Cycads are ornamental plants that in some parts of the world are used as fresh food or raw material for producing flour with a high nutritional value. However, they also contain active compounds, including methylazoxymethanol, β-methylamino-l-alanine, β-alanine-l-oxalylamino and cycasin, which may produce neurotoxic effects. Some studies have associated consuming cycads and their derivatives with neurodegenerative diseases such as amyotrophic lateral sclerosis/Parkinsonism dementia complex, and other diseases characterised by motor impairment. Therefore, we must not forget that any product, no matter how natural, may present health risks or benefits depending on the chemical compounds it contains and the susceptibility of those who consume it.
DevelopmentWe completed a literature analysis to evaluate the neurotoxic properties of cycads and their association with neurological diseases in order to provide structured scientific information that may contribute to preventing health problems in people who use these plants.
ConclusionCycads contain neurotoxic compounds that may contribute to the development of neurological diseases when ingested improperly. We must be mindful of the fact that while some plants have a high nutritional value and may fill the food gap for vulnerable populations, they can also be toxic and have a negative impact on health.
Las cícadas son plantas que en algunas partes del mundo son empleadas como alimento fresco o materia prima para la elaboración de harina con alto valor nutricional. Sin embargo, contienen principios activos como metilazoximetanol, β-metilamino-L-alanina, β-oxalilamino-L-alanina y cicasina, entre otros, que pueden producir efectos neurotóxicos. El consumo de cícadas y sus derivados se ha asociado con enfermedades neurodegenerativas, como el complejo demencia-parkinsonismo-esclerosis-lateral amiotrófica y otras enfermedades caracterizadas por alteraciones en la motricidad. Por lo tanto, no debemos perder de vista que todo producto, aunque sea de origen natural, puede ser benéfico o perjudicial para la salud, lo cual dependerá de sus componentes químicos y de la vulnerabilidad de quienes los consumen.
DesarrolloSe realizó un análisis de la literatura sobre las propiedades neurotóxicas de las cícadas y su asociación con enfermedades neurológicas, con el fin de proporcionar información estructurada a la población para contribuir a la prevención de problemas de salud en quienes interactúan con estas plantas.
ConclusiónLas cícadas contienen neurotóxicos que contribuyen al desarrollo de enfermedades neurológicas cuando son ingeridas inadecuadamente, por lo que debemos considerar que si bien algunos vegetales pueden tener un alto valor nutricional y subsanar el déficit alimentario en las poblaciones vulnerables, también pueden ser tóxicos e impactar negativamente sobre la salud.
Cycads are gymnosperms that are considered living fossils because they have been present in different parts of the world ever since they emerged in the Mesozoic era. Researchers have described some 185 species of cycads, most of which are endemic. While these species are frequently cultivated as exotic ornamentals, they also provide food for humans. Due to poor management of these resources, and to the plants’ complex reproductive cycles, many cycads are threatened or endangered. As such, international trade in cycads is regulated by the Convention on Trade in Endangered Species (CITES). While the seeds of some cycads are used as fresh food or as raw material used to make highly nutritious meal,1 some contain significant amounts of highly toxic chemical compounds.2 Scientists have found an association between consumption of cycad seeds/derivatives and motor and electroencephalographic changes in animal models (Table 1). In humans, cycad consumption has been linked to neurodegenerative diseases such as amyotrophic lateral sclerosis-Parkinsonism dementia complex (ALSPD) and other motor diseases.2,3 Neurotoxic active ingredients identified in cycad seeds include methylazoxymethanol acetate (MAM), β-methylamino-l-alanine (l-BMAA), β-oxalylamino-l-alanine (l-BOAA), and cycasin. These substances have also served as tools for exploring possible aetiologies of ALSPD, a complex presenting frequently on the island of Guam, where the native diet included certain cycad derivatives.3 This study therefore reviews the link between cycad toxins and the development of neurodegenerative disease in order to inform at-risk populations and warn about health problems in those who come into contact with these plants.
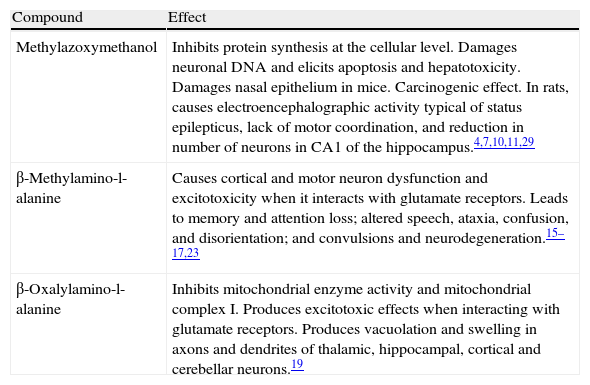
Principal toxic compounds identified in selected Cycadales
| Compound | Effect |
| Methylazoxymethanol | Inhibits protein synthesis at the cellular level. Damages neuronal DNA and elicits apoptosis and hepatotoxicity. Damages nasal epithelium in mice. Carcinogenic effect. In rats, causes electroencephalographic activity typical of status epilepticus, lack of motor coordination, and reduction in number of neurons in CA1 of the hippocampus.4,7,10,11,29 |
| β-Methylamino-l-alanine | Causes cortical and motor neuron dysfunction and excitotoxicity when it interacts with glutamate receptors. Leads to memory and attention loss; altered speech, ataxia, confusion, and disorientation; and convulsions and neurodegeneration.15–17,23 |
| β-Oxalylamino-l-alanine | Inhibits mitochondrial enzyme activity and mitochondrial complex I. Produces excitotoxic effects when interacting with glutamate receptors. Produces vacuolation and swelling in axons and dendrites of thalamic, hippocampal, cortical and cerebellar neurons.19 |
Cycadales synthesise and store a number of neurotoxic and carcinogenic active ingredients (Table 2) including such glucosides as MAM.4 The active ingredient is released by the main glucoside through enzymatic processes occurring in digestion. Cycasin, a β-d-glucoside of MAM, has also been identified in cycads.5,6 Cycasin is the most common glucoside in all types of cycads; others, present in smaller percentages, include macrozamin and neocycasin. The toxic part of cycasin is the methyl-azoxy group present in its structure; this is released as MAM when cycasin is metabolised in the digestive system by the β-glucosidase enzyme produced by normal bacterial flora of the small intestine. For this reason, cycasin only exerts a toxic effect when it is ingested.7 MAM is a product of macrozamin metabolism and the presence of the azoxy group in its chemical structure renders it highly toxic and carcinogenic. At the cellular level, this compound inhibits protein synthesis and affects the DNA of vulnerable neurons, in which it also induces apoptosis. Botanist Knut Norstog8 observed that cycads endemic to Guam produce abundant pollen, which contains high concentrations of cycasin and l-BMAA. When pollen comes into contact with nasal epithelium, cycasin and other toxins can be transported to brain tissue, where they induce neurotoxic effects.9 In mice, intranasal administration of MAM damages the olfactory epithelium10 and may affect brain tissue over the long term. These findings suggest that the respiratory system may be a route of entry for cycad toxins; therefore, long periods of environmental exposure to the pollen of these cycads may result in high concentrations of the toxin in the nasal epithelium. Cycasin has the most thoroughly studied toxicology of all of the azoxyglycosides; it paved the way for studies of mutagenicity and carcinogenicity of other azoxyglycosides, such as macrozamin and neocycasin.11 Azoxyglycosides present risk of mutagenesis when they are ingested because intestinal flora is able to deglycosylate them and render them highly toxic. Human carcinogenicity of certain cycad compounds is supported by laboratory results that show that cycasin and MAM are carcinogenic to various organs, and also in numerous species.12,13 Other studies have not detected associations between consumption of cycad flour and cancer,14 but these studies do not rule out the possibility that other cycad flour samples or different cycad derivatives may contain this toxin and constitute a health risk for consumers.
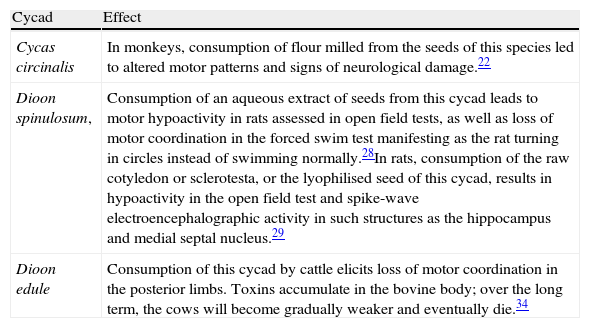
Some effects associated with cycad seed or cycad derivative consumption in experimental animals
| Cycad | Effect |
| Cycas circinalis | In monkeys, consumption of flour milled from the seeds of this species led to altered motor patterns and signs of neurological damage.22 |
| Dioon spinulosum, | Consumption of an aqueous extract of seeds from this cycad leads to motor hypoactivity in rats assessed in open field tests, as well as loss of motor coordination in the forced swim test manifesting as the rat turning in circles instead of swimming normally.28In rats, consumption of the raw cotyledon or sclerotesta, or the lyophilised seed of this cycad, results in hypoactivity in the open field test and spike-wave electroencephalographic activity in such structures as the hippocampus and medial septal nucleus.29 |
| Dioon edule | Consumption of this cycad by cattle elicits loss of motor coordination in the posterior limbs. Toxins accumulate in the bovine body; over the long term, the cows will become gradually weaker and eventually die.34 |
For humans, the most frequent route of exposure to cycad toxins is through foods prepared with this plant; however, the level of azoxyglycosides may be low if cycads and their derivatives are prepared properly. In contrast, cycad foodstuffs that are prepared improperly may be toxic and negatively impact people's health.
Cycads also contain another chemical compound with neurotoxic activity: l-BMAA, a non-proteinogenic amino acid recognised by NMDA receptors. Its chemical structure is similar to that of glutamate. This amino acid is found in seeds of Cycas circinalis (C. circinalis) and it has even been identified in flour made from these seeds.15 Although the effect of l-BMAA on the body is not yet fully understood, many studies have shown the compound's excitotoxic effect when it interacts with glutamate receptors. People with l-BMAA toxicity develop cortical and motor neuron dysfunction (a characteristic of Parkinson's disease) as well as behavioural anomalies that are associated with motor neuron degeneration.16 The effects of l-BMAA on the CNS arise from the compound's anticholinergic activity and produce behavioural changes. Characteristic syndromes include memory and attention loss, speech alterations, ataxia, confusion, and disorientation,17 that is, some of the changes typically seen in patients with Alzheimer disease.18
Another toxin present in C. circinalis is l-BOAA, which decreases mitochondrial enzymatic activity (NADH-dehydrogenase and lactate dehydrogenase) and elicits marked inhibition of mitochondrial complex I.19l-BOAA has a strong affinity for glutamate receptors, especially AMPA, which is what makes it excitotoxic. Some data indicate that presence of l-BOAA produces vacuolation, axon and dendrite swelling in neurons in the thalamus, and to a lesser extent, in the hippocampus, cortex and cerebellum as well; this results in a neurodegenerative process.19 The above points to the neurotoxicity of chemical compounds found in cycads; acting together, they may contribute to the development of neurodegenerative diseases associated with cycad consumption.
Cycads and their association with neurodegenerative diseasesFor years, the study of diseases endemic to specific populations has been viewed as an opportunity for identifying the aetiology and underlying mechanisms in each particular case. These ‘natural experiments’, as they have been called, have let researchers resolve questions about the aetiology and epidemiology of certain diseases.13,20 An excellent example of this systematic search for factors and mechanisms that trigger neurodegenerative disorders can be found in the western Pacific. After World War II, tortillas made with flour milled from C. circinalis seeds were commonly eaten in Guam. Reliance on this foodstuff was followed by a major epidemic of what was known as Guam dementia syndrome.20 Whiting12 was the pioneer researcher of the link between dementia in Guam and the neurotoxins in cycads. In their search for factors that would elicit the neurodegeneration affecting natives of Guam, Vega and Bell21 identified a non-proteinogenic amino acid which they named l-BMAA in the seeds of C. circinalis. In the 1970s, different groups of researchers became involved in identifying other potentially neurotoxic chemical compounds in cycad seeds; they found the glucosides MAM and sterol β-d-glucoside. In the 1980s, Steele and Guzmán assessed the neurotoxic effects of the l-BMAA in C. circinalis flour on motor activity in selected monkey species. They reported altered motor patterns in animals exposed to this chemical compound.22 Duncan et al.23 performed a series of studies to measure the quantity of the neurotoxin 2-amino-3-(methylamino)-propanoic acid (l-BMAA) in C. circinalis seed flour from Guam. Their results found that more than 87% of the l-BMAA content was eliminated by the flour preparation process; these researchers therefore ruled out C. circinalis flour as a cause of gradual neuron degeneration in the ALSPD complex cases identified in Guam. On this basis, they hypothesised that the true trigger of the neurodegenerative diseases found on the island could be attributed to the bioaccumulation of cycad neurotoxins.24 Inhabitants of Guam often ate fruit bats whose diet basically consisted of cycad seeds. High levels of toxic metabolites were present in the meat, and these metabolites were then transferred to humans who ate the bats.25 This theory may have substance since studies have shown that incidence of the ALSPD complex has decreased over the same period as bat consumption has decreased in Guam.25 Although different studies have attempted to explain the endemic nature of the disease found among natives of Guam –a progressive disease with symptoms similar to those of Lou Gehrig disease or ALS and accompanied by dementia in its final stages– data are currently inconclusive. Meanwhile, the contribution of toxic compounds in cycads has yet to be ruled out.
As a result of these studies, l-BMAA has been found to act as a glutamate receptor agonist, which causes excitotoxicity, convulsions, and neurodegeneration.15 In fact, intracerebroventricular administration of l-BMAA to rats elicits loss of motor activity and will even incapacitate them to the point of preventing locomotion.26 One outstanding finding was the discovery that l-BMAA may be produced by cyanobacteria of the Nostoc genus. These bacteria are symbionts on cycad roots and seeds,24 which could also explain the high content of this amino acid in cycad roots. Coincidentally, a Canadian study described patients with Alzheimer disease associated with high levels of l-BMAA due to consumption of water contaminated with cyanobacteria.27 The above leads us to believe that neurodegenerative diseases associated with cycad consumption may be due to high levels of l-BMAA or other neurotoxins, and this hypothesis is still being explored.
Studies of the neurotoxic effect of Dioon spinulosum seeds and methylazoxymethanolStudies by our working group support the hypothesis that cycad seeds cause motor alterations which may be associated with neural damage. A series of rat models found that chronic consumption (40 days) of an aqueous extract of Dioon spinulosum (D. spinulosum) elicited abnormal motor patterns in open field tests. Furthermore, when rats took the forced swimming test they spun in circles and were unable to keep their balance in order to swim normally.28 The above suggests altered motor control in the rat's limbs interfering with its balance; this may be due to the toxic compounds present in the seeds of this cycad. Our results are consistent with earlier reports that identified motor alterations in rats performing an open field test after a diet of D. spinulosum seeds. These alterations were associated with changes in electroencephalographic activity29 characterised by the high-voltage, high-frequency waves typical in status epilepticus. Interestingly enough, these effects on motor and electroencephalographic activity were reproduced using cortical administration of MAM.29 Results suggest that this compound may be responsible for neural damage and therefore altered behaviour, although the participation of other compounds could not be ruled out. However, changes in rat motor patterns associated with cycad seed consumption can be prevented by concomitantly administering progesterone,28 a neurosteroid whose neuroprotective effect has been proved clinically and experimentally.30
Another series evaluated the effect of microinjections of MAM in CA1 of the hippocampus on swimming patterns in Wistar rats. As could be expected, administering MAM to the hippocampus produced progressive deterioration of motor activity in the rats, which showed an increased tendency to spin in circles and increased immobility time during the test. This was similar to the effect of the glutamate microinjection.31 In addition, animals receiving the MAM or glutamate microinjection had a lower neuron count in CA1 and CA3 of the hippocampus than a control group.31 The above indicates that both substances damage neurons, possibly due to their excitatory effects, and this may be related to motor alterations associated with the consumption of cycads and cycad products.
Closing comments and observationsCycad plants are regarded as living fossils that have survived countless climate change events. The neurotoxins in these plants include cycasin, macrozamin, MAM, l-BMAA, and l-BOAA. These toxins have been linked to neurological diseases including ALS, Parkinson's disease, and Alzheimer disease. Beginning of 1960s, different studies were undertaken to identify the cause of ALSPD complex in natives of Guam. At that time, the disease was known as the ‘epidemic of the century’, and people following the lifestyle and customs of the western Pacific, including eating cycads and cycad products, were considered to be at high risk. The epidemiological consequences were unimaginably devastating; entire families were ill in some cases, regardless of age or such other factors as malnutrition or exposure to infectious agents. Diseased individuals became reclusive because of their limitations, and they even had difficulty recognising themselves. Current evidence suggests that these neurological diseases may have been caused by neurotoxins in flour milled from cycad seeds. Based on this discovery and the important technological breakthroughs of the past and current centuries, researchers have managed to identify neurotoxins that may be involved in the process. The introduction of selective mutations and use of animal models (rat models work the best) to emulate some of the traits of ALSPD have allowed us to gain a better understanding of the complex and draw closer to its underlying mechanism. Guam dementia syndrome, at one point in history, caused more cases of cognitive and motor impairment than any other neurodegenerative disease. The conclusion is clear; many developing countries are undergoing epidemiological transition processes of their own. In this sense, different types of dementia have become full-blown health problems, and therefore require in-depth analysis on many levels, whether clinical, diagnostic, or causal. Much remains to be clarified about diseases affecting the brain, and precisely those countries whose populations suffer the most exposure to neurotoxins engage in the least research on that subject. We should point out that the parts of Mexico in which the most cycad seeds are consumed as a corn substitute are the northern parts of Veracruz and Oaxaca. However, the Secretariat of Health has not yet received any official reports describing outbreaks of neurodegenerative diseases in those states. However, anecdotal evidence from the same Mexican states reports that cattle feeding on fresh cycad leaves will develop poor motor coordination of the posterior limbs. People exposed to cycads within enclosed spaces, such as greenhouses, report headaches and decreased liver function over the long term. Likewise, Mixtecs in the Mexican state of Oaxaca describe a disease, enchamalamiento, characterised by atrophy, deformation of the joints, and musculoskeletal changes, and attributed to excessive consumption of cycads.2 These data underline the need for epidemiological studies including neurologists and other specialists and to detect potential neurological manifestations in populations that consume cycad products.
Lastly, this review clearly states that many natural products that may be highly nutritive and provide an alternative food source to help vulnerable populations during shortages may also be highly toxic. When processed incorrectly, these foodstuffs may have a negative impact on the health of consumers. These phenomena have been witnessed with other crops such as manioc/cassava. Since the plant is easy to cultivate and adapts well to different climates, production is increasing despite reports that consuming improperly processed manioc may be linked to developing neurological diseases.32,33 As a result, we must not forget that all products, whether or not they are natural, may be beneficial or harmful to the body depending on the chemical compounds they contain and the vulnerability of the person consuming them.
ConclusionsCycads contain neurotoxins that contribute to the development of neurological diseases when they are ingested improperly. Doctors must consider whether vegetable matter that may be highly nutritive and helpful for alleviating shortages in vulnerable populations may also be toxic and harmful, particularly from the perspective of developing neurological disease.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rivadeneyra-Domínguez E, Rodríguez-Landa JF. Las cícadas y su relación con algunas enfermedades neurodegenerativas. Neurología. 2014;29:517–522.