Some treatments are inappropriate for patients with cognitive decline. We analyse their use in 500 patients and present a literature review.
DevelopmentBenzodiazepines produce dependence, and reduce attention, memory, and motor ability. They can cause disinhibition or aggressive behaviour, facilitate the appearance of delirium, and increase accident and mortality rates in people older than 60. In subjects over 65, low systolic blood pressure is associated with cognitive decline. Maintaining this figure between 130 and 140mmHg (145 in patients older than 80) is recommended. Hypocholesterolaemia<160mg/dL is associated with increased morbidity and mortality, aggressiveness, and suicide; high-density lipoprotein (HDL)-cholesterol<40mg/dL is associated with memory loss and increased vascular and mortality risks. Old age is a predisposing factor for developing cognitive disorders or delirium when taking opioids. The risks of prescribing anticholinesterases and memantine to patients with non-Alzheimer dementia that is not associated with Parkinson disease, mild cognitive impairment, or psychiatric disorders probably outweigh the benefits. Anticholinergic drugs acting preferentially on the peripheral system can also induce cognitive side effects. Practitioners should be aware of steroid-induced dementia and steroid-induced psychosis, and know that risk of delirium increases with polypharmacy. Of 500 patients with cognitive impairment, 70.4% were on multiple medications and 42% were taking benzodiazepines. Both conditions were present in 74.3% of all suspected iatrogenic cases.
ConclusionsPolypharmacy should be avoided, if it is not essential, especially in elderly patients and those with cognitive impairment. Benzodiazepines, opioids and anticholinergics often elicit cognitive and behavioural disorders. Moreover, systolic blood pressure must be kept above 130mmHg, total cholesterol levels over 160mg/dL, and HDL-cholesterol over 40mg/dL in this population.
Algunos fármacos resultan inconvenientes en pacientes con deterioro cognitivo. Se analiza su uso en 500 pacientes y se revisa la bibliografía.
DesarrolloLas benzodiacepinas producen dependencia y reducen la atención, memoria y agilidad motora. Pueden inducir desinhibición o agresividad, facilitan los episodios confusionales e incrementan los accidentes y la mortalidad en mayores de 60 años. En mayores de 65, la presión sistólica baja se asocia a deterioro cognitivo. Es recomendable mantenerla en 130-140 mmHg (145 en ≥ 80 años). La colesterolemia<160mg/dl se asocia a mayor morbimortalidad, agresividad y suicidio, y el colesterol unido a las lipoproteínas de alta densidad (c-HDL) < 40mg/dl empeora la memoria y aumenta el riesgo vascular y la mortalidad. La edad avanzada predispone para que los opioides produzcan alteración cognitiva y confusión. En demencias no Alzheimer y no asociadas a Parkinson, deterioro cognitivo ligero y enfermedades psiquiátricas, los efectos adversos de anticolinesterásicos y memantina probablemente superan al beneficio. La alteración cognitiva por anticolinérgicos de acción preferentemente periférica también es posible. Hay que conocer la demencia o psicosis por corticoides, y saber que la polifarmacia facilita el síndrome confusional. El 70,4% de 500 pacientes con disfunción cognitiva analizados recibía polifarmacia y el 42%, benzodiacepinas. Los que compartían ambas situaciones representaron el 74,3% de los casos en los que se sospechó iatrogenia.
ConclusionesEn personas con edad avanzada o deterioro cognitivo, es necesario evitar la polifarmacia innecesaria y tener presente que las benzodiacepinas, los opioides y los anticolinérgicos producen frecuentemente alteraciones cognitivas y conductuales. Además, deben evitarse la presión sistólica < 130 mmHg, el colesterol < 160mg/dl y el colesterol HDL < 40mg/dl.
Certain treatment plans, although apparently coherent, may be harmful to elderly patients or patients with cerebrovascular diseases, mild cognitive decline, or dementia. The present article reviews the disadvantages of treating certain patients with benzodiazepines (BZD), opioid analgesics (OA), or anticholinergics (AC), as well as the risks of excessively strict treatment with hypolipidaemic or antihypertensive drugs.
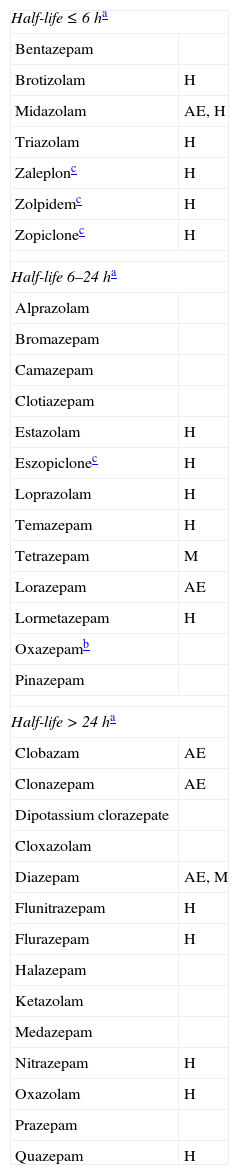
ProcedureAre BZD the best treatment option for anxiety, agitation, or insomnia?In many clinical situations, BZDs and non-BZD hypnotics constitute the first-choice treatment for fighting anxiety, insomnia, and other related symptoms. Some active ingredients in this category are short-, medium-, or long-acting. Some of them have significant muscle relaxant or anticonvulsant properties, and different degrees of sedative or hypnotic efficacy (Table 1). Given this wide range of properties, target symptoms can be controlled with proper know-how. Short-term feedback from the patient and household members generally conveys satisfaction, which increases the doctor's confidence in his or her prescribing habits. This may partially explain why BZD consumption has been increasing gradually. Data from the Spanish Agency for Medicines and Medical Devices1 report that 30.25 DDD were administered per 1000 inhabitants in 1992 (DDD=defined daily dose, or the average maintenance dose for a specific indication), whereas by 2006 it had risen to 62.14 DDD per 1000 inhabitants (an increase of 105%). Use of non-BZD sedative-hypnotics (zaleplon, zolpidem, zopiclone) rose from 1.64 DDD to 6.71 DDD per 1000 inhabitants (an increase of 309%).
Principal benzodiazepines and non-benzodiazepine sedative-hypnotics
| Half-life≤6ha | |
| Bentazepam | |
| Brotizolam | H |
| Midazolam | AE, H |
| Triazolam | H |
| Zaleplonc | H |
| Zolpidemc | H |
| Zopiclonec | H |
| Half-life 6–24ha | |
| Alprazolam | |
| Bromazepam | |
| Camazepam | |
| Clotiazepam | |
| Estazolam | H |
| Eszopiclonec | H |
| Loprazolam | H |
| Temazepam | H |
| Tetrazepam | M |
| Lorazepam | AE |
| Lormetazepam | H |
| Oxazepamb | |
| Pinazepam | |
| Half-life>24ha | |
| Clobazam | AE |
| Clonazepam | AE |
| Dipotassium clorazepate | |
| Cloxazolam | |
| Diazepam | AE, M |
| Flunitrazepam | H |
| Flurazepam | H |
| Halazepam | |
| Ketazolam | |
| Medazepam | |
| Nitrazepam | H |
| Oxazolam | H |
| Prazepam | |
| Quazepam | H |
AE: anticonvulsant and antiepileptic effect; H: significant hypnotic effect; M: significant muscle relaxant effect.
We must be aware of the disadvantages of indiscriminate use of these agents. These drugs act on GABAA receptors, inhibiting many cerebral functions. They decrease attention, certain memory functions, mental activation, and motor skills.2,3 From treatment onset,4 the rate of falls and fractures increases,4–6 and this trend rises more sharply with increasing age, dose, and drug half-life.4,6 Additionally, these drugs raise the incidence of accidents of all kinds (traffic, domestic, sport-related, and work-related).3,7 They can also generate paradoxical reactions, such as uninhibited or aggressive behaviours.8 On the other hand, BZDs and non-BZD sedative-hypnotics have an anterograde amnesic effect, aggravate memory deficits,9 and increase the risk of delirium episodes.10 Several experts suggest that adverse effects of sedative hypnotics exceed their benefits for people over 60, in addition to increasing mortality.11,12 In elderly people and those with cognitive decline, BZDs may be beneficial as treatment for agitated states. However, if the treatment length exceeds 3 weeks, patients may develop dependence (drug tolerance and symptoms of withdrawal syndrome),13 and some secondary effects may appear at an earlier time.4 Therefore, it is advisable to choose a different treatment when behavioural disorders are continuous or very frequent. Among alternative maintenance treatments, we should mention mood stabiliser anticonvulsants (such as pregabalin, whose indications include generalised anxiety disorder).14,15 Another class is constituted by antidepressants with an anxiolytic or sedative effect (especially when symptoms of depression are present): escitalopram,16 venlafaxine,17 duloxetine,18 trazodone,19,20 and mirtazapine.21 Additional alternative treatments include melatonin (for treating behavioural changes associated with circadian rhythm disturbances)22; and atypical neuroleptics previously administered to patients with this profile (for example, low doses of quetiapine, i.e. 12.5-200mg/days in 1 or 2 daily doses).23 Atypical neuroleptics are more effective in patients also experiencing delusional thinking and hallucinations. These neuroleptic agents must be selected correctly and long-term use must be avoided, since each drug is accompanied by predictable adverse effects and a potentially increased risk of mortality.24–26 Regardless of the treatment chosen, it is necessary to examine patients frequently in order to reduce doses or discontinue the drug as soon as possible. Adjuvant non-pharmacological treatments are also useful.27
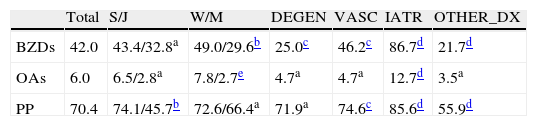
The study began with a register of 500 patients aged 19 to 104 (mean age 75.2, median 77, all with cognitive dysfunction and 141 with symptoms of dementia) treated by a cognitive neurology department. Patients were asked whether they were being treated with BZDs or any non-BZD sedative-hypnotics at the time of the initial consult. Results are listed in Tables 2–5. A high percentage of patients (42%) were under treatment with one of these; this was more frequent in women and across all diagnostic groups (Table 4). For 74.2% of the patients receiving the treatment, the doctor estimated that the drugs were involved in the aetiology of cognitive symptoms, and that BZDs had an impact on 86.7% of all patients presenting totally or partially iatrogenic symptoms (Table 4).
Age, sex, and diagnostic groups of the selected 500 patients
| W | S | DEGEN | VASC | IATR | OTHER_DX |
| 318 (63.6) | 430 (86) | 64 (12.8) | 338 (67.6) | 180 (36) | 143 (28.6) |
Percentages in brackets.
DEGEN: clinical diagnosis of degenerative disease; IATR: an adverse drug effect is considered to have contributed to the cognitive symptoms; W: woman; OTHER_DX: any other diagnosis of cognitive impairment that is neither degenerative, vascular, nor iatrogenic in origin; S: 65 and older; VASC: clinical diagnosis of cerebrovascular disease.
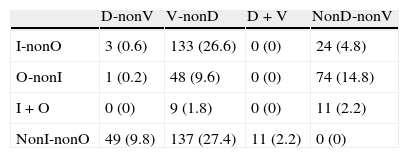
Distribution of diagnoses including cases of combined aetiology (percentages given in brackets)
| D-nonV | V-nonD | D+V | NonD-nonV | |
| I-nonO | 3 (0.6) | 133 (26.6) | 0 (0) | 24 (4.8) |
| O-nonI | 1 (0.2) | 48 (9.6) | 0 (0) | 74 (14.8) |
| I+O | 0 (0) | 9 (1.8) | 0 (0) | 11 (2.2) |
| NonI-nonO | 49 (9.8) | 137 (27.4) | 11 (2.2) | 0 (0) |
D: clinical diagnosis of degenerative disease; I: an adverse drug effect is considered to have contributed to the cognitive symptoms; O: any other diagnosis of cognitive impairment that is neither degenerative, vascular, nor iatrogenic in origin; V: clinical diagnosis of cerebrovascular disease.
Distribution of the 500 patients according to types of treatment observed (data shown as percentages)
| Total | S/J | W/M | DEGEN | VASC | IATR | OTHER_DX | |
| BZDs | 42.0 | 43.4/32.8a | 49.0/29.6b | 25.0c | 46.2c | 86.7d | 21.7d |
| OAs | 6.0 | 6.5/2.8a | 7.8/2.7e | 4.7a | 4.7a | 12.7d | 3.5a |
| PP | 70.4 | 74.1/45.7b | 72.6/66.4a | 71.9a | 74.6c | 85.6d | 55.9d |
DEGEN: clinical diagnosis of degenerative disease; IATR: an adverse drug effect is considered to have contributed to the cognitive symptoms; J: younger than 65; W: woman; OTHER_DX: any other diagnosis of cognitive impairment that is neither degenerative, vascular, nor iatrogenic in origin; PP: polypharmacy; S: 65 and older; M: man; VASC: clinical diagnosis of cerebrovascular disease.
Chi-square test:
a Not significant.
Hypertension in midlife increases the risk of developing dementia in later life,28 and treating the condition probably reduces that risk.29 However, the optimum ranges for systolic and diastolic blood pressure have changed over time, and they are still subject to debate.30
There is an association between low blood pressure, whether systolic or diastolic, and cognitive decline, especially in the elderly population.31–33 This association is stronger for systolic blood pressure.28,31 Attention and executive functions are more sensitive to changes in blood pressure than are other cognitive functions, such as language or memory functions.34
Patients with low cardiac ejection fraction show cognitive impairment and reduced motor skill due to cerebral hypoperfusion. Such deficiencies are more marked when patients also exhibit systolic hypotension.35
According to a consensus document by experts from the American societies of Neurology, Geriatrics, Preventive Cardiology, Hypertension, and Nephrology, systolic blood pressure should not exceed 140mmHg in people aged 65 to 79, and it should be maintained between 140 and 145mmHg in people aged 80 or older.36 Most of the authors addressing this topic recommend that systolic blood pressure in this population should not be lower than 130mmHg, except in patients with both heart failure and coronary artery disease.
Should cholesterol levels be kept as low as possible to decrease cardiovascular risk?Prolonged high levels of cholesterol increase the risk of developing coronary artery disease and cerebral infarction.37 Therefore, lowering cholesterol is clearly beneficial in secondary prevention of adverse events associated with coronary artery disease. However, its efficacy in the primary prevention of cerebrovascular or cardiovascular accidents is less evident, especially for people over 75. Treatment must be selected carefully according to the risk level.38,39
On the other hand, low cholesterol levels (<160mg/dL) are harmful to mental health and associated with higher morbidity and mortality rates.40,41 Low cholesterol is also associated with aggressive behaviour and higher incidence of suicide.42 Some studies have shown that reducing low-density lipoprotein cholesterol (LDL-C) to less than 120mg/dL does not reduce cardiovascular risk.43 Furthermore, an excessive decrease in high-density lipoprotein cholesterol (HDL-C<40mg/dL) gives rise to memory decline44 and increases risk of mortality and also of stroke and coronary artery disease (especially in patients with diabetes).45,46 As a result, statin treatment in patients with dyslipidaemia must be adjusted to maintain cholesterol levels between 160 and 200mg/dL, and niacin or fibrates must be included for patients with low HDL-C levels.39
Should chronic pain therapy be aggressive?Chronic pain is common among the elderly and it reduces quality of life.47 In addition to attempting to target the cause, symptomatic treatment includes practical recommendations and medication, which may be administered orally or topically, or injected at the pain location.48 Pharmacological treatment comprises simple analgesics (paracetamol or metamizole), anti-inflammatory agents, and opiate analgesics (strong OAs, such as morphine, hydromorphone, buprenorphine, methadone, fentanyl, oxycodone, meperidine, and tapentadol, and weak OAs, such as tramadol, codeine, dihydrocodeine and dextropropoxyphene). There are also adjuvant drugs including coanalgesics and coadjuvants. Coanalgesics lessen certain types of pain and include certain anticonvulsants (pregabalin, gabapentin, lamotrigine, carbamazepine, oxcarbazepine, clonazepam, topiramate), certain muscle relaxant drugs (baclofen, tizanidine), selected antidepressants (especially amitriptyline and other tricyclic antidepressants), and selective norepinephrine and serotonin reuptake inhibitors (duloxetine, venlafaxine; mirtazapine). On the other hand, coadjuvant drugs indirectly lessen pain or its repercussions (anxiolytics and non-analgesic antidepressants when anxiety or depression are involved, muscle relaxants for contractures, antibiotics for infections, laxatives for constipation, etc.). The previously mentioned disadvantages of BZD treatment must be considered when adjuvant drugs are prescribed. Additionally, we should take into account that tricyclic antidepressants have an AC effect, which may worsen cognitive state in elderly patients or those whose condition progresses with cholinergic deficiencies. BZDs may also lead to orthostatic hypotension or severe complications in patients with arrhythmia or atrioventricular block. Furthermore, combinations such as tramadol and antidepressant agents may lead to serotonin syndrome.49
Correct use of analgesic drugs (including OAs) in patients with long-term pain may improve functional status and social engagement in the elderly.50 Easy-to-use OAs with an increasingly better tolerability appeared on the market in the past 25 years. Some examples are hydromorphone (sustained-release oral morphine), oral tramadol, transdermal fentanyl patches, and tapentadol. Since tapentadol acts as both an OA and a norepinephrine reuptake inhibitor, it is effective with fewer secondary effects. Changes in the pharmacopoeia resulted in an 1284% increase in OA use in Spain between 1992 and 2006 (from 0.32 to 4.43 DDD per 1000 inhabitants).51
In elderly individuals, OAs can alter cognition and perception, especially in cases of renal failure or dehydration.52 These drugs may also be dangerous in patients with an underlying pulmonary condition and in those whose medication causes respiratory depression.52 Due to their potential adverse effects and ability to cause dependence, they are restricted to cases of acute or chronic pain that does not respond to other types of analgesic treatment.
Several review articles on iatrogenic delirium show that OA use increases the likelihood of developing the disease by a factor of 2 or 3 among patients at risk.10 Risk factors include age over 65, cognitive decline, severe illness, hip fracture in the acute phase, poor nutrition, dehydration, alcohol or drug abuse, visual or hearing impairment, sleep deprivation, and low level of activity.53 Precipitating factors include intense pain, infections, alcohol or drug withdrawal, stress, various medical procedures and electrolyte disturbances. Furthermore, Solomon et al.54 noticed that elderly people on OA treatment had an increased risk of cardiovascular accidents, fractures, and mortality compared to elderly NSAID users.
Therefore, OAs must be avoided when alternative drugs offer reasonably effective pain treatment, especially in elderly patients and those with decline in cognitive function. Considering that pain itself can trigger delirium, opioids may be used when available alternatives are ineffective. In such cases, we should be mindful that low to moderate doses (equivalent to less than 40mg/day of morphine) have a more beneficial impact on quality of life than high doses.55 In fact, many elderly people require down-adjustment of dosage due to comorbidities and drug-drug interactions.48 Doctors must examine patients frequently to identify any adverse effects as soon as possible.
In the group of 500 patients analysed in the present study, 30 patients were treated with opioids (6%), and most were women (Table 4). It is believed that drug therapy was partially responsible for cognitive symptoms in 23 patients, all of whom were polymedicated (12.7% of the cases included iatrogenic effects) (Tables 4–6).
Should cholinesterase-inhibitor treatment be tested in patients with mild cognitive impairment associated with Alzheimer disease or with non-Alzheimer dementia?Patients who meet clinical criteria for Alzheimer disease (AD) must start treatment with cholinesterase inhibitors (ChEI) unless specific circumstances contraindicate these drugs. Memantine is prescribed in intermediate stages of dementia. Clinical diagnosis of typical AD focuses on verifying that the patient has hippocampal amnesia (difficulty in registering new information). Complementary tests will show a pathophysiological marker of AD (decreased amyloid-β42 levels and increased phosphorylated tau levels in cerebrospinal fluid, temporo-parietal hypoactivity in functional neuroimaging scans, or hippocampal atrophy in structural neuroimaging scans). At present, and perhaps as long as there are no available drugs which modify the clinical course of the disease, current healthcare practice usually focuses on testing the reported episodic memory alterations and using magnetic resonance imaging (MRI) techniques to reveal hippocampal thinning and confirm absence of other cerebral lesions which may justify all the symptoms.
AD is not the only cause of hippocampal amnesia. The hippocampus and its limbic circuits can be altered, therefore causing related amnesia symptoms in patients affected by trauma or surgery, or patients with anoxia, ischaemia, herpesviral encephalitis, tumours, hypothyroidism, and autoimmune diseases (for example, neurological lupus or autoimmune limbic encephalitis).56–59 Therefore, AD diagnosis cannot be based solely on neuropsychological examination. On the other hand, hippocampal atrophy shown by MRI does not necessarily point to underlying AD. Alterations may be caused by hippocampal sclerosis of another aetiology (vascular, anoxic, or due to idiopathic or non-specific tauopathies),60 old age,61 Cushing syndrome and other diseases associated with increased glucocorticoid activity (recurrent major depression, some cases of prolonged post-traumatic stress disorder),62,63 chronic bilateral vestibular loss,64 prolonged treatment with valproic acid,65 prior history of perinatal hypoxia-ischaemia,66 or they may be due to unknown causes.67 Subcortical ischaemic vascular dementia may also result in hippocampal atrophy.68
Trials of ChEIs in patients with mild cognitive impairment have shown that this treatment does not delay onset of dementia and is associated with more adverse effects than placebo.69 For example, ChEIs are associated with a four-fold increase in the risk of syncope.70 In patients with pure vascular dementia, donepezil and galantamine improve cognition, but not all examined aspects of functional status.71,72 Rivastigmine also lacks efficacy, in addition to being associated with an increase in vascular adverse effects and mortality.73 In 2011, the British Association for Psychopharmacology advised against ChEIs for treating frontotemporal dementia.74 These drugs have only been officially indicated or mentioned in guidelines for the treatment of AD, dementia associated with Parkinson's disease, and Lewy body dementia. Further investigations are needed to prescribe ChEIs for other diseases such as Down syndrome, progressive supranuclear palsy, Huntington disease, multiple sclerosis, epilepsy, acute confusional syndrome, traumatic brain injury, sleep disorders, schizophrenia and bipolar disorder.75 Before prescribing drugs off-label, doctors must consider the patient's risk of adverse effects. Cholinergic stimulation of the cerebral cortex, striate body, and brainstem, and increased peripheral cholinergic activity may cause a number of symptoms, whether cognitive-behavioural (confusion, agitation, anxiety), extrapyramidal (tremor), sleep-related (drowsiness, insomnia, nightmares), cardiorespiratory (bradycardia, syncope, exacerbation of symptoms associated with asthma or chronic obstructive pulmonary disease), and digestive (nausea, vomiting, diarrhoea, abdominal pain, anorexia, weight loss). It may also cause weakness, muscle cramps, or urinary incontinence.70,76,77 Elderly people and those under treatment with conventional or atypical antipsychotics are more likely to develop severe adverse effects.78
In many pathological states of the nervous system, excitotoxicity promotes functional and structural damage. This is why experiments have shown that memantine has neuroprotective effects in patients with cerebral ischaemia, intraparenchymal cerebral haemorrhage, HIV-associated dementia, head trauma, carbamate insecticide poisoning, glaucoma, motor neuron diseases, Huntington disease, Parkinson's disease, and AD. At present, AD is the only disease with sufficient clinical and therapeutic evidence to support use of this treatment. In patients with mild AD, trials of memantine showed no significantly beneficial effects.79 In patients with Parkinson's disease and dementia, or Lewy body dementia, overall clinical impression improved substantially whereas cognition and functional performance did not.80,81 In contrast, memantine showed a positive impact on cognition in patients with mild to moderate vascular dementia, although this effect did not significantly improve the overall clinical impression or functional performance.82,83 A pilot study in patients with Huntington disease showed that memantine was associated with less pronounced choreic movements, but not with improved cognition, behaviour, or functional performance.84 In a phase II trial, memantine did not improve neuropsychological performance in patients with HIV-associated cognitive decline.85 In another trial including patients with amyotrophic lateral sclerosis, memantine treatment showed no efficacy.86 A glutamatergic dysfunction is present in schizophrenia and in mood, anxiety, and obsessive-compulsive disorders. This being the case, treatment with memantine has been studied (and sometimes prescribed off-label) in patients with depression, bipolar disorder, schizophrenia, pervasive developmental disorder, obsessive-compulsive disorder, bulimia, and substance addictions. Some findings show treatment to be potentially beneficial, for example, in alcoholic patients or patients with catatonic schizophrenia. However, the quality of evidence is insufficient to draw conclusions since there are few large studies and results are frequently contradictory.87 Before prescribing a drug off-label, we must bear in mind that while memantine is generally well-tolerated, it can cause such adverse effects as headache and constipation, and it may slightly increase the risk of seizures.76
MiscellaneousIt seems logical that ACs acting mainly on the brain, such as antiparkinsonian drugs (orphenadrine, biperiden, trihexyphenidyl, benzatropine, procyclidine, tetrabenazine), are harmful to cognition in patients with diseases that cause cholinergic deficiencies (AD, Lewy body dementia, some cases of vascular dementia, etc.). ACs may also alter cognition or behaviour in elderly people because this group presents cholinergic deficiencies resulting from physiological decreases in cellularity in cholinergic nuclei and in the density of muscarinic receptors. A more obscure fact is that certain ACs with a more selective peripheral action can penetrate the blood-brain barrier and cause this kind of effect. Even more surprisingly, this may occur when drugs are administered topically or by inhalation.88 Such is the case for drugs to treat overactive bladder, which exhibit certain differences. They range from oxybutynin, which penetrates the blood-brain barrier more easily and has a lower tolerability than the rest, to trospium chloride, which barely enters the central nervous system.89 AC eye drops,90 sublingual atropine to reduce sialorrhoea in terminal patients and those with Parkinson's disease,91,92 and scopolamine to reduce terminal bronchial secretions or to prevent motion sickness92,93 are all unusual treatments with potential central or peripheral AC effects. There are case reports of delirium triggered by inhaled AC bronchodilators, but such cases are very rare since absorption into the bloodstream is minimal. AC action has been identified in drugs pertaining to other pharmacological groups (OAs, tricyclic antidepressants, antihistamines, antipsychotics, BZDs, some antibiotics and immunosuppressants, captopril, cyproheptadine, chlortalidone, codeine, corticosteroids, furosemide, digoxin, diltiazem, isosorbide dinitrate, dipyridamole, disopyramide, hydrochlorothiazide, metoclopramide, nifedipine, paroxetine, quinidine, ranitidine, theophylline, triamterene, warfarin). These drugs can exert an adjuvant AC effect when used simultaneously or with more powerful ACs.89,94
It should be mentioned that treatment with steroids (even budesonide) can occasionally, and for short periods of time, cause dementia (steroid dementia) and/or psychotic symptoms (steroid psychosis).95,96
At times, the cognitive or behavioural adverse effects are not caused by improper drug selection, but rather by excessively large doses (a common occurrence with elderly patients) or prolonged use (which results in higher risk in the case of sedative and/or addictive drugs).
According to the WHO, polypharmacy is the use of more than 3 drugs per patient at one time. This is necessary on some occasions in patients with multiple diseases. In other cases, the patient's list of drugs contains a drug that is not indicated, not effective, whose action overlaps or is incompatible with that of another drug, or that is contraindicated in that patient. In polypharmacy patients, interactions are sometimes unpredictable due to the great variety of combined chemical formulas. Interactions are more frequent in elderly people, who do not usually metabolise and eliminate drugs in an optimal way. More specifically, polypharmacy promotes development of confusional syndrome; at times, the drug provoking the disorder may directly or indirectly prolong a confusional state.97
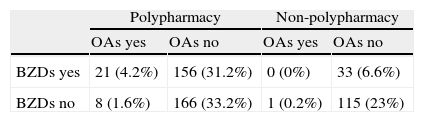
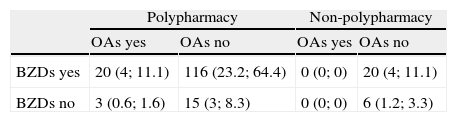
The 500 patients examined here were taking between 0 and 19 drugs (mean 5.5). Polypharmacy patients accounted for 70.4% of the total. Polypharmacy was more frequent in patients 65 or older with no diagnosis of degenerative disease (Table 4). Both BZDs and OAs were more frequently associated with polypharmacy (P<.001 in both cases; Table 5). However, in the case of OAs, only the frequency of polypharmacy was significantly associated with drug-related cognitive symptoms (P<.05) (Table 6).
Distribution of types of treatment observed in the 180 patients whose medication was considered to have contributed to their cognitive symptoms
| Polypharmacy | Non-polypharmacy | |||
| OAs yes | OAs no | OAs yes | OAs no | |
| BZDs yes | 20 (4; 11.1) | 116 (23.2; 64.4) | 0 (0; 0) | 20 (4; 11.1) |
| BZDs no | 3 (0.6; 1.6) | 15 (3; 8.3) | 0 (0; 0) | 6 (1.2; 3.3) |
X (y; z): X=number of patients; y=percentage of the total of 500 patients; z=percentage out of the 180 patients whose diagnosis included an iatrogenic effect.
In daily practice we must be aware that treatments, dosage and duration must be carefully determined for elderly patients who present cognitive impairment, cerebrovascular disease, severe diseases, or diseases affecting systemic metabolism or the integrity of the blood-brain barrier. Polypharmacy should also be avoided when certain drugs are not necessary or suitable. BZDs and other sedative drugs, OAs, and any drug with AC effects are especially likely to cause acute or chronic cognitive-behavioural disturbances in vulnerable patients. Additionally, systolic blood pressure should not be allowed to drop below 130mmHg in patients with atheromatosis and a loss of elasticity in the arteries leading to the brain, and cholesterol levels should not be reduced excessively (total cholesterol<160mg/dL, HDL-C<40mg/dL).
In the sample of 500 patients with altered cognitive functions, large numbers of patients were treated with BZDs (42%, mostly women) or on a polypharmacy regimen (70.4%, more frequent in patients 65 and older). Polypharmacy patients taking BZDs accounted for 74.3% of the cases in which an iatrogenic factor was suspected in the aetiology of cognitive symptoms.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Robles Bayón A, Gude Sampedro F. Prescripciones inconvenientes en el tratamiento del paciente con deterioro cognitivo. Neurología. 2014;29:523–532.