Carpal tunnel syndrome is the most common peripheral neuropathy. It is characterised by the compression of the median nerve in the carpal tunnel. It presents a high prevalence and is a disabling condition from the earliest stages. Severe cases are usually treated surgically, while conservative treatment is recommended in mild-to-moderate cases. The aim of this systematic review is to present the conservative treatments and determine their effectiveness in mild-to-moderate cases of carpal tunnel syndrome in the last 15 years.
MethodsA systematic review was performed according to PRISMA criteria. We used the Medline, PEDro, and Cochrane databases to find and select randomised controlled clinical trials evaluating the effects of conservative treatment on the symptoms and functional ability of patients with mild-to-moderate carpal tunnel syndrome; 32 clinical trials were included. There is evidence supporting the effectiveness of oral drugs, although injections appear to be more effective. Splinting has been shown to be effective, and it is also associated with use of other non-pharmacological techniques. Assessments of the use of electrotherapy techniques alone have shown no conclusive results about their effectiveness. Other soft tissue techniques have also shown good results but evidence on this topic is limited. Various treatment combinations (drug and non-pharmacological treatments) have been proposed without conclusive results.
ConclusionsSeveral conservative treatments are able to relieve symptoms and improve functional ability of patients with mild-to-moderate carpal tunnel syndrome. These include splinting, oral drugs, injections, electrotherapy, specific manual techniques, and neural gliding exercises as well as different combinations of the above. We have been unable to describe the best technique or combination of techniques due to the limitations of the studies; therefore, further studies of better methodological quality are needed.
El Síndrome del Túnel Carpiano [STC] es la neuropatía periférica más común. Consiste en la compresión del nervio mediano a nivel de túnel carpiano. Tiene una alta prevalencia y genera una situación muy discapacitante desde las primeras fases. En los casos graves el tratamiento suele ser quirúrgico, mientras que en los leves y moderados el tratamiento es conservador. El objetivo de esta revisión es conocer los tratamientos conservadores, así como su efectividad, en pacientes con STC leve y moderado, en los 15 últimos años.
DesarrolloSe realizó una revisión sistemática según los criterios de PRISMA. Se emplearon las bases de datos Medline, PEDro y Cochrane. Se seleccionaron aquellos ensayos clínicos controlados y aleatorizados que analizasen los efectos del tratamiento conservador sobre los síntomas y la función en pacientes con STC leve o moderado. Se incluyeron 32 ensayos clínicos. Existe evidencia sobre la efectividad de los fármacos orales aunque las infiltraciones parecen ser más efectivas. El uso de férulas ha mostrado ser efectiva y asociada a otras técnicas no farmacológicas también. Las técnicas de electroterapia no han mostrado resultados concluyentes sobre la efectividad de forma aislada. Otras técnicas de tejido blando también han mostrado buenos resultados pero es escasa la evidencia en este campo. También se han propuesto varias combinaciones de tratamiento farmacológico con no farmacológico sin resultados concluyentes.
ConclusionesExisten varios tratamientos conservadores capaces de mejorar los síntomas y la función de los pacientes con STC leve y moderado. Éstos incluyen el uso de férulas, fármacos orales, infiltraciones, técnicas de electroterapia, técnicas manuales específicas y ejercicios de deslizamiento neural, así como la combinación de varias de ellas. No ha sido posible describir la mejor técnica o combinación de técnicas debido a las limitaciones de los estudios, por lo que es necesario realizar más estudios con una calidad metodológica adecuada.
Carpal tunnel syndrome (CTS), the most common peripheral neuropathy, is defined as the set of signs and symptoms caused by compression of the median nerve at the level of the wrist, as it passes through the carpal tunnel.1
The condition is thought to affect 3.8%-4.9% of the general population,1 with a higher prevalence in women2; peak prevalence occurs between the ages of 50 and 59.1
Symptoms vary and often present bilaterally.3,4 CTS is characterised by pain, numbness, and/or a tingling sensation in the hand, wrist, and the first 3 digits (sometimes extending beyond these areas), and has a tendency to worsen at night.3,4 In advanced stages, patients display reduced hand strength and function. One noteworthy aspect of the syndrome is that there is not always a direct connection between the degree of nerve conduction impairment and symptom severity; some patients with mild-or-moderate nerve damage display severe symptoms and reduced functional capacity.5
Researchers agree that when aetiology is related to a systemic condition, treatment should aim to control these factors.6 The severity of the condition must also be taken into account when selecting a therapeutic approach. Surgery is the preferred treatment for severe CTS,7 whereas mild-to-moderate cases should be managed with conservative treatments. Although numerous conservative treatments are available,7 there is no consensus as to the best non-surgical techniques for the treatment of mild to moderate CTS.8
In the light of the high prevalence and incidence, the significant functional limitations, and the socioeconomic costs associated with the condition (it is considered an occupational disease),9 we reviewed articles published in the last 15 years in order to assess the effectiveness of conservative treatment for patients with mild-to-moderate CTS.
DevelopmentWe performed a systematic review in accordance with the criteria set out in the PRISMA statement. We conducted literature searches of clinical trials on the Medline, PEDro, Cochrane, and Scopus databases, using the following MESH terms: “carpal tunnel syndrome,” “treatment outcome,” and “physical therapy.”
The search terms were combined in order to obtain the greatest possible number of results.
Inclusion criteriaWe included clinical trials published since 2000 and meeting the following criteria:
- –
Study includes patients diagnosed with CTS who were not pregnant or affected by any systemic disease (e.g. osteoarthritis, rheumatoid arthritis, diabetes mellitus) or by acute neck or upper limb trauma to which CTS may be attributed.
- –
Study includes patients with CTS and no history of surgery for the condition.
- –
Treatment was administered to patients with mild-to-moderate CTS, and electroneurography results were compared to those of a control group or a group of patients receiving a different treatment or a placebo. We accepted authors’ criteria for the classification of CTS severity, in order to be able to use the data provided in the different studies.
- –
Studies evaluated pain, function, or some variable reflecting patient recovery.
- –
Studies were published in English, French, or Spanish.
Following the literature search, two independent reviewers applied the above-listed inclusion criteria to select potentially relevant studies based on the title and abstract. The reviewers reached a consensus on which articles to include. A third reviewer resolved doubts or disagreements arising from the study selection process.
Assessment of study methodology qualityWe assessed methodological quality using the PEDro scale, based on the Delphi list developed by Verhagen and colleagues, from Maastricht University's Department of Epidemiology. This scale enables us to rapidly identify trials with appropriate levels of validity. Fig. 1 details the criteria of the PEDro scale. We awarded studies one point for each “yes” and no points for each “no” response. Points were only awarded where criteria were clearly met, with the maximum possible score being 10.
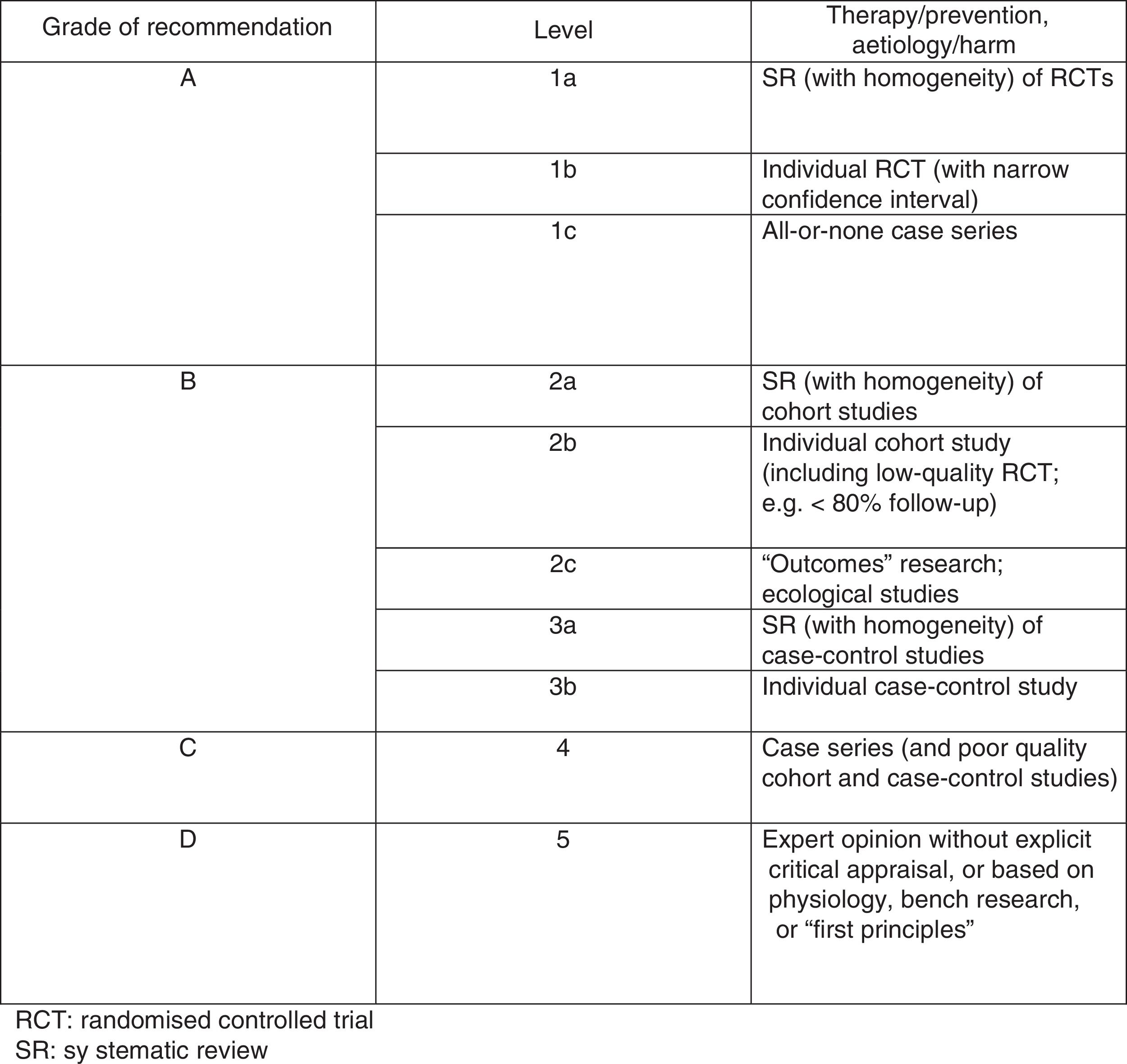
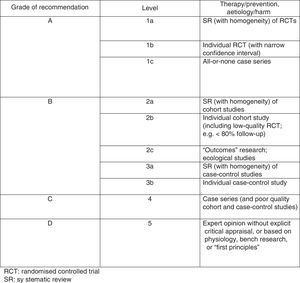
The Oxford scale (Fig. 2)10 was also used to assess the level of evidence. The scale evaluates evidence by thematic area or clinical scenario, and by study type. Evidence is graded according to the best design for each clinical scenario. The studies with the highest levels of evidence are systematic reviews of randomised controlled trials and individual trials with narrow confidence intervals.
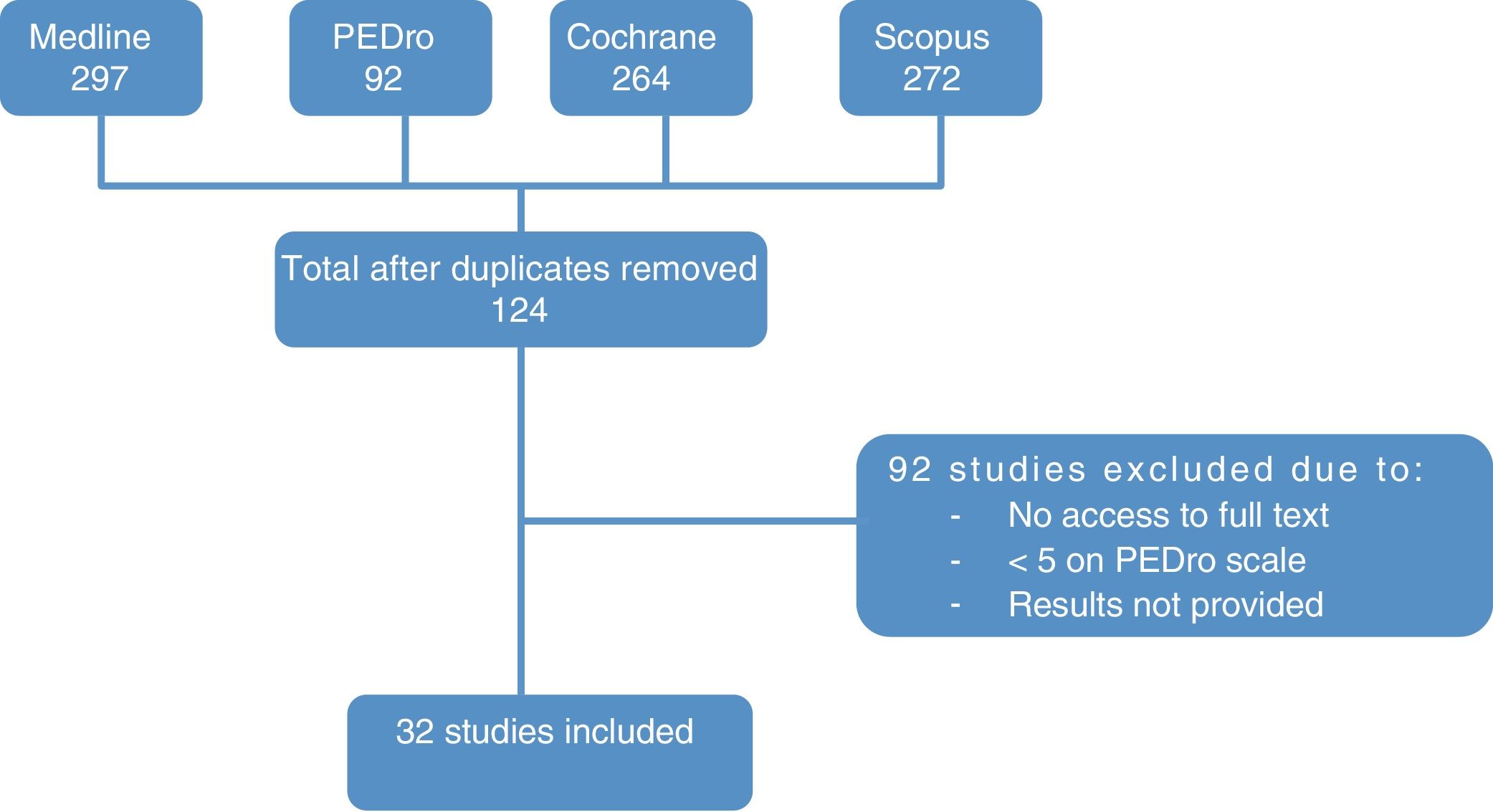
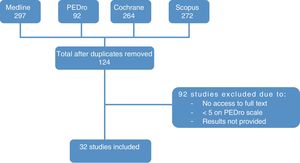
ResultsStudy selectionThe literature search yielded 676 papers. Duplicates were excluded, leaving a total of 124 articles (83 from Medline, 34 from the Cochrane database, and 7 from the PEDro database). After reading the titles and abstracts, we discarded those articles that were not relevant to the study and whose full texts we were unable to access.
Finally, we analysed the full texts of the remaining articles to confirm that they met the inclusion criteria. This final step left a total of 32 articles for evaluation. The flow diagram in Fig. 3 illustrates the article selection process.
Methodological quality of the studies includedEleven studies scored 8 or higher on the PEDro scale. The remaining articles scored 5 or higher, and featured aspects that could be improved, such as heterogeneous samples at baseline, or an absence of data on the results obtained.
On the Oxford scale, 17 studies were graded 1B, 14 were graded 1C, and one was graded 3B; no article was graded A.
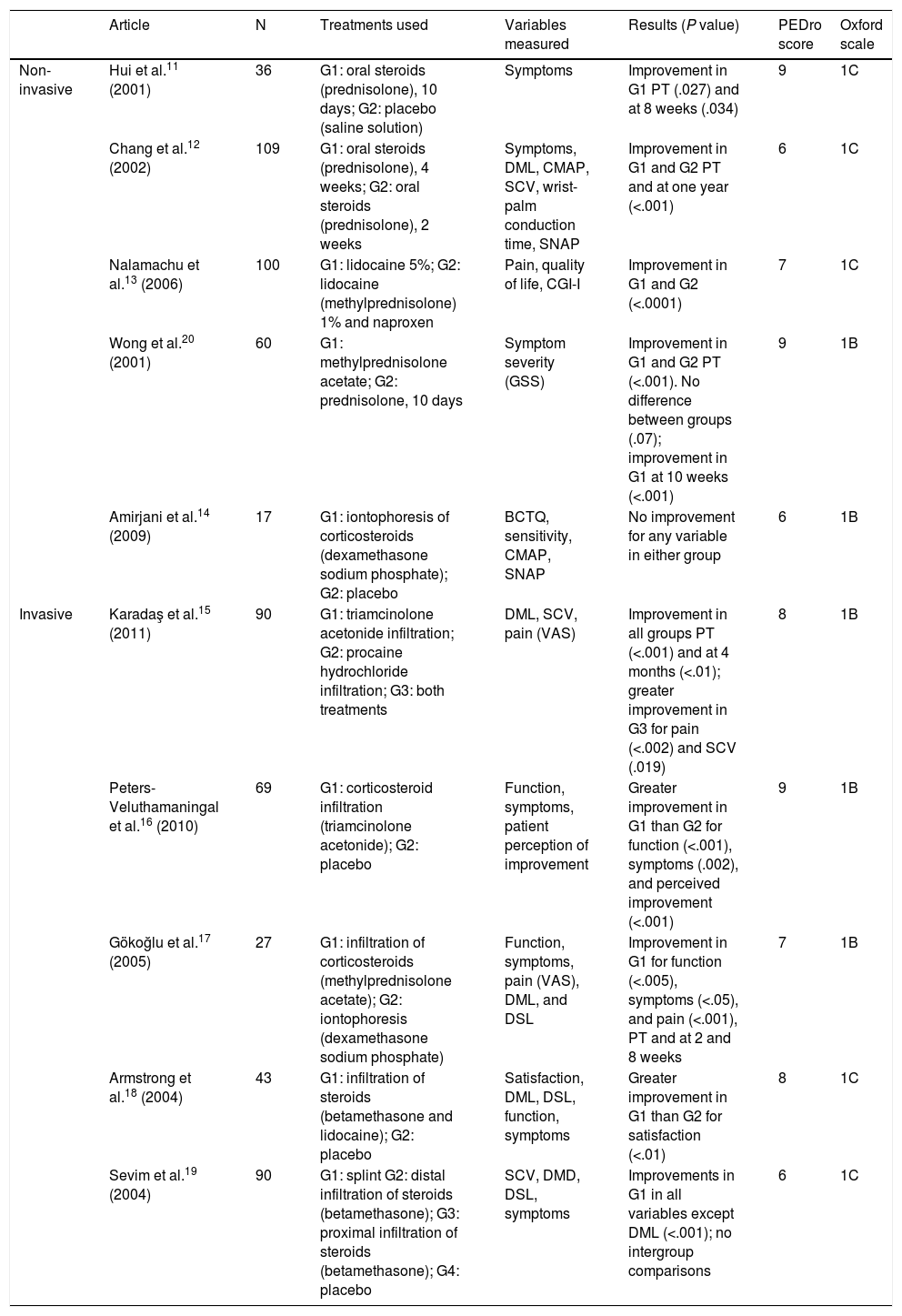
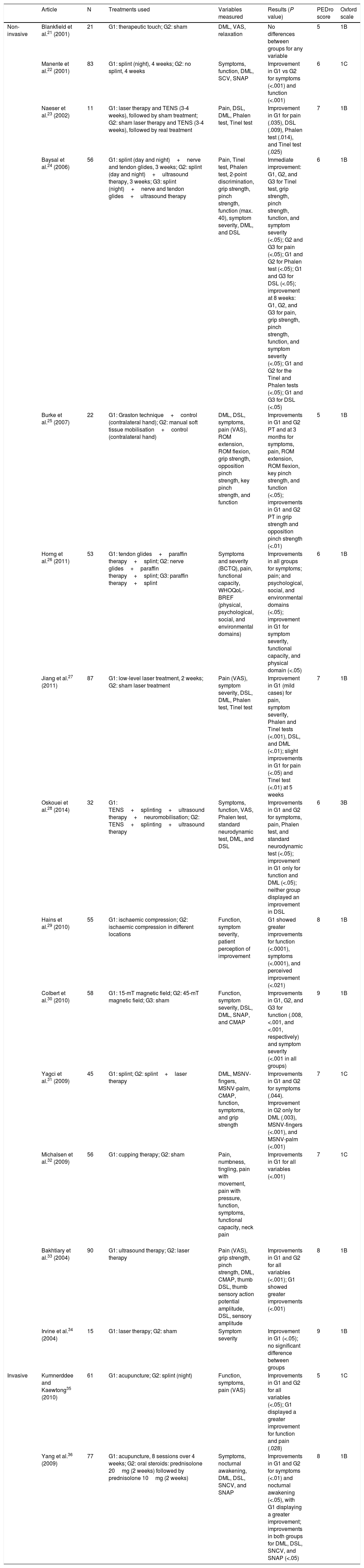
Study characteristicsOf the 32 studies analysed, 10 used pharmacological treatments11–20 (Table 1), 16 used non-pharmacological treatments21–36 (Table 2), and 6 used a combination of pharmacological and non-pharmacological treatments37–42 (Table 3). Tables 1–3 show the detail of each of the studies analysed.
Summary of results. Pharmacological treatments.
| Article | N | Treatments used | Variables measured | Results (P value) | PEDro score | Oxford scale | |
|---|---|---|---|---|---|---|---|
| Non-invasive | Hui et al.11 (2001) | 36 | G1: oral steroids (prednisolone), 10 days; G2: placebo (saline solution) | Symptoms | Improvement in G1 PT (.027) and at 8 weeks (.034) | 9 | 1C |
| Chang et al.12 (2002) | 109 | G1: oral steroids (prednisolone), 4 weeks; G2: oral steroids (prednisolone), 2 weeks | Symptoms, DML, CMAP, SCV, wrist-palm conduction time, SNAP | Improvement in G1 and G2 PT and at one year (<.001) | 6 | 1C | |
| Nalamachu et al.13 (2006) | 100 | G1: lidocaine 5%; G2: lidocaine (methylprednisolone) 1% and naproxen | Pain, quality of life, CGI-I | Improvement in G1 and G2 (<.0001) | 7 | 1C | |
| Wong et al.20 (2001) | 60 | G1: methylprednisolone acetate; G2: prednisolone, 10 days | Symptom severity (GSS) | Improvement in G1 and G2 PT (<.001). No difference between groups (.07); improvement in G1 at 10 weeks (<.001) | 9 | 1B | |
| Amirjani et al.14 (2009) | 17 | G1: iontophoresis of corticosteroids (dexamethasone sodium phosphate); G2: placebo | BCTQ, sensitivity, CMAP, SNAP | No improvement for any variable in either group | 6 | 1B | |
| Invasive | Karadaş et al.15 (2011) | 90 | G1: triamcinolone acetonide infiltration; G2: procaine hydrochloride infiltration; G3: both treatments | DML, SCV, pain (VAS) | Improvement in all groups PT (<.001) and at 4 months (<.01); greater improvement in G3 for pain (<.002) and SCV (.019) | 8 | 1B |
| Peters-Veluthamaningal et al.16 (2010) | 69 | G1: corticosteroid infiltration (triamcinolone acetonide); G2: placebo | Function, symptoms, patient perception of improvement | Greater improvement in G1 than G2 for function (<.001), symptoms (.002), and perceived improvement (<.001) | 9 | 1B | |
| Gökoğlu et al.17 (2005) | 27 | G1: infiltration of corticosteroids (methylprednisolone acetate); G2: iontophoresis (dexamethasone sodium phosphate) | Function, symptoms, pain (VAS), DML, and DSL | Improvement in G1 for function (<.005), symptoms (<.05), and pain (<.001), PT and at 2 and 8 weeks | 7 | 1B | |
| Armstrong et al.18 (2004) | 43 | G1: infiltration of steroids (betamethasone and lidocaine); G2: placebo | Satisfaction, DML, DSL, function, symptoms | Greater improvement in G1 than G2 for satisfaction (<.01) | 8 | 1C | |
| Sevim et al.19 (2004) | 90 | G1: splint G2: distal infiltration of steroids (betamethasone); G3: proximal infiltration of steroids (betamethasone); G4: placebo | SCV, DMD, DSL, symptoms | Improvements in G1 in all variables except DML (<.001); no intergroup comparisons | 6 | 1C |
BCTQ, Boston Carpal Tunnel Syndrome Questionnaire; CGI-I, Investigator Clinical Global Impression of Improvement; CMAP, compound muscle action potential; DML, distal motor latency; DSL, distal sensory latency; G, group; GSS, Global Symptom Score; PT, post-treatment; SCV, sensory conduction velocity; SNAP, sensory nerve action potential; VAS, Visual Analogue Scale.
Summary of results. Non-pharmacological treatments.
| Article | N | Treatments used | Variables measured | Results (P value) | PEDro score | Oxford scale | |
|---|---|---|---|---|---|---|---|
| Non-invasive | Blankfield et al.21 (2001) | 21 | G1: therapeutic touch; G2: sham | DML, VAS, relaxation | No differences between groups for any variable | 5 | 1B |
| Manente et al.22 (2001) | 83 | G1: splint (night), 4 weeks; G2: no splint, 4 weeks | Symptoms, function, DML, SCV, SNAP | Improvement in G1 vs G2 for symptoms (<.001) and function (<.001) | 6 | 1C | |
| Naeser et al.23 (2002) | 11 | G1: laser therapy and TENS (3-4 weeks), followed by sham treatment; G2: sham laser therapy and TENS (3-4 weeks), followed by real treatment | Pain, DSL, DML, Phalen test, Tinel test | Improvement in G1 for pain (.035), DSL (.009), Phalen test (.014), and Tinel test (.025) | 7 | 1B | |
| Baysal et al.24 (2006) | 56 | G1: splint (day and night)+nerve and tendon glides, 3 weeks; G2: splint (day and night)+ultrasound therapy, 3 weeks; G3: splint (night)+nerve and tendon glides+ultrasound therapy | Pain, Tinel test, Phalen test, 2-point discrimination, grip strength, pinch strength, function (max. 40), symptom severity, DML, and DSL | Immediate improvement: G1, G2, and G3 for Tinel test, grip strength, pinch strength, function, and symptom severity (<.05); G2 and G3 for pain (<.05); G1 and G2 for Phalen test (<.05); G1 and G3 for DSL (<.05); improvement at 8 weeks: G1, G2, and G3 for pain, grip strength, pinch strength, function, and symptom severity (<.05); G1 and G2 for the Tinel and Phalen tests (<.05); G1 and G3 for DSL (<.05) | 6 | 1B | |
| Burke et al.25 (2007) | 22 | G1: Graston technique+control (contralateral hand); G2: manual soft tissue mobilisation+control (contralateral hand) | DML, DSL, symptoms, pain (VAS), ROM extension, ROM flexion, grip strength, opposition pinch strength, key pinch strength, and function | Improvements in G1 and G2 PT and at 3 months for symptoms, pain, ROM extension, ROM flexion, key pinch strength, and function (<.05); improvements in G1 and G2 PT in grip strength and opposition pinch strength (<.01) | 5 | 1B | |
| Horng et al.26 (2011) | 53 | G1: tendon glides+paraffin therapy+splint; G2: nerve glides+paraffin therapy+splint; G3: paraffin therapy+splint | Symptoms and severity (BCTQ), pain, functional capacity, WHOQoL-BREF (physical, psychological, social, and environmental domains) | Improvements in all groups for symptoms; pain; and psychological, social, and environmental domains (<.05); improvement in G1 for symptom severity, functional capacity, and physical domain (<.05) | 6 | 1B | |
| Jiang et al.27 (2011) | 87 | G1: low-level laser treatment, 2 weeks; G2: sham laser treatment | Pain (VAS), symptom severity, DSL, DML, Phalen test, Tinel test | Improvement in G1 (mild cases) for pain, symptom severity, Phalen and Tinel tests (<.001), DSL, and DML (<.01); slight improvements in G1 for pain (<.05) and Tinel test (<.01) at 5 weeks | 7 | 1B | |
| Oskouei et al.28 (2014) | 32 | G1: TENS+splinting+ultrasound therapy+neuromobilisation; G2: TENS+splinting+ultrasound therapy | Symptoms, function, VAS, Phalen test, standard neurodynamic test, DML, and DSL | Improvements in G1 and G2 for symptoms, pain, Phalen test, and standard neurodynamic test (<.05); improvement in G1 only for function and DML (<.05); neither group displayed an improvement in DSL | 6 | 3B | |
| Hains et al.29 (2010) | 55 | G1: ischaemic compression; G2: ischaemic compression in different locations | Function, symptom severity, patient perception of improvement | G1 showed greater improvements for function (<.0001), symptoms (<.0001), and perceived improvement (<.021) | 8 | 1B | |
| Colbert et al.30 (2010) | 58 | G1: 15-mT magnetic field; G2: 45-mT magnetic field; G3: sham | Function, symptom severity, DSL, DML, SNAP, and CMAP | Improvements in G1, G2, and G3 for function (.008, <.001, and <.001, respectively) and symptom severity (<.001 in all groups) | 9 | 1B | |
| Yagci et al.31 (2009) | 45 | G1: splint; G2: splint+laser therapy | DML, MSNV-fingers, MSNV-palm, CMAP, function, symptoms, and grip strength | Improvements in G1 and G2 for symptoms (.044). Improvement in G2 only for DML (.003), MSNV-fingers (<.001), and MSNV-palm (<.001) | 7 | 1C | |
| Michalsen et al.32 (2009) | 56 | G1: cupping therapy; G2: sham | Pain, numbness, tingling, pain with movement, pain with pressure, function, symptoms, functional capacity, neck pain | Improvements in G1 for all variables (<.001) | 7 | 1C | |
| Bakhtiary et al.33 (2004) | 90 | G1: ultrasound therapy; G2: laser therapy | Pain (VAS), grip strength, pinch strength, DML, CMAP, thumb DSL, thumb sensory action potential amplitude, DSL, sensory amplitude | Improvements in G1 and G2 for all variables (<.001); G1 showed greater improvements (<.001) | 8 | 1B | |
| Irvine et al.34 (2004) | 15 | G1: laser therapy; G2: sham | Symptom severity | Improvement in G1 (<.05); no significant difference between groups | 9 | 1B | |
| Invasive | Kumnerddee and Kaewtong35 (2010) | 61 | G1: acupuncture; G2: splint (night) | Function, symptoms, pain (VAS) | Improvements in G1 and G2 for all variables (<.05); G1 displayed a greater improvement for function and pain (.028) | 5 | 1C |
| Yang et al.36 (2009) | 77 | G1: acupuncture, 8 sessions over 4 weeks; G2: oral steroids: prednisolone 20mg (2 weeks) followed by prednisolone 10mg (2 weeks) | Symptoms, nocturnal awakening, DML, DSL, SNCV, and SNAP | Improvements in G1 and G2 for symptoms (<.01) and nocturnal awakening (<.05), with G1 displaying a greater improvement; improvements in both groups for DML, DSL, SNCV, and SNAP (<.05) | 8 | 1B |
BCTQ, Boston Carpal Tunnel Syndrome Questionnaire; CMAP, compound muscle action potential; DML, distal motor latency; DSL, distal sensory latency; G, group; MSNV, median sensory nerve conduction velocity; PT, post-treatment; ROM, range of movement; SCV, sensory conduction velocity; SNAP, sensory nerve action potential: SNCV, sensory nerve conduction velocity; TENS, transcutaneous electric nerve stimulation; VAS, visual analogue scale; WHOQoL-BREF, World Health Organisation Quality of Life questionnaire, brief version.
Summary of results. Combined treatments.
| Author (year) | N | Treatments used | Variables measured | Results (P value) | PEDro scale | Oxford scale |
|---|---|---|---|---|---|---|
| Soyupek et al.37 (2012) | 36 | G1: splint+infiltration (betamethasone dipropionate); G2: phonophoresis with corticosteroids (dicofenac diethylammonium); G3: phonophoresis with NSAIDs (betamethasone); duration: 3 months | Pain; symptoms; Tinel and Phalen tests; cross-sectional area and transverse and anteroposterior diameters of the median nerve; MCV; SCV; CMAP; SNAP; DML; and DSL | Improvements in all 3 groups for transverse diameter, MCV, SCV, CMAP, SNAP, DML, and DSL (<.05); improvements in G2 for pain, Tinel and Phalen tests, anteroposterior diameter, and cross-sectional area (<.05); Improvements in G3 for pain and Phalen test (<.05) | 7 | 1B |
| Bardak et al.38 (2009) | 111 | G1: splint+infiltrations (betamethasone); G2: splint+infiltrations (betamethasone)+tendon and nerve glides; G3: tendon and nerve glides | Function, symptoms, 2-point discrimination | Improvement in G1, G2, and G3 for function and symptoms (<.01); G3 displayed worse results | 7 | 1C |
| Bilgici et al.39 (2010) | 49 | G1: ultrasound therapy; G2: steroids (dexamethasone)+splinting | Function, symptoms, pain, grip strength, 2-point discrimination, SNCV, DML | Improvements in G1 and G2 for function (<.001), symptom severity (<.001), pain (<.016), 2-point discrimination (<.016), and SNCV and DML (<.016); G2 displayed greater improvements in symptoms and grip strength (<.05) | 6 | 1C |
| Yildiz et al.40 (2011) | 74 | G1: pulse mode ultrasound with ketoprofen; G2: pulse mode ultrasound with no drug; G3: sham ultrasound | Pain, function, symptoms, DML, DSL | No group displayed any improvement (>.05) | 9 | 1C |
| Celiker et al.41 (2002) | 33 | G1: neutral positioned splint (night)+acemetacin; G2: local infiltration of methylprednisolone acetate | Phalen and Tinel tests, reverse Phalen test, pain, symptoms, DML, and DSL | Improvements in both groups for pain, symptoms, DML, and DSL (<.05); no differences were observed between groups (>.05) | 6 | 1C |
| Gurcay et al.42 (2012) | 52 | G1: phonophoresis with betamethasone+splinting; G2: iontophoresis with betamethasone+splinting; G3: neutral-positioned splint (night) | Symptom severity, grip strength, NHPT score | Improvements in all groups for symptom severity at 3 months (<.05); greater improvement in G1 than G3 for grip strength (.012) | 7 | 1C |
CMAP, compound muscle action potential; DML, distal motor latency; DSL, distal sensory latency; G, group; MCV, motor conduction velocity; NSAID, non-steroidal anti-inflammatory drug; SCV, sensory conduction velocity; SNAP, sensory nerve action potential.
Regarding pharmacological treatment, oral steroids appear to be effective in the short and medium term,11 although we do not know the optimal dose nor which drugs are the most effective. Infiltrations have been demonstrated to improve symptoms, function, and patients’ personal perception of their condition.15–20 The most commonly used drugs are prednisolone, methylprednisolone, dexamethasone sodium phosphate, triamcinolone acetonide, methylprednisolone acetate, lidocaine, and diclofenac diethylammonium, although the best dose and drug for short-term use are not known.13,20
The non-pharmacological approaches which have received the most attention are splinting,22,24,26,28,31,35 electrotherapy,23,24,27,28,30,31,33,34 and manual therapy techniques.25,29,32 Splinting as a sole treatment has been demonstrated to improve function and symptom severity, although no changes have been observed in electroneurographic variables.22 Better results have been associated with splinting in combination with other techniques such as laser therapy,31 transcutaneous electrical nerve stimulation (TENS), neuromobilisation,28 ultrasound therapy,24 and paraffin therapy.26 In some cases, improvements were observed in electroneurographic variables (distal motor latency, sensory nerve action potential, or distal sensory latency); however, the available evidence on this improvement is inconclusive.
There is also no conclusive evidence on the benefits of electrotherapy techniques as a sole treatment. There is insufficient evidence that laser therapy is more effective than control or placebo treatments27,31,34; in combination with TENS, however, it improves pain, peak sensory latency, and Phalen and Tinel test findings.23 Ultrasound therapy appears to have more pronounced effects on pain, strength, and electroneurographic parameters.33
Regarding manual techniques, both ischaemic compression29 and cupping therapy32 significantly improve symptoms and function.
The Graston technique has been shown to be effective for improving range of movement, but not pain, sensitivity, or electroneurographic findings for distal motor latency or sensory conduction velocity.25 Acupuncture is effective for reducing pain and improving function and has shown better results than splinting.35 It has also been found to improve distal motor latency.36
Finally, evidence on the best combination of pharmacological and non-pharmacological techniques is inconclusive.37–42
Some studies found no improvements when non-pharmacological techniques such as splinting were combined with pharmacological treatment.41 Likewise, there is no evidence that it is beneficial to associate drug treatment with non-pharmacological techniques such as ultrasound therapy or splinting.39,42 However, we did generally find that combining both types of treatment was more effective than using a single type.37,38
DiscussionThe aim of this review was to shed light on the effectiveness of non-surgical interventions for the treatment of mild to moderate CTS.
Oral drugs administered to these patients act systemically, reducing interstitial fluid pressure in the carpal tunnel. This treatment has been associated with short- and long-term improvements in symptoms and in some electroneurographic variables.11,12
Local treatments include drug infiltrations which induce anaesthaesia by increasing the threshold of electrical excitability in order to stabilise the sodium channel. This invasive technique involves a series of risks, including iatrogenic lesions.43,44 Furthermore, we are yet to determine the most effective drug and dose.13,15 The only available evidence is that infiltrations are more effective than sham or no treatment.16,18
No short-term difference has been observed between systemic drugs and local treatment, although infiltrations appear to be more effective in the long term.20 However, there is insufficient evidence to confirm this hypothesis. Another approach by which drugs may be administered locally is iontophoresis; however, this technique has not been demonstrated to be effective as the sole treatment for CTS.14 Due to the review's 15-year limit, we found no studies in which patients received gabapentin, an antiepileptic drug frequently prescribed in everyday practice which has a demonstrated analgesic effect in CTS, displaying greater tolerability than such other drugs as opioids or antidepressants.
Splinting was one of the most frequently studied non-pharmacological treatments. Splints are used to keep the wrist in a position that reduces pressure inside the carpal tunnel. No consensus has yet been reached about the best position or type of splint.45 These parameters may be influenced by each patient's individual anthropometric measurements and functional characteristics, making it a challenge to determine the best position and material. Splinting has been shown to improve several clinical variables but not electroneurographic variables.22 However, the studies into this treatment were of relatively poor methodological quality, according to the scales used.
Splinting in combination with nerve glides,24 paraffin therapy, and tendon glides26 has not been demonstrated to be more effective than splinting alone. However, when the technique is combined with ultrasound therapy,24 TENS, or neuromobilisation,28 the effect is more pronounced.
Electrotherapy techniques constitute another widely used group of non-pharmacological treatments. However, the studies performed to date report conflicting results with relation to the effectiveness of these treatments. Laser therapy has been associated with improvements in nerve conduction velocity and the other clinical variables studied in cases of mild CTS.27 However, another study found no difference compared to a control group.34 These discrepancies in the results may be due to methodological differences, as the first study used a sham group, whereas the second used a control group. The different studies also used different outcome variables. If we compare different electrotherapy techniques, ultrasound therapy appears to be more effective than laser therapy; while this conclusion is reported by only one study, that study is of good methodological quality.33 Static magnetic field therapy was not found to be more effective than a sham treatment.30
Manual therapy is another of the non-pharmacological approaches used to treat CTS. Examples of this type of therapy are ischaemic compression, massage, the Graston technique, and cupping therapy. These therapies aim to reduce tension in the muscles and soft tissues adjacent to the affected region in order to improve nerve mobilisation. A study into ischaemic compression of the biceps, pronator teres, and subscapularis muscles found the technique to have some effect in improving function and symptom severity,29 although it did have some methodological flaws, such as each group including a different number of patients. Patients receiving the Graston technique or cupping therapy applied to the forearm muscles showed symptomatic improvements compared to a control group.25,32 However, these studies present methodological issues, for example, with sample size. The scarcity of research and the variability of these interventions make it difficult to determine the most effective technique and even to understand the mechanisms of action. Acupuncture has been found to be somewhat effective, showing greater benefits than drug treatment or splinting for some symptoms, for function, and for some electroneurographic variables.35,36
In clinical practice, pharmacological and non-pharmacological treatments are often combined. Studies have been performed into different combinations of treatments. Several studies support the effectiveness of splinting combined with pharmacological treatment,37 which is demonstrated to be more effective than other non-pharmacological treatments.38,41,42
The combination of ultrasound therapy with oral drugs or steroid infiltration does not perform better than ultrasound as the sole therapy.39,40
Given the limited number and the variability of studies, it is difficult to draw comparisons between treatment techniques and combinations. We are therefore unable to determine whether combining pharmacological and non-pharmacological treatments is more effective than administering a single conservative treatment.
LimitationsOur review has certain limitations, arising from the studies included. It is difficult to draw comparisons between the studies on account of the differences in the variables measured and the types and combinations of treatments studied.
Some articles do not provide specific detail on the methodology followed, which made it difficult to evaluate their methodological quality. Several articles did not compare the results from different groups, preventing us from drawing conclusions about which techniques were more effective.
There is a need for further studies specifically evaluating techniques or combinations of techniques by measuring clinical, functional, and electroneurological variables. Future studies should be of the highest methodological quality, enabling reliable analysis of the results.
ConclusionsThere is evidence that oral steroids constitute an effective treatment for mild-to-moderate CTS in the medium term. Local infiltrations also appear to be effective, although further research is needed to better understand their safety and effectiveness. Splinting also appears to be an effective short-term treatment. Splints are more effective when they are combined with other treatment techniques such as TENS, ultrasound therapy, or nerve gliding, although we are yet to determine the optimal combination. Evidence about electrotherapy techniques is controversial, although ultrasound therapy seems to be more effective than laser therapy. Evidence about manual or instrumental manipulation of the soft tissue is unclear. Acupuncture seems to be effective, despite limited evidence.
It was not possible to identify the best technique or combination of techniques, as the variability between studies in terms of the techniques evaluated and the variables assessed prevented easy comparison. Therefore, we deem it necessary to perform further, higher-quality studies in order to obtain conclusive results.
Ethical standardsProtection of people and animalsThe authors declare that the procedures followed comply with the ethical standards of the relevant committee on human experimentation, the World Medical Association, and the Declaration of Helsinki.
Data protectionThe authors have observed their centre's protocols for the publication of patient data.
Right to privacy and informed consentThe authors obtained informed consent from the patients and/or subjects described in the article. The written informed consent forms are in the possession of the corresponding author.
FundingNo funding was received for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Jiménez del Barrio S, Bueno Garcia E, Hidalgo García C, Estébanez de Miguel E, Tricás Moreno JM, Rodríguez Marco S, Ceballos Laita L. Tratamiento conservador en pacientes con síndrome del túnel carpiano con intensidad leve o moderada. Revisión sistemática. Neurología. 2018;33:590–601.