Multiple sclerosis (MS) is a chronic, inflammatory disease of the central nervous system (CNS), characterised by demyelination, remyelination, and axonal loss; the condition mainly affects young patients.1
Relapsing-remitting MS (RRMS) accounts for 85% of cases. A relapse is the occurrence of new symptoms or worsening of previous symptoms, lasting over 24hours, in the absence of a trigger factor.
Clinically isolated syndrome (CIS) is an initial episode of neurological symptoms characteristic of demyelinating diseases, with potential to progress to RRMS. RRMS is diagnosed only when the patient meets criteria for dissemination in time and space. The most recent formulation is included in the 2010 revised McDonald criteria.
Symptoms of the disease depend on which area of the CNS is affected; by order of frequency, the most characteristic symptoms include sensory alterations, loss of visual acuity, motor impairment, and symptoms of brainstem, cerebellar, and spinal cord involvement.2 Autonomic symptoms are observed in 79% of patients with MS. The urinary system is most frequently affected (65%); 50% of men display erectile dysfunction. Other common symptoms include sudomotor autonomic abnormalities (35%) and gastrointestinal disturbances (33%), with cardiovascular problems occurring in only 8% of cases.3 One of the least frequently described cardiovascular symptoms is left ventricular dysfunction.4
Acute left ventricular dysfunction has previously been reported in patients with RRMS.4 We present a case of cardiogenic shock in a patient with CIS.
Our patient was a 28-year-old woman with no relevant personal or family history, who came to hospital due to a 4-day history of oppressive holocranial headache and proximal hypoaesthesia in the left lower limb. She also reported vertigo, nausea, and vomiting. In the previous 24hours she had also presented right facial hypoaesthesia.
The neurological examination revealed facial asymmetry with effacement of the right nasolabial fold, mild proximal hypoaesthesia in the lateral aspect of the left lower limb, and gait ataxia with inability to walk in tandem; she scored 3.5 (S2, C3) on the Expanded Disability Status Scale (EDSS).
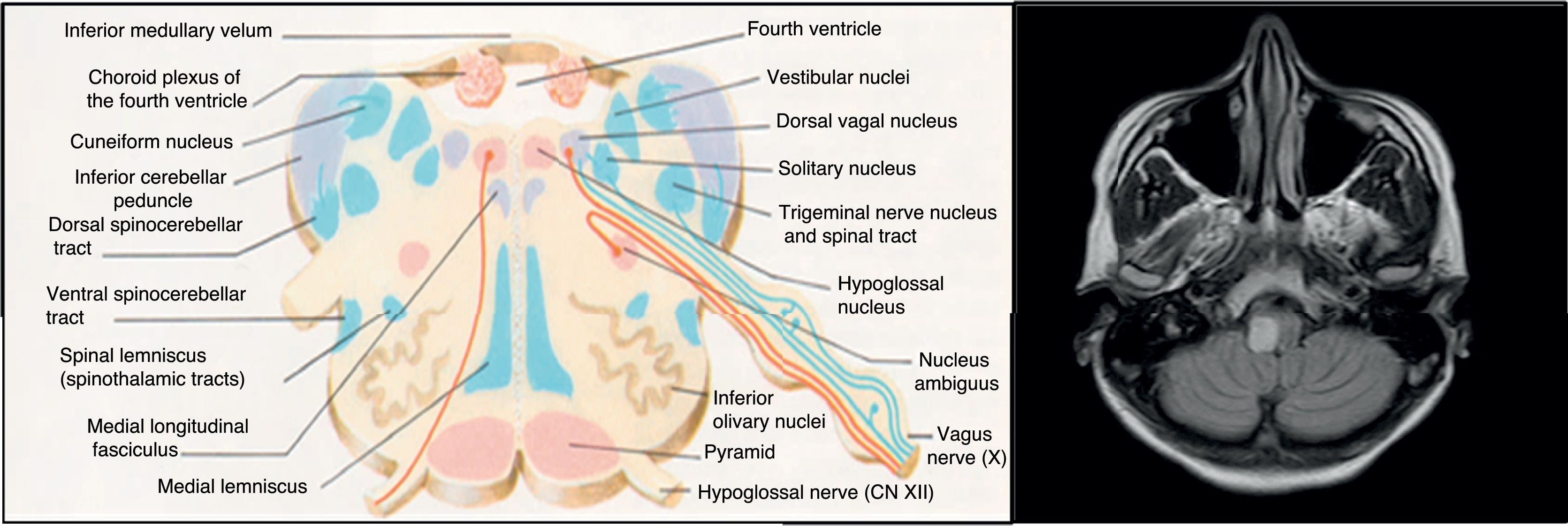
A brain MRI scan identified 2 demyelinating lesions: one, located in the left periventricular area and perpendicular to the axis, appeared to be chronic and was oval in shape, whereas the other lesion was located on the right side of the medulla oblongata and measured 7mm in diameter, was well-defined, and showed gadolinium uptake. The spinal cord MRI study found no alterations. Oligoclonal bands were detected in the CSF but not in the serum.
All other tests yielded unremarkable results; we were able to rule out infectious or autoimmune aetiology. As the patient did not meet the 2010 McDonald criteria for MS, she was diagnosed with CIS. The patient received 5 boluses of intravenous methylprednisolone and showed complete symptom resolution at 10 days (EDSS 0).
Fifteen days after the first episode, the patient presented acute dyspnoea, intense headache, and profuse sweating, which woke her from night-time sleep. The physical examination performed at the emergency department detected sinus tachycardia (135bpm), low blood pressure (60/30mmHg), an oxygen saturation of 95% with an FiO2 of 50%, respiratory failure, laboured breathing, and bilateral, generalised crackling.
An electrocardiography study revealed diffuse ST segment depression. A transthoracic echocardiography showed left ventricle hypocontractility at the basal and mid-cavity sections, severely decreased global systolic function (<25%), a diastolic filling pattern of increased preload, and considerable functional mitral regurgitation. A heart biopsy showed no signs of myocarditis; the patient was therefore diagnosed with cardiogenic shock secondary to acute left ventricular dysfunction due to probable inverted takotsubo cardiomyopathy. She was treated with 1g intravenous methylprednisolone for 3 days and underwent orotracheal intubation, Swan-Ganz catheterisation, and intra-aortic balloon pump placement.
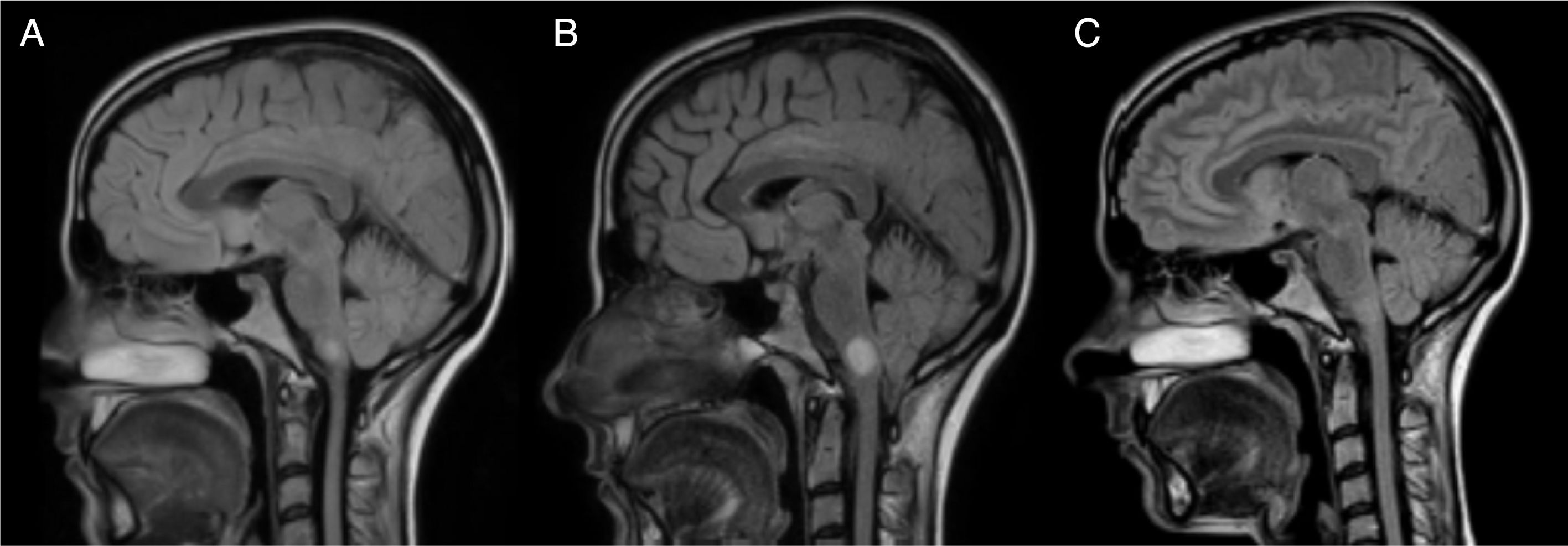
Twelve hours after the episode of acute cardiological symptoms, the patient presented severe vertigo and ataxia, and required 2 crutches to walk (EDSS 6.5). A follow-up brain and spinal cord MRI scan showed that the lesion on the right side of the medulla oblongata had increased in size to 13mm (Figs. 1 and 2); we therefore decided to administer 5 cycles of plasmapheresis. She progressed favourably but continued to display nystagmus in the primary gaze position, oscillopsia, abasia, and gait ataxia (EDSS 3.5). The patient improved progressively over the following months and was left with only mild oscillopsia at extreme gaze positions.
MRI: progression of the lesion to the right side of the medulla oblongata. Image B, taken one month after image A and 15 days after the episode of cardiogenic shock, shows an increase in lesion size from 7mm to 13mm. Image C, taken one year after image B, shows remyelination of the lesion.
After a year of follow-up, the patient has shown no further relapses or new MRI lesions. She has an EDSS score of 2 (brainstem 2) and presents no symptoms of heart disorders; cardiac oedema resolved and ventricular function recovered completely. Subsequent brain MRI scans show nearly complete disappearance of the lesion to the medulla oblongata (Fig. 1). Oligoclonal bands persist in the CSF.
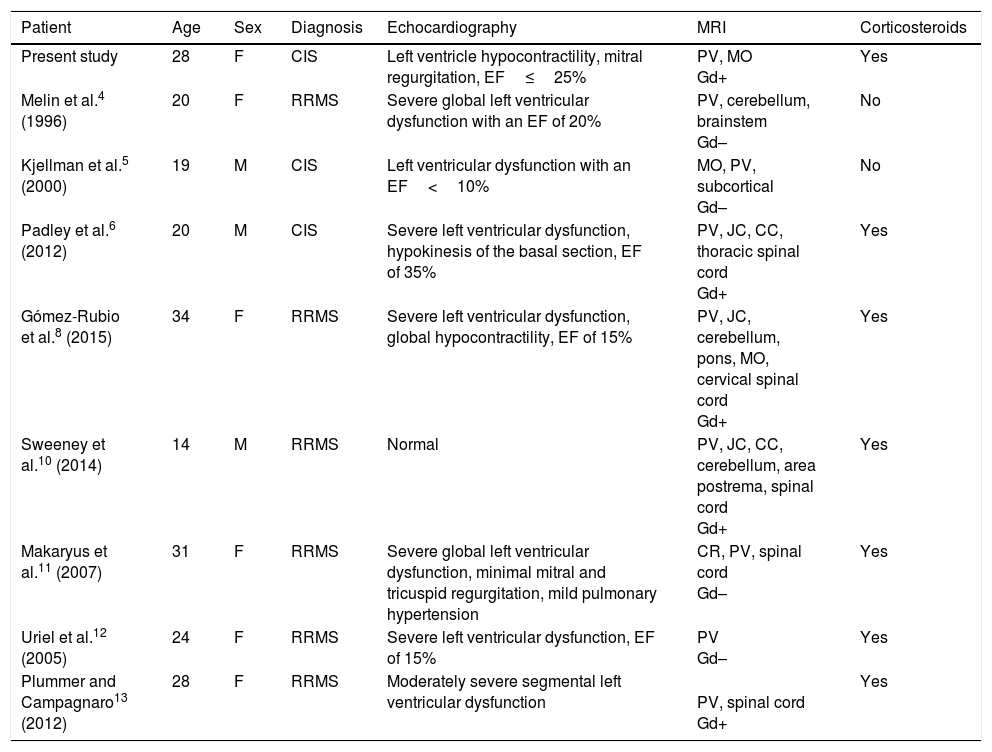
Few cases of left ventricular dysfunction have been reported in patients with MS (Table 1). The condition is even rarer after the first episode of MS-like symptoms (CIS). To our knowledge, only 2 similar cases have been published in the literature.5,6 However, unlike in the case described here, those patients presented significant MRI lesions, meeting the McDonald criteria for RRMS.7 Both patients also presented a second episode soon after the first, and were therefore diagnosed with clinically defined MS.
Clinical characteristics of patients with MS presenting left ventricular dysfunction.
| Patient | Age | Sex | Diagnosis | Echocardiography | MRI | Corticosteroids |
|---|---|---|---|---|---|---|
| Present study | 28 | F | CIS | Left ventricle hypocontractility, mitral regurgitation, EF≤25% | PV, MO Gd+ | Yes |
| Melin et al.4 (1996) | 20 | F | RRMS | Severe global left ventricular dysfunction with an EF of 20% | PV, cerebellum, brainstem Gd– | No |
| Kjellman et al.5 (2000) | 19 | M | CIS | Left ventricular dysfunction with an EF<10% | MO, PV, subcortical Gd– | No |
| Padley et al.6 (2012) | 20 | M | CIS | Severe left ventricular dysfunction, hypokinesis of the basal section, EF of 35% | PV, JC, CC, thoracic spinal cord Gd+ | Yes |
| Gómez-Rubio et al.8 (2015) | 34 | F | RRMS | Severe left ventricular dysfunction, global hypocontractility, EF of 15% | PV, JC, cerebellum, pons, MO, cervical spinal cord Gd+ | Yes |
| Sweeney et al.10 (2014) | 14 | M | RRMS | Normal | PV, JC, CC, cerebellum, area postrema, spinal cord Gd+ | Yes |
| Makaryus et al.11 (2007) | 31 | F | RRMS | Severe global left ventricular dysfunction, minimal mitral and tricuspid regurgitation, mild pulmonary hypertension | CR, PV, spinal cord Gd– | Yes |
| Uriel et al.12 (2005) | 24 | F | RRMS | Severe left ventricular dysfunction, EF of 15% | PV Gd– | Yes |
| Plummer and Campagnaro13 (2012) | 28 | F | RRMS | Moderately severe segmental left ventricular dysfunction | PV, spinal cord Gd+ | Yes |
CC: corpus callosum; CIS: clinically isolated syndrome; CR: corona radiata; EF: ejection fraction; F: female; Gd–: no gadolinium uptake; Gd+: gadolinium uptake; JC: juxtacortical; M: male; MO: medulla oblongata; PV: periventricular; RRMS: relapsing-remitting multiple sclerosis.
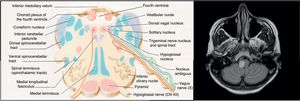
The aetiopathogenesis of left ventricular dysfunction in RRMS is controversial. There are several hypotheses attempting to explain the development of neurogenic pulmonary oedema secondary to left ventricular dysfunction in MS. According to the 4 most relevant hypotheses, it may be due to dysimmune or metabolic mechanisms, autonomic dysfunction, or a stress-induced catecholaminergic storm. The first hypothesis postulates that viral infection activates CNS immune response in MS; this may affect the heart, causing myocarditis and resulting in left ventricular dysfunction.8 In our case, we detected no active viral infections or signs of myocarditis in the cardiac biopsy. The second hypothesis proposes a decrease in levels of high-energy phosphates (phosphocreatine and ATP) in patients with MS not showing clinical signs of heart disease, suggesting subclinical involvement.9 The third hypothesis is linked to the location of demyelinating plaques: the solitary nucleus, area postrema, and caudal spinal cord act as sympathetic connections between the hypothalamus and the spinal cord10; lesions to these areas may alter this pathway. Brainstem involvement is rarely reported in the literature. In our patient, however, the lesion to the medulla oblongata was located near the dorsal vagal nucleus, involved in autonomic regulation, which may explain our patient's autonomic dysfunction, as in the case presented by Gómez-Rubio et al.8 According to those authors, autonomic dysfunction may cause a catecholaminergic storm, triggering cardiogenic shock. This may explain takotsubo syndrome, also known as apical ballooning syndrome or stress cardiomyopathy. The mechanisms by which autonomic dysfunction causes acute reversible left ventricular dysfunction include vasospasm, microvascular dysfunction, and direct myocardial damage by catecholamines.6 Unlike in our case, the patient presented by Gómez-Rubio et al. had RRMS and showed a considerable lesion load on MRI scans.
Some studies addressing left ventricular dysfunction in patients with RRMS report no association with age or EDSS score.14 Left ventricular dysfunction has also been reported in patients with secondary-progressive MS; this comorbidity is therefore not exclusive to RRMS.15
In our patient, initial treatment with high-dose intravenous corticosteroids was insufficient to control progression of the lesion to the medulla oblongata; 5 cycles of plasmapheresis had to be administered. Although our patient's progression was more typical of acute disseminated encephalomyelitis, persistence of oligoclonal bands in the CSF ruled out this diagnosis. To our knowledge, none of the patients described in the literature were treated with plasmapheresis.
It is not known whether this complication may change the natural course of the disease. In our case, there was no connection between severity at onset (due to the lesion to the medulla oblongata) and disease progression; initial severity was not found to be a factor of poor prognosis after a year of follow-up. Early diagnosis and treatment of heart complications and MS relapses is essential.
Please cite this article as: González Mingot C, Juárez Turégano A, Bosch Gaya A, Brieva Ruiz L. Shock cardiogénico en síndrome clínico aislado. Neurología. 2019;34:69–72.