate verbal memory test, OR=2.57 (0.75-8.85); delayed verbal memory test, OR=3.01 (1.06-8.53); serial subtraction test, OR=2.00 (1.19-3.36) |
| Grabe et al.14 | 1059 patients (60-80 years): 497 women and 562 men | Number of remaining teeth, prevalence of edentulism | Cognitive impairment. No dementia, MMSE score>24; mild dementia, MMSE score of 20-24; moderate dementia, MMSE score of 10-19; severe dementia, MMSE score<10 | Age, level of education, income, smoking, AHT, DM, BMI, alcohol consumption, and number of children | A statistically significant association was observed between tooth loss and cognitive impairment in women (P=.002), but not in men. |
| Okamoto et al.15 | 3061 patients: 101 with cognitive impairment and 2960 with no cognitive impairment (>65 years) | Number of remaining teeth (3 categories), CPI, and presence or absence of plaque, calculus, uncleanliness of dentures, or coating of tongue | Cognitive impairment MMSE GDS | Sex, age, level of education | Number of remaining teeth (0-10), OR=1.72 (1.06-2.80) CPI (code 4), OR=1.25 (0.68-2.32) Presence or absence of plaque, calculus, or OH, OR=1.10 (0.76-1.33) |
| Matthews et al.16 | 9853 adults (>45 years) 8546 patients (86.7%), 0 missing teeth 424 patients (4.3%), 1-5 missing teeth 314 patients (3.2%), 6-16 missing teeth 569 patients (5.8%), >16 missing teeth | Number of missing teeth (patient questionnaire) | Cognitive impairment. WLL and delayed verbal memory test (telephone survey) | Demographic factors (region, race, sex, age), BMI, CRPlog, risk factors (DM, AHT, HL, smoking), income, level of education | ß (95% CI) in number of missing teeth > 16; WLL=−0.01 (−0.13 to 0.12). Delayed verbal memory test=−0.01 (−0.17 to 0.16). Loss of 6-16 or >16 teeth is associated with cognitive impairment; no association is observed after adjusting for socio-economic factors (income, level of education) |
| Syrjälä et al.17 | 76 patients with dementia, 278 without dementia (82 years) 101 men and 253 women 174 patients with periodontitis | PD≥4mm | Alzheimer disease (n=49), VD (n=16), other types of dementia (n=11) Mild dementia (n=33), moderate dementia (n=24), and severe dementia (n=19) DSM-III R | Age, sex, level of education, smoking, dementia severity, type of dwelling, and number of remaining teeth | Number of teeth with periodontal pockets Alzheimer disease, RR=1.4 (0.9-2.1), dementia (VD and other types), RR=2.5 (1.5-4.1) |
| Kamer et al.18 | 152 adults (>70 years) | Periodontal disease. MCPI score≥3 in 10% of the remaining teeth (signs of inflammation and PD≥4mm) Tooth loss (0-10 or ≥11 missing teeth) | Cognitive impairment. WAIS: digit span, DST, picture completion, BDT | Sex, level of education, signs of cardiovascular ischaemia, CVDRs (DM, AHT, cholesterol, triglycerides), tooth loss, decayed teeth, smoking, alcohol consumption | DST/periodontal inflammation status, OR=7.00 (1.74-28.16) BDT/missing teeth, OR=7.62 (1.29-44.89) in the 0-10 missing teeth group, OR=0.35 (0.01-17.39) in the >11 missing teeth group |
| Cicciù et al.19 | 158 patients with Alzheimer disease (65-87 years): 57 men and 101 women 87.2% with periodontitis | Periodontitis PD≥4mm, BP, and mobility (type 3) | Alzheimer disease OHIP-14 | Periodontal disease and number of remaining teeth are the most important factors. Percentage of affected teeth: PD>4mm, 87.2%; BP=75%; mobility (type 3)=37.34% | |
| Stewart et al.20 | 1053 patients (70-79 years): 49.8% men and 50.2% women, 34.2% white and 65.8% black | Number of teeth, number of occluding pairs of teeth, PD, percentage of sites with CAL≥3mm, CAL, proportion with loss of attachment, GI, PI, number of BP sites | Cognitive impairment. 3MS, DSST, clock drawing score | Age, sex, level of education, race, year 1 cardiovascular risk, CVI, DM, AHT, smoking, year 1 depression | OR (PD)=0.90 (0.67-1.20), OR (CAL)=0.83 (0.61-1.12), OR (number of teeth)=1.18 (0.95-1.45) |
| Park et al.21 | 438 patients (≥50 years, mean age 64.1 years): 123 men and 315 women | Number of missing teeth | Cognitive impairment. MMSE<24 | Age, sex, level of education, vascular risk factors (AHT, DM, HL, smoking) | OR=1.99 (1.08-3.69) between 6 and 10 missing teeth. OR=2.25 (1.26-4.02) more than 10 missing teeth |
| Naorungroj et al.22 | 9874 adult patients (45-64 years): 44% men and 56% women 50% frequent drinkers 14% smokers 5942 patients with periodontitis 733 with severe periodontitis 2374 with moderate periodontitis 1097 with mild periodontitis | BGI classification: (1) healthy: PD≥3mm; (2) gingivitis: PD≤3mm and BP≥10%; (3) mild periodontitis: one or more sites with PD≥4mm and BP<10%; 4) moderate periodontitis: one or more sites with PD ≥ 4mm and BP 10%-50%; (5) severe periodontitis: one or more sites with PD≥4mm and BP>50% | Cognitive impairment DWR, DSS, WF | Age, sex, race/ethnicity, level of education, income, place of study, smoking, alcohol consumption, DM | Severe periodontitis. ß (SD) DWR=0.047 (0.076) DSS=–0.44 (0.48) WF=–0.78 (0.59) |
| Naorungroj et al.23 | 10050 patients (45-64 years): 44.5% men and 55.5% women, 81.5% white 19.5% black 16.7% severe periodontitis | 3 groups: no or mild periodontitis, moderate periodontitis, and severe peridontitis Number of missing teeth, number of remaining teeth, PD, recession, PI, GI, and questionnaire (OH: brushing, flossing, visits to the dentist, and reason for consultation) | Cognitive impairment DWR, DSS, WF | Age, sex, race, level of education, smoking, alcohol consumption, medical history | Severe periodontitis. OR (95% CI) DWR=0.99 (0.92-1.06) DSS=1.05 (0.97-1.13); WF=0.98 (0.91-1.05) |
| Martande et al.24 | 58 patients with Alzheimer disease and 60 healthy patients (50-80 years) 52 men and 66 women | Periodontal examination PI, GI, PD, CALv, BP % | Alzheimer disease NINCDS-ADRDA. MMSE cognitive impairment degree (4 groups): 1) no dementia, MMSE≥26; 2) mild, MMSE=21-25; 3) moderate, MMSE=11-20; 4) severe, MMSE≤10 | Values for periodontal parameters are significantly higher in patients with Alzheimer disease. Periodontal status deteriorates with Alzheimer disease progression. | |
| Naorungroj et al.25 | 588 patients (52-75 years): 39% men and 61% women, 51% white and 49% black 40%-60% with periodontitis | BGI classification: (1) healthy: PD≤3mm; (2) gingivitis: PD≤3mm and BP≥10%; (3) mild periodontitis: one or more sites with PD≥4mm and BP<10%; (4) moderate periodontitis: PD≥4mm and BP 10%-50%; (5) severe periodontitis: PD≥4mm and BP>50% | Cognitive impairment DWR, DSS, WF | Age, sex, race, level of education, income, smoking, alcohol consumption, DM | Periodontal disease is not associated with cognitive impairment Severe periodontitis. ß (SD) DWR=–0.16 (0.21) DSS=–0.28 (1.56) WF=–0.50 (1.79) |
| Almomani et al.26 | 221 patients (62 years): 121 men and 100 women | Periodontal status: mean CALv in the remaining teeth Number of remaining teeth | Cognitive impairment. MMSE<25, GDS, TAB, DASH | Age, sex, smoking, brushing frequency, DM, AHT, CVI, arthritis, MI, psychiatric disorders | Periodontal disease, (CALv)-DASH Regression coefficient (95% CI)=0.016 (0.004-0.028) Edentulism-MMSE OR (95% CI)=1.83 (1.01-3.30) |
| Kamer et al.27 | 38 patients without dementia (61 years): 12 men (31.6%) and 26 women (68.4%) | Periodontitis. Proximal CAL≥3mm in at least 2 non-adjacent teeth | Alzheimer disease CDR=0, GDS≤2mm, MMSE≥28 | Age, ApoE-4, smoking | Loss of attachment ≥3mm was significantly correlated with PIB uptake in brain areas vulnerable to amyloid-ß accumulation. r=0.50; P=.002; ß=0.011 |
AHT: arterial hypertension; ApoE-4: ApoE-4 genotype; ß: beta coefficient; BDT: Block Design Test; BGI: biofilm-gingival interface; BMI: body mass index; BP: bleeding on probing; CAL: clinical attachment loss; CALv: clinical attachment level; CDR: Clinical Dementia Rating; CI: confidence interval; CPI: Community Periodontal Index; CRP: C-reactive protein; CRPlog: C-reactive protein logarithm; CVDR: cardiovascular disease risk factor; CVI: cardiovascular infarct; DASH: Disability of Arm, Shoulder and Hand test; DM: diabetes mellitus; DSM-III-R: Diagnostic and Statistical Manual of Mental Disorders III; DSS: Digit Symbol Substitution; DSST: Digit Symbol Substitution Test; DST: Digital Symbol Test; DWR: Delayed Word Recall; EU: ELISA units; GDS: Geriatric Depression Scale; GI: gingival index; HL: hyperlipidaemia; MCPI: Modified Community Periodontal Index; MI: myocardial infarct; MMSE: Mini–Mental State Examination; NHANES: National Health and Nutrition Examination Survey; NINCDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; OH: oral hygiene; OHIP-14: Oral Health Impact Profile-14; OR: odds ratio; PD: probing depth; PI: plaque index; PIB: Pittsburgh compound B; Pg: Porphyromonas gingivalis; r: correlation coefficient; RR: relative risk; SD: standard deviation; TAB: Tinetti Assessment Battery for gait and balance; VD: vascular dementia; WAIS: Wechsler Adult Intelligence Scale; WF: word fluency; WLL: word list learning; 3 MS: modified Mini–Mental State Examination.
Periodontal disease is defined in multiple ways in the reviewed studies. This lack of homogeneity in the diagnosis of the condition makes comparison of results difficult. Correct periodontal diagnosis requires a clinical examination evaluating such parameters as periodontal pocket probing depth (PD),6,9,11,12,15,18–20,22–25,27 clinical attachment level,8–11,13,15,23–25,27 presence of bleeding on probing,8,19,22,24 plaque index observed during the examination,24 tooth mobility,19 or radiography values, such as alveolar bone loss,6 which are used as complementary diagnostic methods. However, some publications define periodontitis less reliably and accurately, recording tooth losses or absences at the time of oral examination, without considering the causes.7,14,15,18,20,21,26 Cavities, tooth fracture due to trauma, or orthodontic considerations are possible reasons why dental extraction may be necessary. Another method is the use of a questionnaire administered directly to the patient, in which he or she is asked to indicate the number of missing teeth. This self-evaluation enables researchers to include a large number of subjects in the study, although the periodontal diagnosis is not reliable.16 A study published in 2009 defined the different degrees of periodontal disease according to a microbiological criterion, classifying periodontitis as severe, moderate, or advanced based on the presence of Porphyromonas gingivalis, highly prevalent bacteria in periodontally compromised patients.13
As with periodontitis, the definition of dementia is not homogeneous in the reviewed studies, and few studies differentiate between AD, VD, and other types of dementia.7,17 In other cases, dementia is classified according to the level of disease severity (mild, moderate, or severe).10,14,17,24 Furthermore, different diagnostic tests are used, making it difficult to compare the impairment caused by the disease in each study population. The most widely used test is the Mini–Mental State Examination,6,9,14,15,20,24,26,27 created by Folstein et al.28 in 1975. There was also a lack of homogeneity in the diagnostic criteria used. In some cases, the different versions and revisions of the American Psychiatric Association criteria are used, as well as those included in the Diagnostic and Statistical Manual of Mental Disorders (DSM-II-R, DSM-III-R, DSM-IV-R).29 Other studies use the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association30 for the diagnosis of AD7,9,10,17,24 or the criteria of the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN)31 for VD.7 Isolated tests are used occasionally, whereas other studies use a combination of several tests, such as the Digit Symbol Substitution Test,11,12,18,22,23,25,32 which is part of the battery included in the Wechsler Adult Intelligence Scale.18 Some researchers use diagnostic tests based on a series of questions, such as the Oral Health Impact Profile-14,19 while others use immediate or delayed recall tests and repetition series,13 and in some cases, questionnaires administered by telephone.16
Another factor to take into account is the age of the subjects included in the studies. Some studies establish age ranges between 45 and 87 years14,19,20,22–25; others establish a mean age of 4516 or include only patients aged 65 or older,10,15,17,18,26,27 whereas one prospective study includes younger patients, establishing a range between 28 and 70 years.6 As can be observed in the reviewed publications, prevalence of dementia increases exponentially with age. This is also the case with periodontitis; therefore, both conditions are associated with older populations.
Sample size is also highly heterogeneous. Cross-sectional studies include between 152 and 9853 subjects,16,18 whereas cohort studies include 597 to 697 patients.6,7 Case–control studies use smaller samples, with the number of subjects ranging from 29 to 180 cases9,10 and from 30 to 229 controls.9,10 This great variability makes it difficult to compare results between the different publications.
In most of the studies included, some variables correspond to common risk factors for periodontitis and dementia, and may lead to misinterpretation of the relationship between these 2 processes; therefore, these variables have been considered when performing the statistical analysis. Some of these confounding factors were age,6,7,10,11,14–17,20–23,25,26 sex,10,11,15–18,20–23,25,26 smoking,11,13,14,16–18,20–23,25,26 alcohol consumption,6,11,14,18,22,23,25 such systemic conditions as diabetes mellitus and arterial hypertension, stroke, risk of cardiovascular diseases, hyperlipidaemia, serum cholesterol levels, body mass index, psychiatric disorders,6,7,10,11,13,14,16,18,20–23,25,26 level of education,6,7,10,11,14–18,20–23,25 socioeconomic status,14,16,22,25 the number of remaining teeth or decayed teeth,10,17,18 and oral hygiene habits.10,26
The studies analysed show varying levels of association between periodontitis and dementia. Associations were expressed as odds ratios in retrospective studies and as relative risk in prospective studies. Retrospective studies showed an odds ratio ranging from 0.6911 to 7.62,18 whereas in the prospective study, the relative risk was 1.03 when analysing the radiographic bone loss and 1.04 when such clinical parameters as PD were analysed.6 Data should be analysed in depth, since odds ratios are obtained from the combination of radiographic periodontal variables,6 microbiological variables,13 and clinical variables such as tooth loss,7,15,18,21,26 oral hygiene,15 or PD and the clinical attachment level.6,10,11,15,18,23 The latter 2 parameters are the most reliable at a methodological level. It should be noted that on many occasions, multiple assessments are used to establish a diagnosis of periodontitis.6,7,10,13,15,23 Furthermore, the most widely used neurological tests are the Mini–Mental State Examination,6,15,21,26 the Digit Symbol Substitution Test,11,23 the Wechsler Adult Intelligence Scale,18 and immediate and delayed recall tests and repetition series.13 The cognitive impairment and dementia diagnostic criteria used were those of the DSM-III-R,7,17 DSM-IV-R,10 and NINDS-AIREN.7
Whereas the studies by Yu and Kuo11 and Naorungroj et al.,23 published in 2008 and 2013, respectively, do not report an association between periodontitis and dementia, Okamoto et al.15 and Kamer et al.18 did find a positive association when they established the number of present teeth as main variable. However, when the variable analysed was the presence of plaque, PD, or the clinical attachment level, said association was not significant. The same occurs with other authors, who found a positive association depending on the type of neurological test used.13,21 When associating the number of remaining teeth with Mini–Mental State Examination score,21,26 or the presence of severe periodontitis with DSM-IV-R criteria,10 a positive association was found between periodontitis and cognitive impairment.
Some of the studies analysed lack measures of frequency. Such authors as Grabe et al.14 show their results in clinical terms, concluding that tooth loss presents a statistically significant association with cognitive impairment (this association was only observed in women). In 2013, Cicciú et al.19 confirmed that the oral health issues most influencing quality of life are periodontal disease, PD>4mm, bleeding on probing, and the number of remaining teeth. They conclude that the absence of more than 2 molar teeth increases chewing inability, decreasing the patient's quality of life. Furthermore, patients with AD and severe cognitive impairment present greater PD and extent of periodontal attachment loss ≥3mm than patients with no neurological diseases; these differences are statistically significant.24
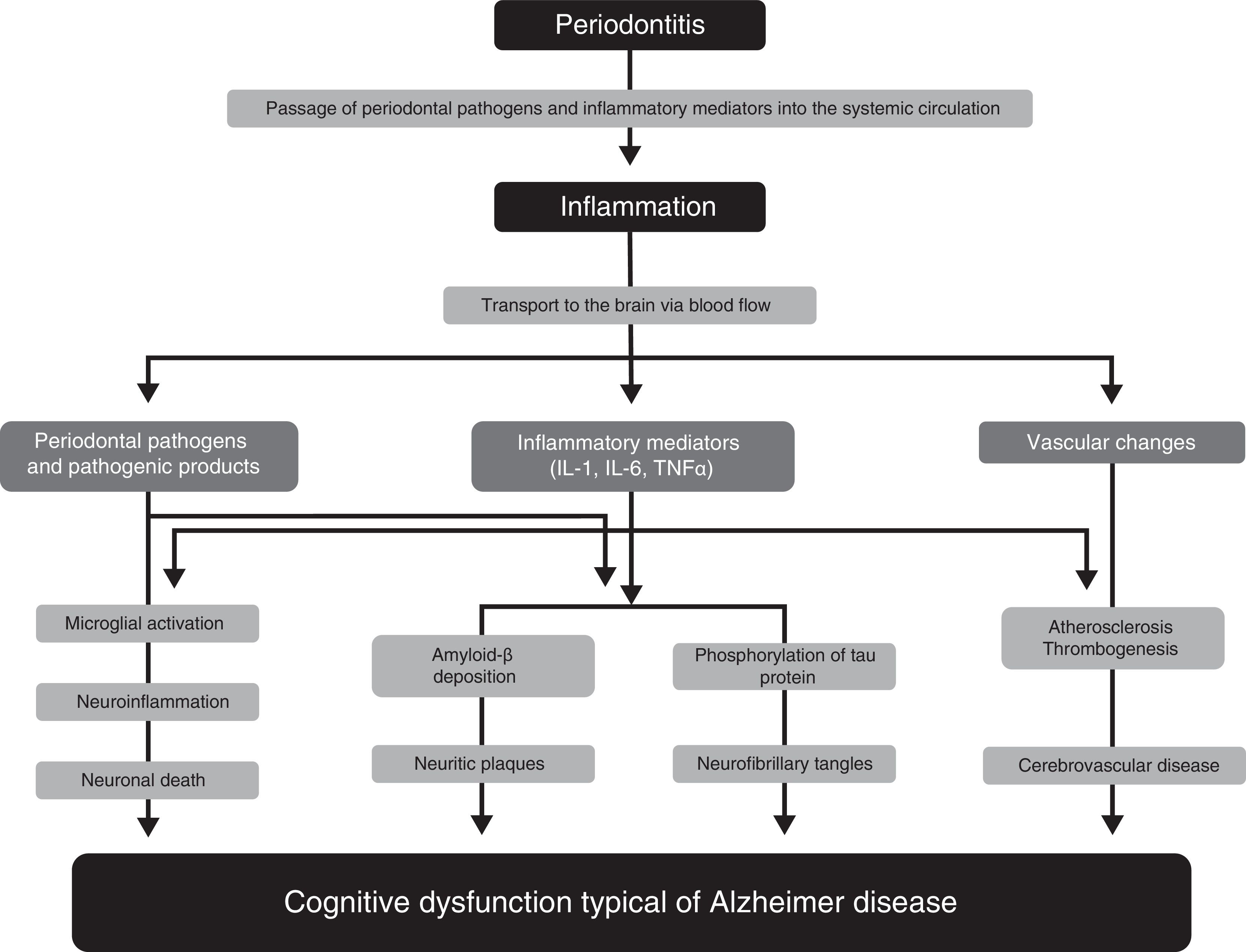
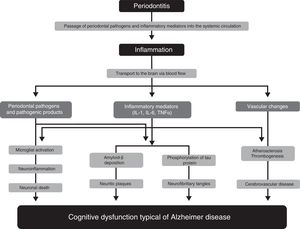
Pathophysiological mechanisms participating in the association between periodontitis and dementiaNumerous studies have proposed several pathophysiological mechanisms that may explain the participation of periodontitis in the pathogenesis of AD33–36 (Fig. 1). One of these mechanisms is the passage of pathogenic agents and inflammatory mediators from the oral cavity to the systemic circulation. When the physical, chemical, or immunological barriers of the oral cavity are affected, patients may develop bacteraemia. There is evidence that during such everyday activities as chewing or brushing or flossing teeth,37,38 periodontal pathogens and their products may induce the production of proinflammatory cytokines, such as interleukin (IL) 1, IL-6, and tumour necrosis factor α. Subjected to a repeated bacterial exposure, receptors of these cytokines may become saturated and cytokines may reach the systemic circulation. In advanced stages of the disease, periodontitis may cause systemic inflammation, as has been shown by some studies in which periodontal patients present higher levels of C-reactive protein than healthy controls.15,17
Pathophysiological mechanisms that potentially explain the association between periodontitis and dementia.
Modified from Uppoor et al.33
Some studies, such as the one by Rai et al.,39 show that total leucocyte, neutrophil, and platelet counts, and the levels of such proinflammatory markers as C-reactive protein, matrix metalloproteinase-8, matrix metalloproteinase-9, and tumour necrosis factor α, were significantly higher in patients with dementia and periodontitis than in controls. The major limitation of this study is that it does not specify the criteria used for the diagnosis of dementia. Some authors report evidence on the role of inflammation as a link between periodontitis and cognitive impairment, and on the benefit of non-steroidal anti-inflammatory drugs in the development of the early stages of dementia.40,41
Another pathophysiological pathway which may link the 2 conditions involves microorganisms from the dental plaque accessing brain tissue, either through direct tissue invasion or through the blood flow or peripheral nerves. Such periodontopathogens as Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, Fusobacterium nucleatum, and Prevotella intermedia have been found to be involved in brain abscesses, demonstrating their capacity to invade brain tissues. One study reports that some oral Treponema species, such as Treponema denticola, are more frequently detected in the brains of AD patients than in subjects without the disease.42
These pathogens, or the inflammatory mediators they produce, may cross the blood-brain barrier and access the brain tissue, where they can trigger a cascade of reactions inducing tissue destruction. The typical brain lesions in AD consist of extracellular neuritic plaques originated by deposition of amyloid-ß, which is derived from abnormal metabolism of the amyloid precursor protein, and neurofibrillary tangles formed by the intracellular accumulation of hyperphosphorylated tau protein. It has been suggested that IL-1 plays a fundamental role in the processing of amyloid precursor protein and favours abnormal deposition of amyloid-ß in the brain. Amyloid-ß triggers the activation of microglial cells which produce acute-phase reactants, prostaglandins, and cytokines (some of which are neurotoxic), stimulating increased production of amyloid-ß and the formation of neurofibrillary tangles. Furthermore, IL-1 increases the production of nitric oxide synthase and acetylcholinesterase; other inflammatory mediators, such as IL-6 and tumour necrosis factor α, modulate the neuroinflammatory cascade.43,44 All these changes initially cause synaptic dysfunction and eventually lead to neuronal death.
Growing evidence supports the important role of vascular factors in AD. Thus, an increased burden of neuritic plaques and neurofibrillary tangles has been described in patients with severe atherosclerotic lesions. The available evidence shows that periodontitis may play a significant role in atherosclerosis and its complications. Such periodontopathogens as P. gingivalis and Streptococcus sanguis increase expression of platelet aggregation-associated protein and may favour the formation of atheromatous plaques, forming thrombi that may cause a cerebrovascular event. Systemic inflammation caused by periodontal pathogens also play a significant role in endothelial dysfunction. As it contributes to the formation of atherosclerotic plaques and the development of endothelial dysfunction, periodontal disease may represent a risk factor for the development of cognitive impairment and its progression to AD.45 This hypothesis is also consistent with the fact that patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) present a higher risk and prevalence of periodontal disease.46
Finally, a bidirectional relationship has also been proposed between periodontal disease and AD.47 According to this hypothesis, AD may predispose to the development of chronic periodontitis. This may be explained by the fact that patients with AD have poorer oral hygiene, either due to decreased or absent manual dexterity for performing mechanical plaque control at home, or because the patient is unable to visit a dentist for professional dental care (for example, oral hygiene instructions, tartrectomy, scaling, and root planing). These factors would lead to the development of periodontal infection, which ultimately causes tooth loss.48,49 Supporting this argument, one study showed that significantly more patients with dementia than individuals without dementia required help brushing their teeth (58.2% vs 0.9%; P<.01). Furthermore, caregivers from the hospital where the study was performed observed that patients with dementia had more difficulties with maintaining proper oral hygiene (for example, refusal to brush teeth, refusal to open the mouth, or patients forgetting to brush their teeth). For this reason, patients diagnosed with dementia presented significantly higher levels of plaque than subjects without dementia at one year of follow-up (P<.01).50 However, we should be cautious when interpreting the results of this study, as the researchers did not assess the key periodontal parameters that define periodontal disease, such as PD, clinical periodontal attachment loss, or bleeding on probing.
ConclusionsIn conclusion, the available evidence does suggest a positive trend towards an association between periodontitis and dementia. However, we do not know the degree of this association or whether there is a causal relationship between these diseases, due to the great heterogeneity in the definitions of both conditions and the methodology used in the studies analysed, and the variety of pathophysiological mechanisms involved. There is a need for prospective observational studies, which should be well-designed, exhaustively classify periodontal disease (type, extension, and severity), and unequivocally define the type of dementia being studied. These studies should also include homogeneous and representative study populations, enabling the risk factors involved in the interaction between these 2 diseases to be identified.
FundingY. Leira is the recipient of a pre-doctoral scholarship granted by the Instituto de Investigación Sanitaria de Santiago de Compostela.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pazos P, Leira Y, Domínguez C, Pías-Peleteiro JM, Blanco J, Aldrey JM. Asociación entre enfermedad periodontal y demencia. Revisión de la bibliografía. Neurología. 2018;33:602–613.