Calcified cerebral embolus (CCE), a rarely reported and underdiagnosed cause of stroke, may be the first manifestation of a vascular or cardiac disease. We describe the characteristics of CCE in a series of 9 cases and review the literature on the subject.
Patients and methodsWe included patients with CCE from 3 different hospitals. We describe the diagnostic approach, neuroimaging findings, origin of the embolism, treatment, and prognosis of these patients.
ResultsWe identified a total of 9 patients presenting spontaneous CCE as the cause of acute ischaemic stroke. In all cases, the middle cerebral artery was affected; all patients underwent CT. A possible calcific source was found in 6 patients (66.6%), originating in the carotid arteries in 3 (33.3%) and in the heart in the other 3 patients (33.3%). Only one patient was treated in the acute phase (trombectomy) and only 11% of patients had modified Ranking Scale scores ≤ 2 at 3 months.
ConclusionsCCE is more frequent than previously thought and, although the condition continues to be underdiagnosed, it is of considerable prognostic relevance in the aetiological study of stroke.
Los embolismos cerebrales cálcicos (ECC) representan una causa de ictus poco descrita e infradiagnosticada, que puede ser la primera manifestación de una enfermedad vascular o cardíaca. El objetivo del presente trabajo es describir las características de los ECC en una serie de casos y revisar la literatura.
Pacientes y métodosTres centros hospitalarios aportaron casos al trabajo. Se evaluaron los métodos diagnósticos, las características de neuroimagen, la fuente embólica, el tratamiento y el pronóstico de los pacientes con ECC.
ResultadosSe recogieron un total de 9 casos con ECC espontáneo como causa de ictus isquémico agudo. Todos afectaron a la arteria cerebral media y se estudiaron mediante TC. Se encontró una posible fuente cálcica en 6 pacientes (66,6%): carotídea en 3 (33,3%) y cardiaca en otros 3 (33,3%) pacientes. Solo 1 paciente se trató en la fase aguda mediante trombectomía y solo un 11% tuvieron un mRS ≤2 a los 3 meses.
ConclusionesLos ECC son más frecuentes de lo que se creía en el pasado y aunque siguen siendo frecuentemente infradiagnosticados, tienen una gran relevancia pronóstica a la hora de dirigir el estudio etiológico del ictus.
Cerebral embolisms may originate from different sites and present a varied histopathological composition. Three main types have been described: arteriogenic (with abundant red blood cells at the centre and platelets covering the fibrin layers at the periphery of the thrombus), cardiogenic (with clusters of platelets disseminated within fibrin-rich clots), and calcified (with large amounts of calcium phosphate).1
Calcified cerebral embolism (CCE) is a poorly described but highly significant cause of stroke: regardless of the sequelae of the cerebrovascular event, it may be the first manifestation of a cardiac or arterial disease. The peculiarity and advantage of CCE is that it may be identified in an initial assessment by non-contrast CT scan, typically performed as the first diagnostic step in most patients with acute stroke.
In this study, we present 9 cases of CCE and describe the associated risk factors, clinical and radiological presentation, and treatment and medium-term prognosis.
Patients and methodsWe gathered data on a total of 9 cases of CCE from 3 hospitals. We retrospectively collected demographic characteristics (age, sex, and vascular risk factors), NIHSS score at admission, CCE characteristics at admission (location, thrombus density in Hounsfield units [HU], and progressive changes in follow-up CT scans), the reperfusion treatment used, and the medium-term prognosis as measured with the modified Rankin Scale (mRS) at 3 months. We used the TOAST classification to define stroke aetiology.2 We also describe complementary test findings potentially involved in the calcified origin of the embolism.
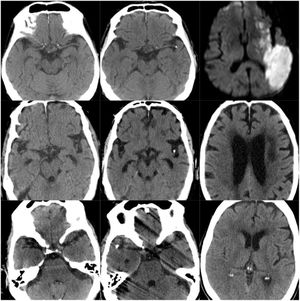
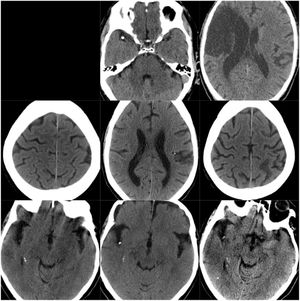
ResultsOf our 9 patients, 5 were women and 4 men, with a mean age of 79.1 years. Baseline imaging assessment of all patients consisted of a CT scan, which showed a unilateral CCE located at the middle cerebral artery (MCA) territory in all cases, with the left MCA affected in 55.5% of cases (Fig. 1–3). The shape of the CCE was rounded or oval in all cases, with a mean attenuation of 211.4 HU. One patient presented distal migration of the hyperdense area attributable to the CCE in a subsequent CT scan, probably due to disintegration of the embolus. The other 8 patients did not show progressive changes in the CCE in neuroimaging studies (Table 1).
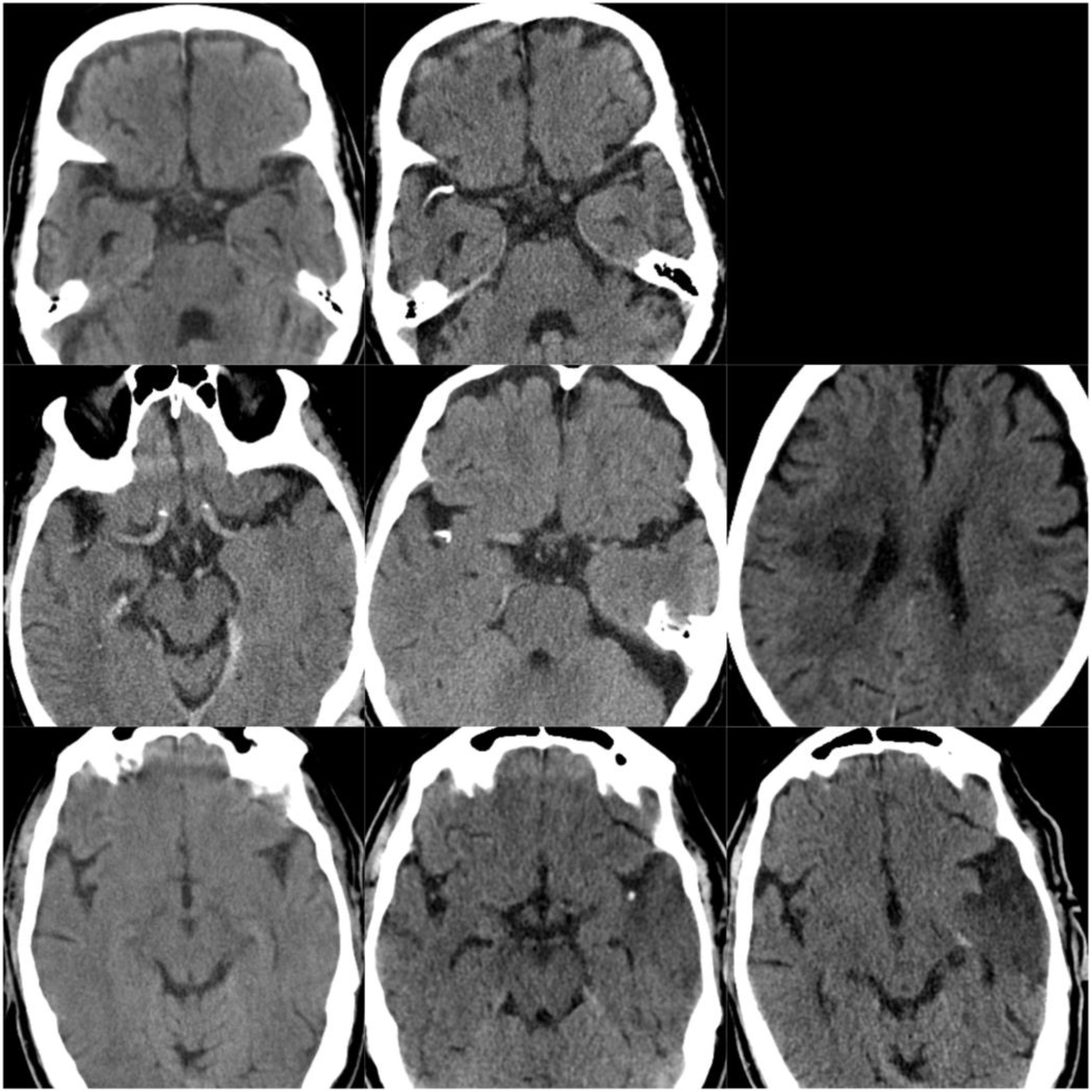
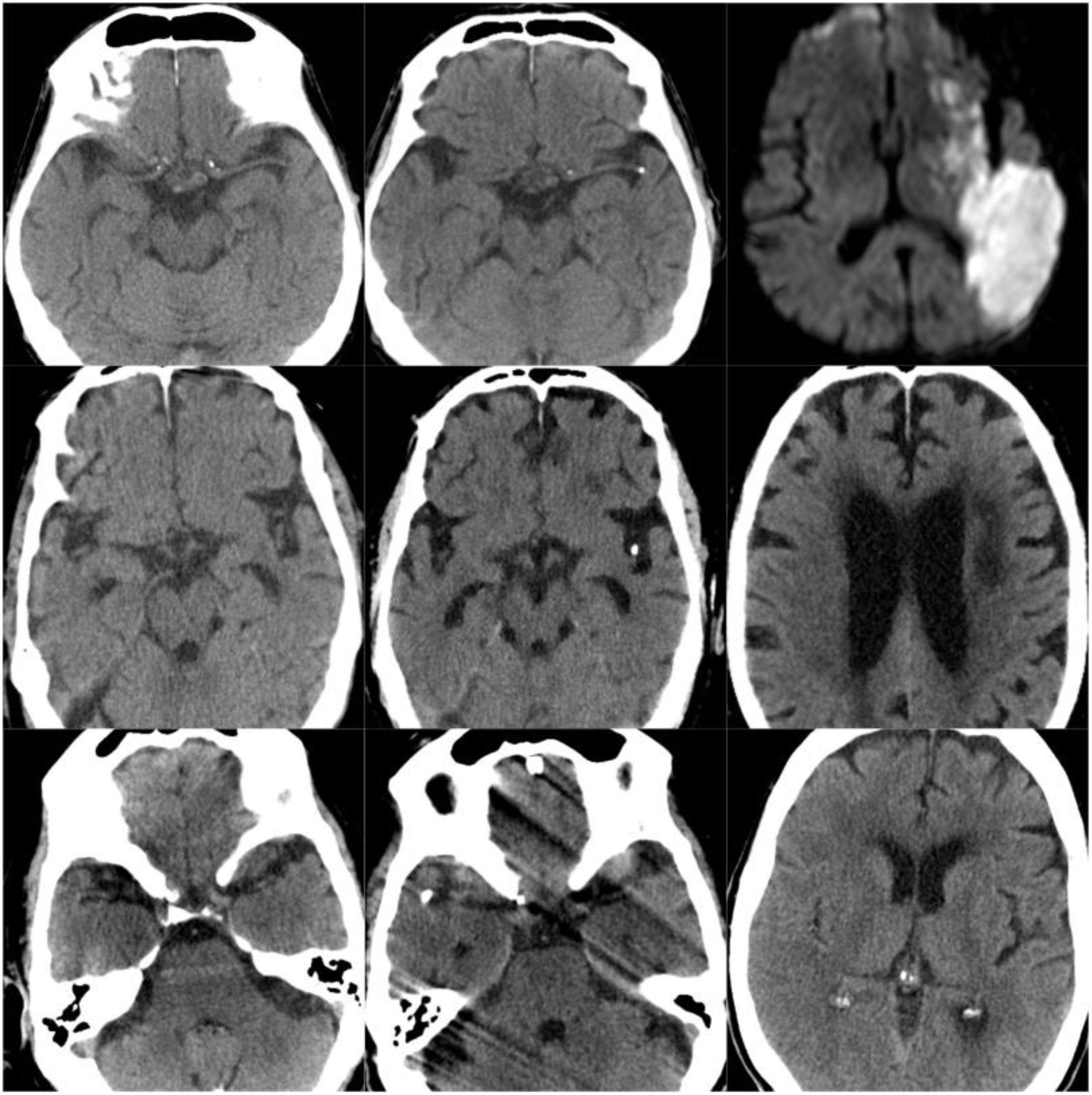
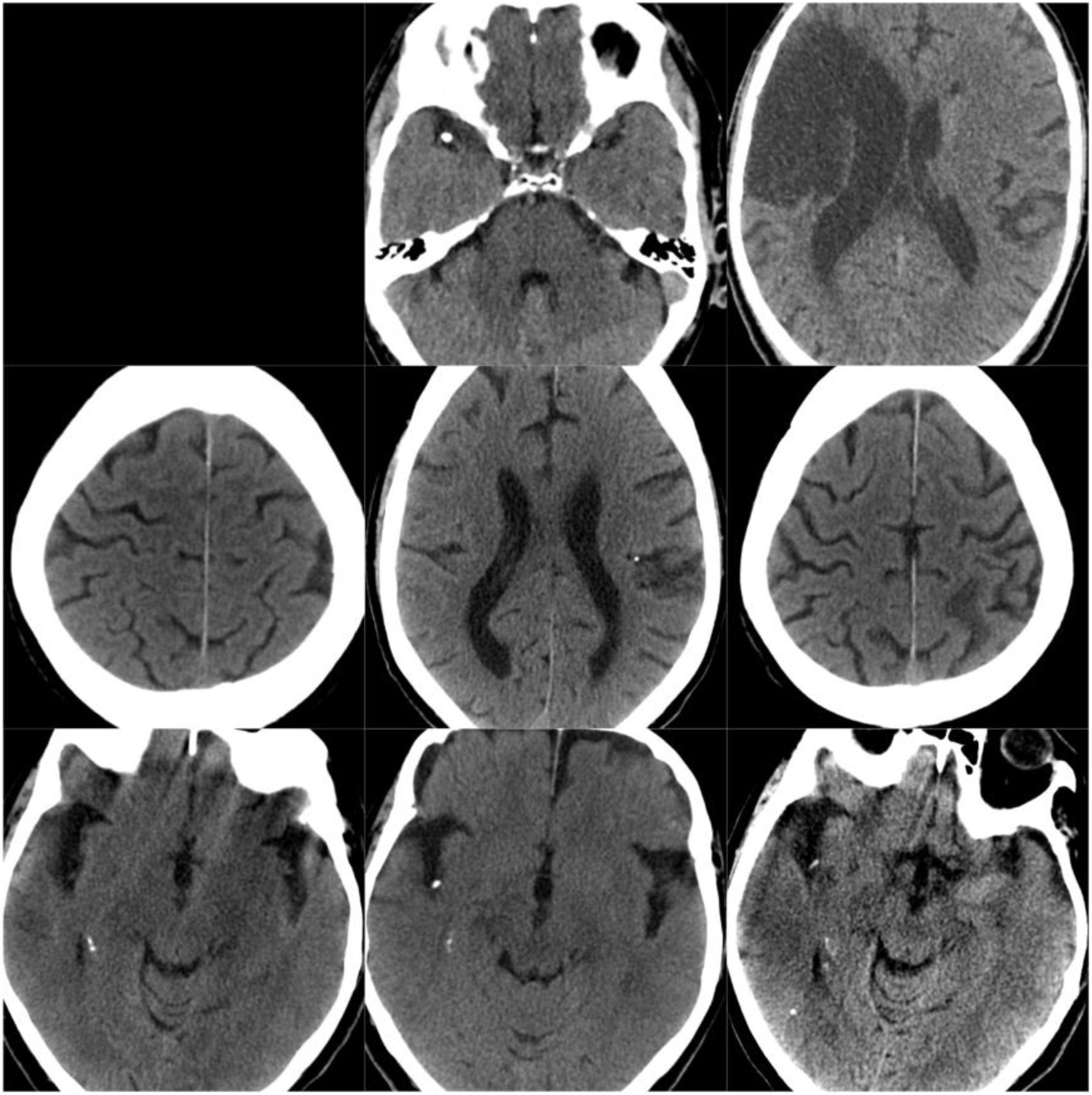
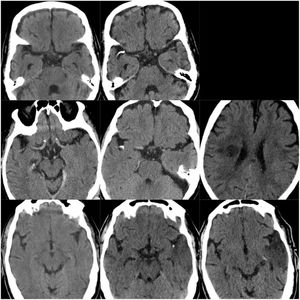
CT scans of CCE in patients 1-3. The first column shows CT scans performed prior to CCE, the second column shows images of the CCE, and the third column shows the image of the corresponding stroke (except for the first patient, for whom the follow-up study after the CCE was not available).
Demographic characteristics of patients with calcified cerebral embolism.
| n (%) | |
|---|---|
| Sex (men) | 4 (44.4) |
| Mean age (years) | 79.1 |
| AHT | 7 (77.8) |
| DM | 2 (22.2) |
| DL | 3 (33.3) |
| Peripheral artery disease | 1 (11.1) |
| AMI | 2 (22.2) |
| AF | 2 (22.2) |
AF: atrial fibrillation; AHT: arterial hypertension; AMI: acute myocardial infarction; DL: dyslipidaemia; DM: diabetes mellitus.
In all cases, embolism was spontaneous, ie, not associated with medical procedures or trauma. According to the TOAST classification, stroke was cardioembolic in 4 cases (44.4%), atherosclerotic in 3 (33.3%), and of undetermined origin in 2 patients (22.2%). We identified the following heart diseases: atrial fibrillation in 2 patients (22.2%), severe aortic stenosis and ischaemic heart disease in one patient (11.1%), and infectious endocarditis in one patient (11.1%) (Table 2).
Clinical and neuroimaging characteristics of calcified cerebral embolism.
| Thrombus location | |
|---|---|
| MCA | 9 (100%) |
| Left MCA | 5 (55.5%) |
| Mean CT thrombus density (HU) | 211.42 |
| Stroke aetiology (TOAST classification) | |
| Cardioembolic | 4 (44.4%) |
| Atherothrombotic | 3 (33.3%) |
| Undetermined | 2 (22.2%) |
| NIHSS score at admission (median, Q1-Q3) | 8 (5-17) |
| Acute-phase mechanical thrombectomy | 1 (11.1%) |
| mRS at 3 months (median, Q1-Q3) | 4 (2-6) |
HU: Hounsfield unit; MCA: middle cerebral artery; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; TOAST: Trial of ORG 10172 in Acute Stroke Treatment classification.
A possible calcified source was found in 6 patients (66.7%) (Table 3). We detected a sclerotic, calcified aortic valve in 2 patients (22.2%) and mitral annular calcification in one patient (11.1%). Three patients (33.3%) presented carotid stenosis as a possible cause of the CCE.
List of possible calcified sources of calcified cerebral embolism.
| Carotid artery, n (%) | 3 (33.3) |
| Type of plaque | |
| Calcification, type III | 3 (33.3) |
| Degree of stenosis | |
| Stenosis < 50% | 1 (11.1) |
| Stenosis 50%-69% | 1 (11.1) |
| Stenosis > 70% | 1 (11.1) |
| Heart, n (%) | 3 (33.3) |
| Sclerotic and calcified aortic valve | 2 (22.2) |
| Mitral annular calcification | 1 (11.1) |
| Unknown, n (%) | 3 (33.3) |
For several reasons, including high baseline mRS scores or progression times outside the therapeutic window, revascularisation by mechanical thrombectomy was only performed in one patient (11.1%), and was unsuccessful. No patient was eligible for intravenous fibrinolysis. Prognosis was fatal in 3 cases (33.3%), and the majority of the remaining patients (4; 44.4%) presented severe functional dependence (mRS ≥ 4); only one patient (11.1%) achieved an mRS score ≤ 2. No recurrence was observed in the 3 months following the ischaemic event.
DiscussionNeuroimaging is increasingly relevant in the aetiological diagnosis of stroke. MRI, specifically, is able to distinguish fibrin-rich thrombi from those with abundant red blood cells.3 However, this differentiation may be complex and have limited benefit in normal clinical practice. However, calcified emboli may be simply and reliably diagnosed by CT, a readily available technique frequently used in the management of stroke patients. Together with the hyperdensity of the occluded vessel, the loss of grey-white differentiation, and sulcal effacement, CCE may be an early CT finding in patients with acute ischaemic stroke.
While CCE is infrequently reported in the literature, recent studies have described considerable prevalence (2.7%) among stroke patients undergoing CT studies.4 The risk of stroke recurrence in these patients is high, at approximately 50%4,5; however, this early recurrence was not observed in any patient in our series. Despite the epidemiological relevance and the prognostic and therapeutic implications of CCE, the condition is underdiagnosed. Up to 27% of patients may be misdiagnosed, and up to 9% of CCEs may go undetected in the preliminary analysis of a CT scan.4
Distinguishing CCE from intrinsic vascular calcification may represent a diagnostic challenge. One of the parameters to be assessed for this purpose is the location of the calcification.5 Thus, within intracranial arteries, calcifications are more frequently located in the carotid siphon and are associated with atherosclerosis, but rarely involve the MCA or anterior cerebral artery.6 The presence of a calcified lesion in the MCA, accompanied by infarction in the territory distal to the lesion, strongly suggests CCE as the most probable cause of stroke. More than 75% of patients had also undergone CT scans prior to the ischaemic event, revealing no calcification, which, together with lesion location, supports the embolic origin of the calcification. CCEs are generally small in size (2-3 mm diameter), with significantly higher density (162 HU) than that of intraluminal thrombi (typically 50-70 HU). Lastly, rounded or oval shapes, rather than linear or tube shapes typical of vessel wall calcifications, also support the diagnosis of CCE.4
Although CCE may be explained by displacement of the embolus after a heart or carotid vascular intervention, catheterisation or surgery,5,7–9 cervical manipulation,10 or cardiopulmonary resuscitation,4 embolism is spontaneous in the great majority of cases, as in our series.4,11
In line with other published studies,4 the embolic source could be identified in most of our patients. In our series, embolisms were classified as atherothrombotic in 4 patients (44.4%), cardioembolic in 3 patients (33.3%), and of undetermined origin in 2 patients (22.2%), according to the TOAST classification. Furthermore, 6 patients (66.6%) presented carotid (33.3%) or cardiac (33.3%) calcifications with potential involvement in the CCE.
Bilateral CCEs suggest a left annular/valvular source or origin in the aortic arch, whereas recurrent ipsilateral events suggest calcified carotid stenosis.12 In our series, 22.2% of patients presented degenerative aortic valve disease with sclerosis and calcification; this contrasts with the results of other series that suggest aortic heart disease as the most frequent cause,13 with up to one-third of patients requiring subsequent surgery.4 In one of our patients, we detected severe aortic insufficiency secondary to endocarditis, together with degenerative signs in the mitral valve. Although CCE is usually related to a long-term complication of endocarditis, hearts with an underlying calcified valve disease may be more vulnerable to infection.14
However, it is frequently difficult to establish a definitive causal relationship with the calcified source, given the high frequency of some of these findings in the elderly population. In fact, the association between CCE and some heart diseases, such as annular calcification, is controversial.15–17 In other cases, as occurred in one-third of our patients, the cause of calcification cannot be identified. Such factors as age and comorbidities may limit the diagnostic screening assessments performed; as a result, some conditions with relevance to CCE may go undetected, with calcified atheromatosis of the aortic arch being one example. Therefore, screening for cardiac and carotid diseases becomes especially relevant in patients with CCE, with a view to indicating surgery for secondary prevention of cerebrovascular disease.
There is little evidence on acute-phase reperfusion therapy in patients with CCE. Regarding intravenous thrombolytic treatment, both negative18,19 and positive results are reported in the literature.4,5 Theoretically, we should not expect thrombolytic treatment to be efficacious, due to the calcified nature of the embolus. However, mechanical thrombectomy, which is able to remove the CCE, is a promising alternative. Nevertheless, this technique has shown limited effectiveness to date, partly due to the hardness of the calcified plaque preventing adequate adaptation to stent mesh, and also to the higher number of reported complications associated with this procedure.20,21 Only one of our patients was treated with mechanical thrombectomy by aspiration, which was unsuccessful. The technological development of more sophisticated stent retrievers or thromboaspiration devices may lead to better results in the future.
CCE is a reasonably frequent condition, although it may go undetected during diagnosis. Recognising the condition is essential, as it may help to determine the aetiology of the ischaemic stroke and, therefore, its treatment and prognosis. New endovascular treatment alternatives for CCE should be explored.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Mosqueira AJ, Canneti B, Martínez Calvo A, Fernández Armendáriz P, Seijo-Martinez M, Pumar JM. Embolia cerebral cálcica: presentación de una serie de 9 casos y revisión de la literatura. Neurología. 2022;37:421–427.