In recent years, there has been an increase of studies dedicated to cognitive rehabilitation in patients with multiple sclerosis (MS); however, few of these analyze the impact on such variables as cognitive reserve. The study aims to explore the effects of a cognitive rehabilitation program comprising a combination of cognitive and physical exercises, as well as group sessions to improve cognitive performance, emotional state, and cognitive reserve index.
MethodFifty patients with MS were subdivided into 2 groups: the control group, which performed aerobic exercise (n=25), and the experimental group (n=25), which participated in the integrated cognitive rehabilitation program (ICRP). All participants were evaluated 3 times (baseline, post-treatment, and long-term) with the Brief Repeatable Battery of Neuropsychological Tests, Cognitive Reserve Scale, Beck Depression Inventory, and a scale evaluating trait and state anxiety.
ResultsCompared with the control group, patients in the experimental group showed improvements in cognitive function, with significant changes in measures of information processing speed, attention, memory, cognitive reserve index, and long-term mood.
ConclusionsThe ICRP was effective in improving cognitive and emotional function in MS, and increased the cognitive reserve index.
En los últimos años se ha observado un interés creciente por la rehabilitación cognitiva en pacientes con esclerosis múltiple. Sin embargo, pocos estudios han analizado su impacto en variables como la reserva cognitiva. Analizamos el efecto de un programa de rehabilitación cognitiva que incluye ejercicios físicos y cognitivos, así como sesiones en grupo enfocadas a mejorar el rendimiento cognitivo, el estado emocional y el índice de reserva cognitiva.
MétodosNuestro estudio incluyó a 50 pacientes con esclerosis múltiple, divididos en 2 grupos: un grupo control (n = 25), en el que los pacientes realizaban ejercicio aeróbico, y un grupo experimental (n = 25), al que se administró un programa integral de rehabilitación cognitiva. Evaluamos a todos los pacientes en 3 momentos diferentes (al inicio, tras el tratamiento, y a largo plazo) con la Batería Neuropsicológica Breve, la Escala de Reserva Cognitiva, el Inventario de Depresión de Beck y una escala para medir la ansiedad rasgo y la ansiedad estado.
ResultadosLos pacientes del grupo experimental mostraron un mejor rendimiento cognitivo que los controles, con cambios significativos en medidas de velocidad de procesamiento de la información, atención, memoria, índice de reserva cognitiva y estado de ánimo a largo plazo.
ConclusiónNuestros resultados demuestran la eficacia del programa de rehabilitación cognitiva para mejorar las funciones cognitiva y emocional de los pacientes con esclerosis múltiple y aumentar el índice de reserva cognitiva.
Multiple Sclerosis (MS) is characterized, among other aspects, by the presence of cognitive deficits that affect approximately 65% of patients diagnosed with the disease.1,2 Among the most frequent cognitive disorders in patients with MS are, the decreasing in processing speed, attention deficits, memory disorders and executive disfunctions.1,3 In patients with MS, these cognitive deficiencies can affect the performance of various daily activities.
Recently, several studies aimed at evaluating the efficacy of cognitive rehabilitation in MS patients. Interventions have included actions to improve attention, learning, memory and executive functions,4–7 while other researchers have used multimodal intervention strategies that combine cognitive stimulation and psychotherapeutic actions.8
However, the effectiveness of cognitive rehabilitation strategies in patients with MS is contradictory. This is due, among other factors, to the heterogeneity in the groups studied, the diversity of rehabilitation programs implemented and the measures used to assess outcomes.9,10
Currently, rehabilitation studies in MS patients focus on verifying the effectiveness of multidisciplinary intervention programs, understanding the concept of neurorehabilitation from a broad perspective. This field includes cognitive rehabilitation programs that use a combination of different strategies, for example, the use of physical and cognitive exercises,11,12 group sessions to learn how to use compensatory strategies and home computer training,7 psychoeducation, self-regulation and compensatory training,13 and enrichment programs for promoting different cognitive leisure activities.14,15
Programs focused on the enrichment of leisure activities are usually developed around the cognitive reserve construct. Cognitive Reserve (CR) The concept of CR refers to differences in cognitive processes that explain differential susceptibility to functional impairment in the presence of pathology. CR can therefore be considered an active model of reserve where the brain actively attempts to cope with brain damage by using pre-existing cognitive processes or by enlisting compensatory processes.16
From this perspective, it has been observed that MS patients with a higher level of cognitive reserve, compared to patients with a lower reserve, take longer to show cognitive decline.17,18 High levels of CR were associated with better cognitive task performance in verbal and spatial memory, attention, processing speed, verbal fluency, and inhibitory control. The results indirectly emphasize the value of early cognitive assessment of cognitive status and CR levels to enable timely initiation of cognitive interventions to increase cognitive reserve in MS in patients with low levels of CR.19
Currently, some authors conceive CR as a dynamic concept that can be modified and enriched throughout a lifespan. For this reason, CR construct has been incorporated from a multidisciplinary approach into rehabilitation programs.20–21
However, there are still few studies of neurorehabilitation aimed at building cognitive reserve in patients with MS. In the present study, a cognitive rehabilitation program called the Integrated Program of Cognitive Rehabilitation (PIRCO) is proposed (PIRCO is the acronym for the name of the program in Spanish: Programa Integrado de Rehabilitación Cognitiva).
The objective of this study was to explore the effect of a multimodal program, in which cognitive exercises were combined with physical exercises; as well as group sessions aimed at improving cognitive performance, reserve-building activities and emotional states.
MethodsParticipantsA total of 50 patients with Multiple Sclerosis (Relapsing-Remitting phenotype) who regularly attend to the Hospital de Rehabilitación “Dr. Faustino Pérez Hernández”, in Sancti Spiritus province, Cuba were evaluated. The patients had clinically defined MS according to the McDonald's criteria22 and the criteria of the clinical forms division proposed by Lubling and Reingold.23
All the patients were assessed at the neurological level, using the Expanded Disability Status Scale (EDSS). Additionally, the following inclusion criteria was considered: age between 18 years and 65 years, duration of the disease ≤20 years (ability to understand and comply with the cognitive rehabilitation program) and EDSS score ≤6. Patients with a severe psychiatric disorder, acute relapses (outbreaks one month before the evaluation or during the investigation that are treated with steroids or immunosuppressive drugs and due to their functional impact limit participation in the study), and other neurological disorders that could affect cognition, were excluded.
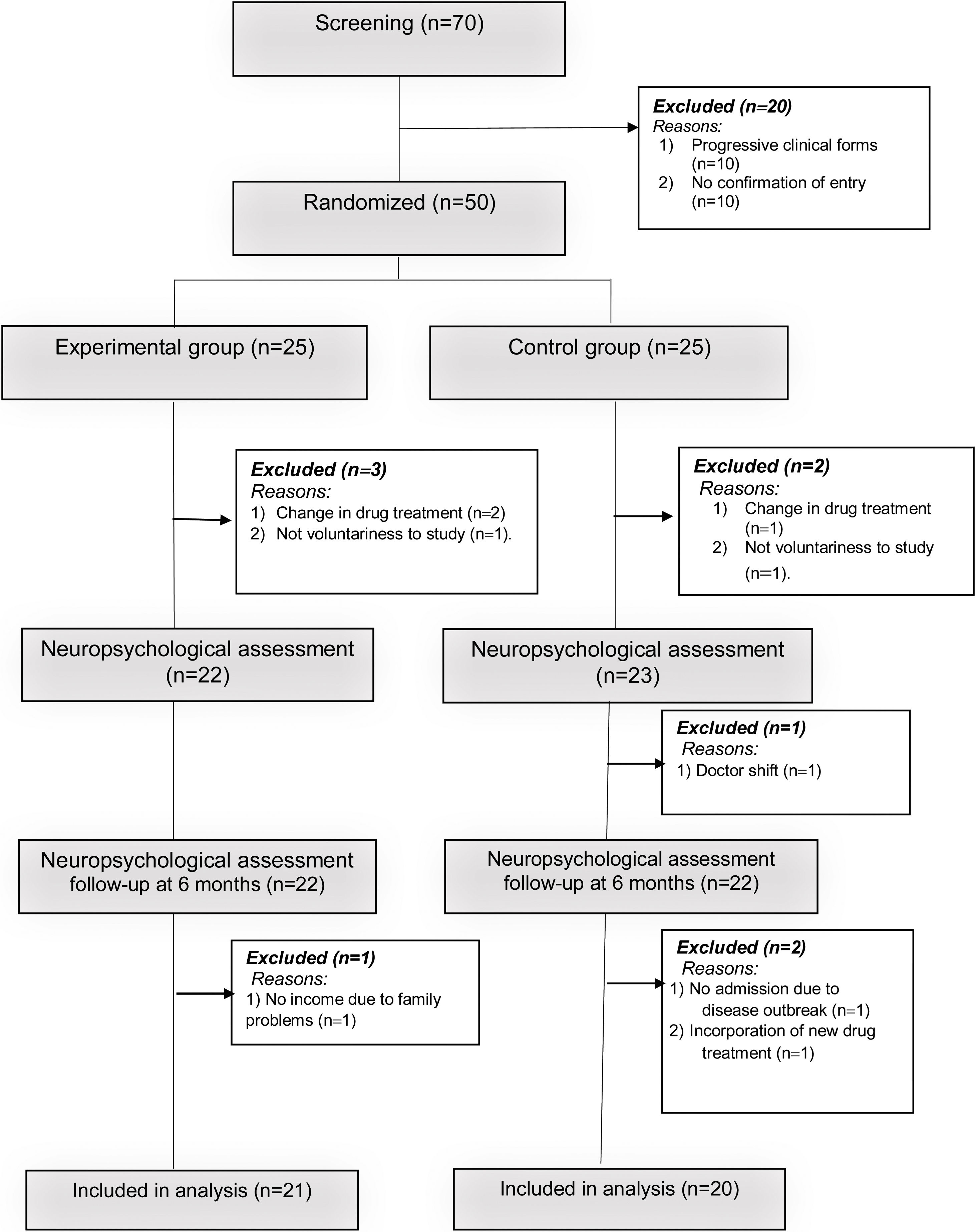
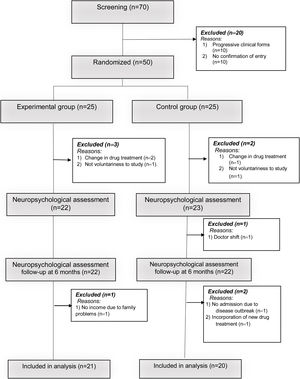
A simple recruitment process was carried out where participants were randomly assigned (in a ratio of 1:1) into an experimental group (EG: n=21) and a control group (CG: n=20).
The reasons for exclusion of some patients in the research process are described in Fig. 1. The study was approved by the ethics committee of the Hospital Provincial de Rehabilitación. All patients were informed about the importance of the research and signed the informed consent in accordance with the ethical principles of the Helsinki declaration.
Neuropsychological assessmentThe brief repeatable batteryThe EG and CG participants completed the Brief Repeatable Battery, translated and adapted to the Spanish-speaking population by Sepulcre et al.,24 The Brief Repeatable Battery (BRB) includes the Selective Reminding Test (SRT) (verbal long-term learning and memory), the Spatial Recall Test (SPART 10/36) (visuospatial long-term learning and memory), the Symbol Digit Modalities Test (SDMT) (attention and speed of information processing), the Paced Auditory Serial Addition Test (PASAT – 2 and 3) (speed of information processing and working memory), and the Word List Generation (WLG) (phonetic and semantic verbal fluency). The patients had never been evaluated using the Brief Repeatable Battery before. The results were considered by the individual measures of the BRB tests.
Cognitive reserve scaleThis scale is made up of 25 items divided into four types of activities: basic daily activities, training and information activities, hobbies and activities of social life.25,26 The scale explores three periods of life using a five-option response scale (Likert) (0, never; 1, once or several times a year; 2, once or several times a month; 3, once or several times a week; 4, three times or more in the week). The scale score range is 0–96.
Emotional statusThe emotional state of the patients was explored using the Spanish version of the Beck Depression Inventory (BDI)27,28 and the State-Trait Anxiety Inventory (STAI).29
Integrated cognitive rehabilitation program (PIRCO)General considerationsThe PIRCO program was implemented in patients who conform the experimental group. The cognitive rehabilitation program is theoretically based on an individualized approach that is built on the basis of the individual's strengths and works to compensate for deficit areas in order to increase the person's ability to participate more fully in daily life activities.30
The program implies three fundamental axes of intervention offered by a multidisciplinary team, where aerobics training, cognitive training, and group sessions from a cognitive and ecological perspective were combined. The training (physical and cognitive) involved the repeated practice of specific tasks designed to reflect the underlying cognitive processes. The main ingredient is repetitive work and explicit teaching of cognitive tasks.30 Group sessions offer activities aimed at self-knowledge work, compensation to improve memory and executive functions, stress management, and promotion of cognitive leisure activities.
The program was implemented during six weeks, with a daily frequency. In the morning session, the treadmill aerobic exercises were performed on daily basis, followed by Dynamic board game of cubes and signs (TaDiCS®). In the afternoon session the ergometric bicycle was performed daily. After the aerobic exercises, the Modified PASAT tasks training (Monday, Tuesday and Friday) and group sessions (Tuesday and Thursday) were alternated. The CG patients practiced only the aerobic exercises, with the same duration, frequency and intensity as the EG (morning session, the treadmill aerobic exercises, and the ergometric bicycle in afternoon session). These patients did not receive any form of cognitive training.
Structure of individual sessionsAerobics trainingA graduated resistance program was implemented, by combining training on the WNQ-7000ª treadmill and the ergometric bicycle (ERGOCIT-AT), using as a reference the training protocol developed by Sandroff et al.,31 The dosage in both workouts was gradual, reaching a maximum time of 30min in the sixth week. Intensity (heart rate reserve) and exercise duration (time in minutes) were controlled. The increasing in intensity and duration of the exercise was carried out based on the physical conditions and the stability of the heart pulse of each patient. If the patients presented a greater motor impairment that limited the initial phase of training (EDSS score 5–6), the dose was adjusted according to their rehabilitation needs, and they were accompanied at all times to control gait dynamics (treadmill training). All sessions were conducted by licensed physical therapists.
Cognitive trainingDynamic board game of cubes and signs (TaDiCS®)The TaDiCS® was used with the aim of training attention and the solution of practical-constructive problems. Its design is based on the clinical model of attention developed by Sohlberg and Mateer,32 as well as the problem solving model proposed by Luria.33 The cognitive training was carried out from Monday to Friday, in the morning session, during 45min. Tasks of visual tracking and visuomotor speed (visual modality), sustained and selective attention (auditory and visual modality), inhibitory control (auditory and visual modality) and practical-constructive problem solving were used.11 These activities were always performed after the aerobic exercises practiced in the morning.
The modified PASAT (Paced Auditory Serial Addition Task) and PVSAT (Paced Visual Serial Addition Task) tasksThe Modified PASAT tasks were implemented through the Computerized Cognitive Rehabilitation and Management System (GERCO®). The speed of information processing, sustained attention and working memory were trained.
Firstly, auditory tasks and then visual tasks were trained. The cognitive tasks had different indicators of complexity: type of tasks (auditory and visual), number of digits, complexity of the digits, duration of the stimulus, interval between stimuli and distractors. This training was carried out three times a week (Monday, Wednesday and Friday) in the afternoon session after the aerobic exercises and with a duration of 30min. The modified PASAT and PVSAT tasks have shown effectiveness in the cognitive rehabilitation of MS patients.34,35
In general, the cognitive training of attention tasks, problem solving, speed of information processing and working memory was used, because they are functions that are usually affected early in MS.
Group work sessionsA total of 10 group sessions were held with two weekly frequencies (Tuesday and Thursday). In each session, 60min were dedicated to group work with the patients and 30min to inform their caregivers on the topics discussed. Caregivers participated with prior informed consent. The topics of the sessions were the following: (1) how to improve cognitive performance through daily life activities; (2) what is cognitive reserve and how to enrich it; (3) how to improve learning and memory, (4) promotion of cognitive leisure activities and self-generation; (5) how to improve attention/concentration capacity; (6) successful behavior planning and self-regulation; (7) coping with stress and (8) development of leisure activities and cognitive health.
These group activities have been designed ad-hoc by program researchers with a psychoeducational and compensatory approach in the field of cognitive rehabilitation. The objective of the sessions with the caregivers was to provide information on the same content that was discussed with the patients; as well as to inform about the compensation strategies used during the work session. All sessions were coordinated by an experienced therapist and co-therapist.
Implementation of the intervention program: General procedureDuring the first week of treatment, a one-hour briefing session was held. During the session, the characteristics of the intervention program were explained to the patients and caregivers of the EG and CG (number and distribution of sessions, type of activities, and evaluation periods). In the case of the EG participants, the characteristics of the multimodal cognitive rehabilitation activities were presented individually and in groups. Both groups (EG and CG) were evaluated three times: baseline, post-intervention (6 weeks), and long-term follow-up (6 months). For the evaluations, the same brief battery of cognitive tests was used, but with different versions (version A, B and A) to control the effect of learning. It should be noted that the patients that participated in the program had not previously carried out cognitive assessments that included PASAT or other cognitive tests.
The post-intervention assessment was performed at the end of the sixth week and the follow-up stage (long-term assessment) was performed 30 weeks later (6 months after completing the intervention). The research process was developed in a double-blind manner. Both PIRCO participants, as well as the evaluators and analysts remained blind to the other activities of the program.
Analysis of dataThe data were processed using SPSS/Windows, version 21. Descriptive statistics were used to explore the demographic and clinical characteristics of the participants. The Chi-square test was used to explore the distribution between genders. Before selecting the t-test or ANOVA method, we explored the distribution of the data and checked that the homogeneity of variances was not violated (Levene's test). An independent t-test sample was performed to compare demographic and clinical variables between groups. A repeated measures ANOVA (2 (GE and GC)×3 (baseline assessment (T1), post-intervention (T2) and long-term evaluation (T3) was performed to check the effect of the program in EG patients in comparison to CG patients. A two-way ANOVA with repeated measures on one factor was conducted. The Mauchly's test of sphericity was used to assess whether or not the assumption of sphericity was met. The Greenhouse–Geisser effect correction was employed when sphericity was violated.36 The effect sizes were calculated using omega-squared (ω2). The reference values for omega-squared were .01, .06, .14 (small, medium and large effect size respectively).37 An additional independent-samples t-test was conducted to compare the differences in the scores recorded at the baseline and time 3 (change score). Effect sizes were calculated using Cohen's d. Cohen classifies .2 as a small effect, .5 as a medium effect and .8 as a large effect.38 Values of p<.05 were considered significant.
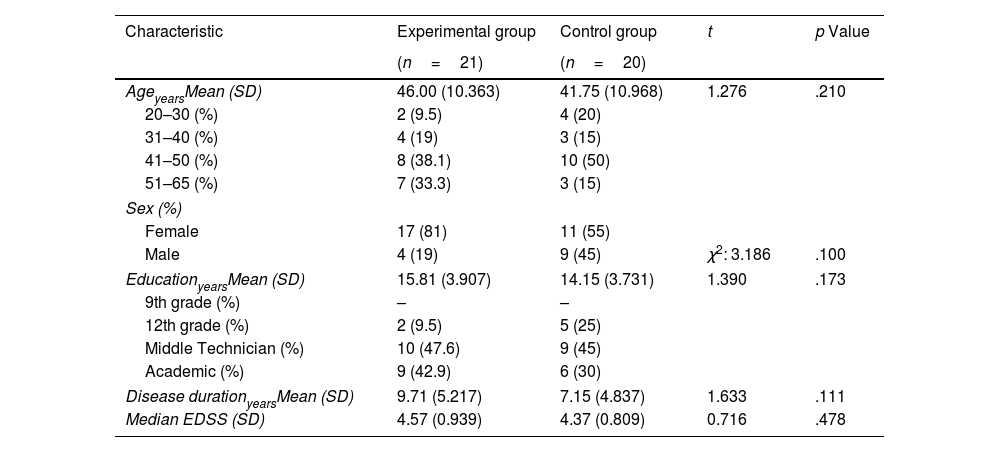
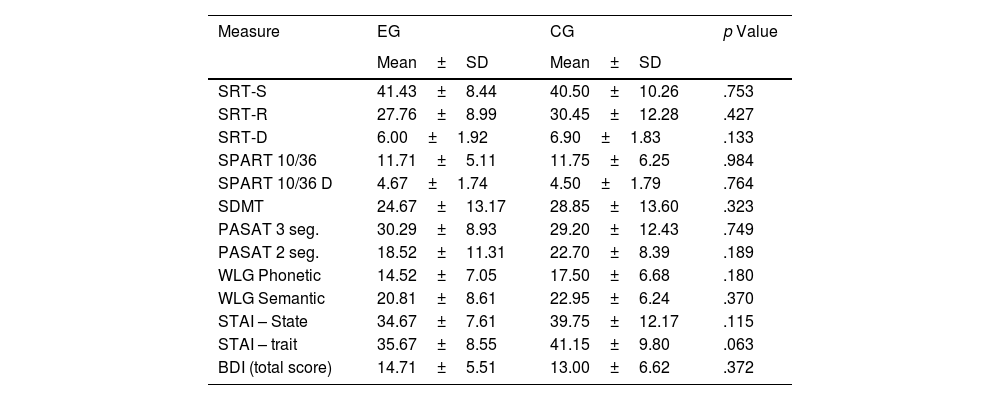
ResultsCharacteristics of the sampleThe demographic and clinical characteristics of the study groups are shown in Table 1. As shown in Table 1, no significant differences were observed in the different variables explored. An equivalent cognitive and emotional profile was observed between EG and CG before starting the cognitive rehabilitation program (Table 2).
Demographical and clinical characteristics of the experimental and control group.
| Characteristic | Experimental group | Control group | t | p Value |
|---|---|---|---|---|
| (n=21) | (n=20) | |||
| AgeyearsMean (SD) | 46.00 (10.363) | 41.75 (10.968) | 1.276 | .210 |
| 20–30 (%) | 2 (9.5) | 4 (20) | ||
| 31–40 (%) | 4 (19) | 3 (15) | ||
| 41–50 (%) | 8 (38.1) | 10 (50) | ||
| 51–65 (%) | 7 (33.3) | 3 (15) | ||
| Sex (%) | ||||
| Female | 17 (81) | 11 (55) | ||
| Male | 4 (19) | 9 (45) | χ2: 3.186 | .100 |
| EducationyearsMean (SD) | 15.81 (3.907) | 14.15 (3.731) | 1.390 | .173 |
| 9th grade (%) | – | – | ||
| 12th grade (%) | 2 (9.5) | 5 (25) | ||
| Middle Technician (%) | 10 (47.6) | 9 (45) | ||
| Academic (%) | 9 (42.9) | 6 (30) | ||
| Disease durationyearsMean (SD) | 9.71 (5.217) | 7.15 (4.837) | 1.633 | .111 |
| Median EDSS (SD) | 4.57 (0.939) | 4.37 (0.809) | 0.716 | .478 |
SD: standard deviation; EDSS: Expanded Disability Status Scale.
Cognitive tests and emotional states in the experimental and control group.
| Measure | EG | CG | p Value |
|---|---|---|---|
| Mean±SD | Mean±SD | ||
| SRT-S | 41.43±8.44 | 40.50±10.26 | .753 |
| SRT-R | 27.76±8.99 | 30.45±12.28 | .427 |
| SRT-D | 6.00±1.92 | 6.90±1.83 | .133 |
| SPART 10/36 | 11.71±5.11 | 11.75±6.25 | .984 |
| SPART 10/36 D | 4.67±1.74 | 4.50±1.79 | .764 |
| SDMT | 24.67±13.17 | 28.85±13.60 | .323 |
| PASAT 3 seg. | 30.29±8.93 | 29.20±12.43 | .749 |
| PASAT 2 seg. | 18.52±11.31 | 22.70±8.39 | .189 |
| WLG Phonetic | 14.52±7.05 | 17.50±6.68 | .180 |
| WLG Semantic | 20.81±8.61 | 22.95±6.24 | .370 |
| STAI – State | 34.67±7.61 | 39.75±12.17 | .115 |
| STAI – trait | 35.67±8.55 | 41.15±9.80 | .063 |
| BDI (total score) | 14.71±5.51 | 13.00±6.62 | .372 |
SD: Standard deviation; SRT-S: Selective Reminding Test (storage), SRT-R: Selective Reminding Test (recovery); SRT-D: Selective Reminding Test (Delayed Recall); SPART 10/36: Spatial Recall Test; SPART 10/36D: Spatial Recall Test (Delayed Recall); SDMT: Symbol Digit Modalities Test; PASAT 2–3: Paced Auditory Serial Addition Task 2 and 3 segundos; WLG: Word List Generation; STAI: State-Trait Anxiety Inventory; BDI: The Beck Depression Inventory.
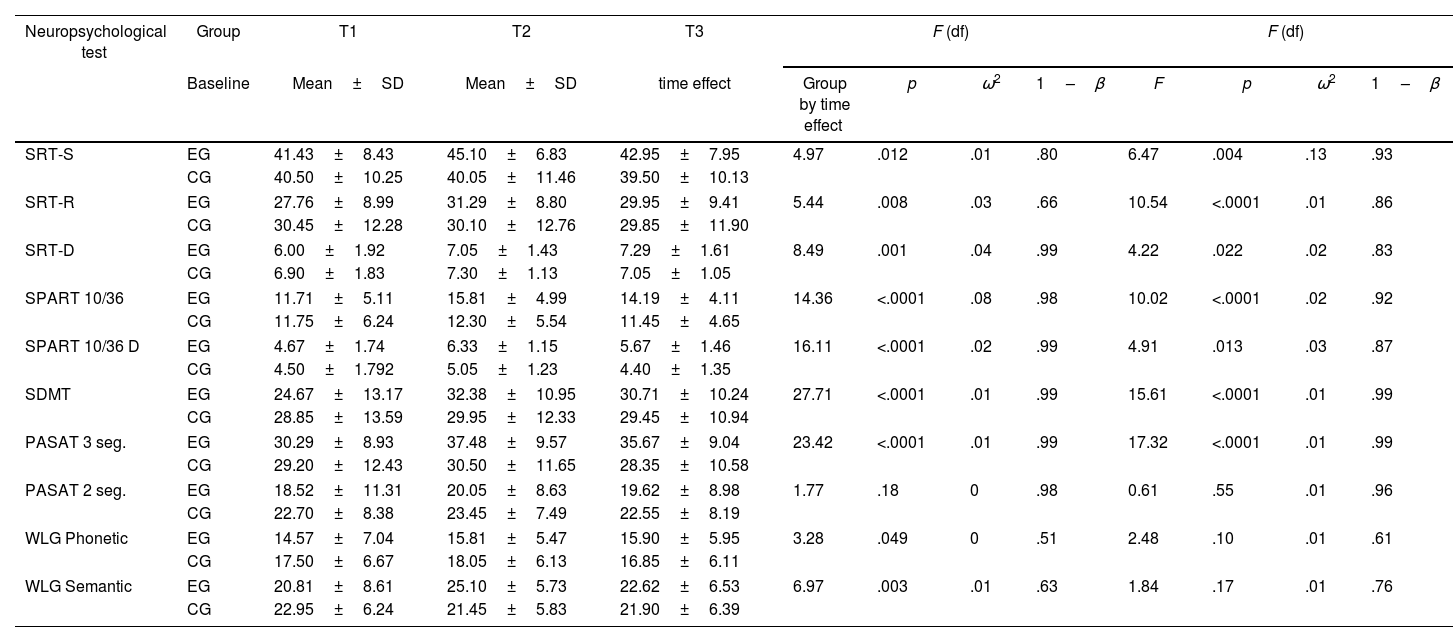
The ANOVA results showed significant changes over time in nine of the ten neuropsychological tests (Table 3). Compared to the baseline (T1), a significant difference was observed in the experimental group according to the scores obtained in T3. Significant differences were observed in verbal memory (SRT) and visuospatial memory (SPART), processing speed (SDMT), attention and working memory (PASAT-3), and verbal fluency (WLG). No significant differences were observed in the control group.
Evaluation of cognitive functions by repeated measures analysis (ANOVA) between the experimental group (EG) and the control group (CG).
| Neuropsychological test | Group | T1 | T2 | T3 | F (df) | F (df) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Mean±SD | Mean±SD | time effect | Group by time effect | p | ω2 | 1–β | F | p | ω2 | 1–β | |
| SRT-S | EG | 41.43±8.43 | 45.10±6.83 | 42.95±7.95 | 4.97 | .012 | .01 | .80 | 6.47 | .004 | .13 | .93 |
| CG | 40.50±10.25 | 40.05±11.46 | 39.50±10.13 | |||||||||
| SRT-R | EG | 27.76±8.99 | 31.29±8.80 | 29.95±9.41 | 5.44 | .008 | .03 | .66 | 10.54 | <.0001 | .01 | .86 |
| CG | 30.45±12.28 | 30.10±12.76 | 29.85±11.90 | |||||||||
| SRT-D | EG | 6.00±1.92 | 7.05±1.43 | 7.29±1.61 | 8.49 | .001 | .04 | .99 | 4.22 | .022 | .02 | .83 |
| CG | 6.90±1.83 | 7.30±1.13 | 7.05±1.05 | |||||||||
| SPART 10/36 | EG | 11.71±5.11 | 15.81±4.99 | 14.19±4.11 | 14.36 | <.0001 | .08 | .98 | 10.02 | <.0001 | .02 | .92 |
| CG | 11.75±6.24 | 12.30±5.54 | 11.45±4.65 | |||||||||
| SPART 10/36 D | EG | 4.67±1.74 | 6.33±1.15 | 5.67±1.46 | 16.11 | <.0001 | .02 | .99 | 4.91 | .013 | .03 | .87 |
| CG | 4.50±1.792 | 5.05±1.23 | 4.40±1.35 | |||||||||
| SDMT | EG | 24.67±13.17 | 32.38±10.95 | 30.71±10.24 | 27.71 | <.0001 | .01 | .99 | 15.61 | <.0001 | .01 | .99 |
| CG | 28.85±13.59 | 29.95±12.33 | 29.45±10.94 | |||||||||
| PASAT 3 seg. | EG | 30.29±8.93 | 37.48±9.57 | 35.67±9.04 | 23.42 | <.0001 | .01 | .99 | 17.32 | <.0001 | .01 | .99 |
| CG | 29.20±12.43 | 30.50±11.65 | 28.35±10.58 | |||||||||
| PASAT 2 seg. | EG | 18.52±11.31 | 20.05±8.63 | 19.62±8.98 | 1.77 | .18 | 0 | .98 | 0.61 | .55 | .01 | .96 |
| CG | 22.70±8.38 | 23.45±7.49 | 22.55±8.19 | |||||||||
| WLG Phonetic | EG | 14.57±7.04 | 15.81±5.47 | 15.90±5.95 | 3.28 | .049 | 0 | .51 | 2.48 | .10 | .01 | .61 |
| CG | 17.50±6.67 | 18.05±6.13 | 16.85±6.11 | |||||||||
| WLG Semantic | EG | 20.81±8.61 | 25.10±5.73 | 22.62±6.53 | 6.97 | .003 | .01 | .63 | 1.84 | .17 | .01 | .76 |
| CG | 22.95±6.24 | 21.45±5.83 | 21.90±6.39 | |||||||||
SD: Standard deviation; F-tests: Wilks’ lambda statistic; SRT-S: Selective Reminding Test (storage), SRT-R: Selective Reminding Test (recovery); SRT-D: Selective Reminding Test (Delayed Recall); SPART 10/36: Spatial Recall Test; SPART 10/36D: Spatial Recall Test (Delayed Recall); SDMT: Symbol Digit Modalities Test; PASAT 2–3: Paced Auditory Serial Addition Task 2 and 3s; WLG: Word List Generation; ω:2 omega-squared; 1–β: observed power.
On the other hand, Time×Group interactions showed significant differences. The effect of the iterations was significant in the measures of verbal memory (SRT) and visuospatial (SPART), speed of processing (SDMT), attention and working memory (PASAT-3). The analysis of the calculation of effect on changes in cognitive performance (effect×time), showed a small effect in all the cognitive variables studied (ω2<0.06) except for SPART 10/36 where the effect size was medium (ω2=0.06–0.14) (Table 3). In the case of time×group interactions showed a small effect in all the cognitive variables studied. For SRT-S values the effect sizes were medium.
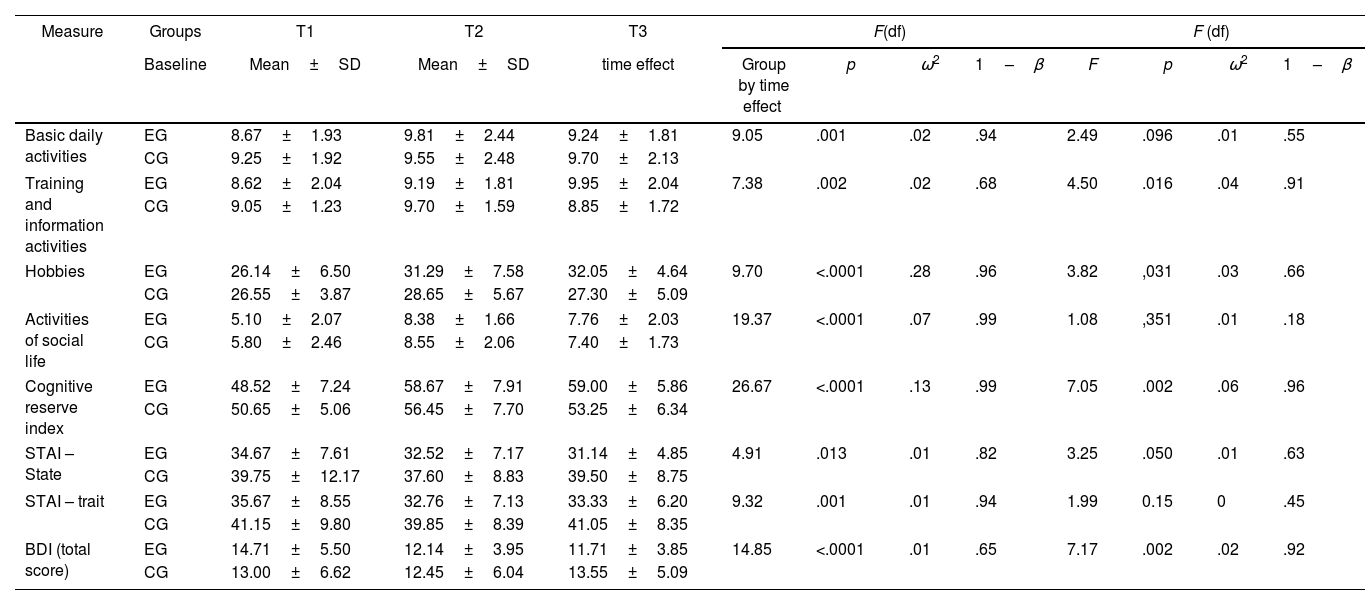
Cognitive reserveThe analysis of the effect×time interactions (Table 4), revealed significant changes in the cognitive reserve index. It was found that the training and information activities, hobbies, and social life showed significant changes after the 6-month follow-up. The time×group interaction was also observed. A significant effect in the cognitive reserve index was verified; as well as self-report measures of basic daily activities, activities that involved training and information, and hobbies. The effect size of the effect×time interactions was small (ω2<0.06) except for cognitive reserve index where the effect size was medium (ω2=0.13) and hobbies where the effect size was large (ω2=0.28). In the time×group interaction, analysis showed a small effect in all the reserve variables studied (ω2<0.06).
Evaluation of the cognitive reserve index, cognitively stimulating activities and emotional states through repeated measures between the experimental group (EG) and the control group (CG).
| Measure | Groups | T1 | T2 | T3 | F(df) | F (df) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Mean±SD | Mean±SD | time effect | Group by time effect | p | ω2 | 1–β | F | p | ω2 | 1–β | |
| Basic daily activities | EG | 8.67±1.93 | 9.81±2.44 | 9.24±1.81 | 9.05 | .001 | .02 | .94 | 2.49 | .096 | .01 | .55 |
| CG | 9.25±1.92 | 9.55±2.48 | 9.70±2.13 | |||||||||
| Training and information activities | EG | 8.62±2.04 | 9.19±1.81 | 9.95±2.04 | 7.38 | .002 | .02 | .68 | 4.50 | .016 | .04 | .91 |
| CG | 9.05±1.23 | 9.70±1.59 | 8.85±1.72 | |||||||||
| Hobbies | EG | 26.14±6.50 | 31.29±7.58 | 32.05±4.64 | 9.70 | <.0001 | .28 | .96 | 3.82 | ,031 | .03 | .66 |
| CG | 26.55±3.87 | 28.65±5.67 | 27.30±5.09 | |||||||||
| Activities of social life | EG | 5.10±2.07 | 8.38±1.66 | 7.76±2.03 | 19.37 | <.0001 | .07 | .99 | 1.08 | ,351 | .01 | .18 |
| CG | 5.80±2.46 | 8.55±2.06 | 7.40±1.73 | |||||||||
| Cognitive reserve index | EG | 48.52±7.24 | 58.67±7.91 | 59.00±5.86 | 26.67 | <.0001 | .13 | .99 | 7.05 | .002 | .06 | .96 |
| CG | 50.65±5.06 | 56.45±7.70 | 53.25±6.34 | |||||||||
| STAI – State | EG | 34.67±7.61 | 32.52±7.17 | 31.14±4.85 | 4.91 | .013 | .01 | .82 | 3.25 | .050 | .01 | .63 |
| CG | 39.75±12.17 | 37.60±8.83 | 39.50±8.75 | |||||||||
| STAI – trait | EG | 35.67±8.55 | 32.76±7.13 | 33.33±6.20 | 9.32 | .001 | .01 | .94 | 1.99 | 0.15 | 0 | .45 |
| CG | 41.15±9.80 | 39.85±8.39 | 41.05±8.35 | |||||||||
| BDI (total score) | EG | 14.71±5.50 | 12.14±3.95 | 11.71±3.85 | 14.85 | <.0001 | .01 | .65 | 7.17 | .002 | .02 | .92 |
| CG | 13.00±6.62 | 12.45±6.04 | 13.55±5.09 | |||||||||
SD: Standard deviation; F-tests: Wilks’ lambda statistic; STAI: State-Trait Anxiety Inventory; BDI: The Beck Depression Inventory; ω2: omega-squared; 1–β: observed power.
After 6 months, significant changes were also found in the effect×time interactions in the levels of trait and state anxiety, as well as in depression in both groups (Table 4). In the time×group interaction analysis, it was found that most of the emotional variables changed significantly, except for anxiety levels as a trait. In the time×group interaction, analysis revealed a small effect in all the emotional variables studied (ω2<0.06).
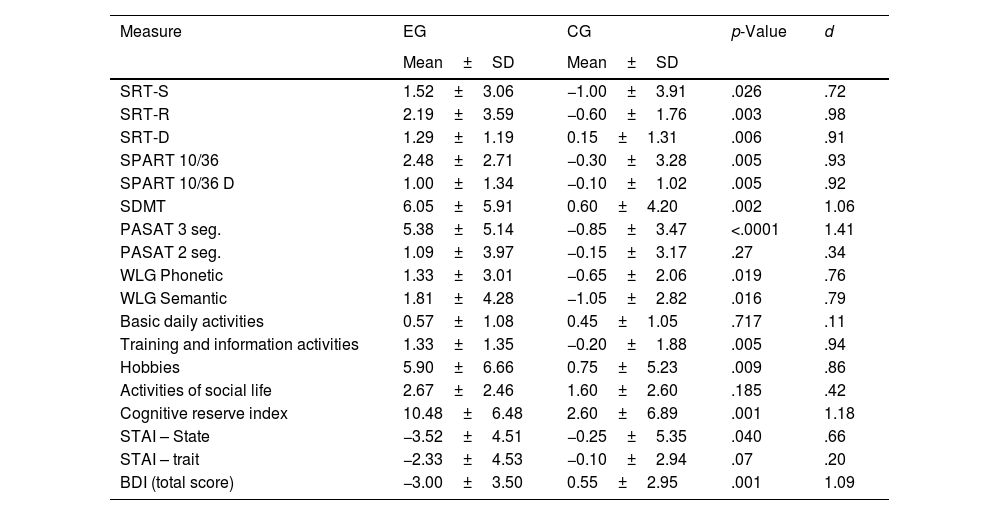
Analysis of change score from T1 to T3The data showed an increase in the performance of all neuropsychological tests in the EG, indicating values (+1); unlike the CGwhere a decline was observed in the execution of most of these tests (−0) (Table 5). The difference between the two groups was significant. In a special way, in the SRT-R tests (p=.003; d=.98); SRT-D (p=.006; d=.91); SPART 10/36 (p=.005; d=.93); SPART 10/36D (p=.005; d=.92); SDMT (p=.002; d=1.06); PASAT-3 (p<.001; d=1.41).
Change score (baseline to time 3) in cognitive tests, cognitive reserve and emotional states in both groups.
| Measure | EG | CG | p-Value | d |
|---|---|---|---|---|
| Mean±SD | Mean±SD | |||
| SRT-S | 1.52±3.06 | −1.00±3.91 | .026 | .72 |
| SRT-R | 2.19±3.59 | −0.60±1.76 | .003 | .98 |
| SRT-D | 1.29±1.19 | 0.15±1.31 | .006 | .91 |
| SPART 10/36 | 2.48±2.71 | −0.30±3.28 | .005 | .93 |
| SPART 10/36 D | 1.00±1.34 | −0.10±1.02 | .005 | .92 |
| SDMT | 6.05±5.91 | 0.60±4.20 | .002 | 1.06 |
| PASAT 3 seg. | 5.38±5.14 | −0.85±3.47 | <.0001 | 1.41 |
| PASAT 2 seg. | 1.09±3.97 | −0.15±3.17 | .27 | .34 |
| WLG Phonetic | 1.33±3.01 | −0.65±2.06 | .019 | .76 |
| WLG Semantic | 1.81±4.28 | −1.05±2.82 | .016 | .79 |
| Basic daily activities | 0.57±1.08 | 0.45±1.05 | .717 | .11 |
| Training and information activities | 1.33±1.35 | −0.20±1.88 | .005 | .94 |
| Hobbies | 5.90±6.66 | 0.75±5.23 | .009 | .86 |
| Activities of social life | 2.67±2.46 | 1.60±2.60 | .185 | .42 |
| Cognitive reserve index | 10.48±6.48 | 2.60±6.89 | .001 | 1.18 |
| STAI – State | −3.52±4.51 | −0.25±5.35 | .040 | .66 |
| STAI – trait | −2.33±4.53 | −0.10±2.94 | .07 | .20 |
| BDI (total score) | −3.00±3.50 | 0.55±2.95 | .001 | 1.09 |
SD: Standard deviation; d: Effect sizes were calculated using Cohen's d: SRT-S: Selective Reminding Test (storage), SRT-R: Selective Reminding Test (recovery); SRT-D: Selective Reminding Test (Delayed Recall); SPART 10/36: Spatial Recall Test; SPART 10/36D: Spatial Recall Test (Delayed Recall); SDMT: Symbol Digit Modalities Test; PASAT 2–3: Paced Auditory Serial Addition Task 2 and 3s; WLG: Word List Generation; STAI: State-Trait Anxiety Inventory; BDI: The Beck Depression Inventory.
Training and information activities (p=.005; d=.94), hobbies – hobbies (p=.009; d=.86) and the cognitive reserve index (p<.001; d=1.18) showed a significant difference between the groups on increasing frequency after the end of the program. No significant changes were observed in basic daily activities and social life activities. On the other hand, significant differences were found in the values of IDARE (State) (p=.040; d=.66) and IDB (p=.001; d=1.09) reflecting a decrease in depression in EG (−3); however, an increase in this variable was observed in the CG during follow-up (+0.55) (Table 5).
The calculation of the magnitude of the differences between the score change (T1 to T3) is generally high (d>.80) between the EG and the CG.
DiscussionThis study provides evidence on the efficacy of PIRCO to help improving long-term cognitive reserve and functioning in MS patients by generalize cognitive leisure activities.
Previous works have described that physical exercises31,39 as well as cognitive rehabilitation programs40,41 are profitable in optimizing cognitive functions in patients with MS. Nevertheless, other authors state that combining this two type of intervention (physical and cognitive) could be applied in the context of a cognitive reserve bettering, in a global form, the ill effects on motor and cognitive disability in MS.12,31 Our studies are in line with previous one, because we have observed that the combined training was effective, improving some diverse functions, such as verbal and visuospatial memory, information processing speed, attention, and working memory. Recent studies on MS agree on the positive effect of the motor-cognitive approach on visuospatial memory performance and the speed to process information, attention, and working memory compared to the group that received physical training only.11,12
Another important variable that can influence the effectiveness of the PIRCO rehabilitation program was group work. During these activities, the EG participants had the opportunity to reinforce their behavior; as well as the development of knowledge and skills necessary to configure and transfer cognitive and leisure activities into daily life one. These group interventions could contribute to increase up the cognitive reserve index once the rehabilitation program is concluded (T2), as well as the benefits observed in the long term (T3), and to reinforce the importance of transferring the benefits that education provides for cognitive health and compensation strategies to activities of daily living. These results coincide with few studies that examine the importance of group sessions included in cognitive rehabilitation programs in MS, which seem to reinforce the learning of new compensation strategies taught by therapists7,42 and also help their successful experience in MS. daily life.7
The effect observed through PIRCO on the increase of cognitive reserve and leisure activities, in the participants, was a key factor in reinforcing the hypothesis that premorbid physical and intellectual activities not only act as a buffer for limiting the MS-related damage but also as functional reserve that can be retrieved by task-oriented training to promote recovery through rehabilitation.43 These results are consistent to a study to identify patients with MS, a relationship among leisure activities generating new learnings (Ex. Reading and writing) with changes been related to brain reserves (larger volume in the hippocampus), as well as bettering memory functions up.14 Schwartz et al.44 in a longitudinal study proved that leisure and cognitive activities been more profitable in patients having larger active cognitive reserves, were: self-willing activities, attending religious or spiritual organizations and social participation. As some authors suggest15 these results are an evidence of the continuous participation in cognitive stimulating activities are capable of creating a cognitive reserve and acting as a protector against worsening disability related to MS, been summed up to previous cognitive reserve in patients.
It is also important to point out the effect caused by training in such a relevant aspect as the well affair. These results suggest that this multimodal rehabilitation approach, creates a notorious effect on the depressive symptoms present in MS patients. These results are in consonance to previous studies carried out by our research group,11 as well as studies carried out by other authors.12
To conclude, it is necessary, to point out an important limitation in this study. The inclusion of a control group made up of MS patients treated with cognitive treatment but not with group therapy; in addition, a group with equivalent demographic characteristics but without MS would have yielded more consistent data of short- and long-term effectiveness in improving cognitive reserve in daily live activities. Hence, although the results are conclusive, it is not possible to conclude, in a certain way, the value of combination therapy. However, it is possible to conclude that this study provides evidence of the adequate benefits of a rehabilitation program, in which cognitive exercises were combined with physical exercises, as well as group sessions; aimed at improving cognitive performance, reserve-building activities, and emotional states.
Regarding the control of confounding variables, there is no significant difference in education between EG and CG. However, the EG group shows a greater number of participants with academic studies and fewer participants with low education (in our case, 9th grade) than the CG group. Due to the relationship between cognitive reserve and education level, the random block design could be useful in future studies.
Finally, although it was not the objective of our study, the analysis of the caregivers’ work could be of vital importance in the effect of the intervention. Therefore, the control of some family factors in the rehabilitation process could validate the approach of the program in the future.
Conflict of interestsThe authors declare that they have no conflict of interest.