Medication overuse headache is a secondary headache in which the regular or frequent use of analgesics can increase the frequency of the episodes, causing the transition from episodic to chronic headache. The prevalence of medication overuse headache is approximately 1%-2%, with higher rates among women aged 30-50 years and with comorbid psychiatric disorders such as depression or anxiety, or other chronic pain disorders. It is important to be familiar with the management of this disease. To this end, the Spanish Society of Neurology’s Headache Study Group has prepared a consensus document addressing this disorder.
DevelopmentThese guidelines were prepared by a group of neurologists specialising in headache after a systematic literature review and provides consensus recommendations on the proper management and treatment of medication overuse headache. The treatment of medication overuse headache is often complex, and is based on 4 fundamental pillars: education and information about the condition, preventive treatment, discontinuation of the drug being overused, and treatment for withdrawal symptoms. Follow-up of patients at risk of recurrence is important.
ConclusionsWe hope that this document will be useful in daily clinical practice and that it will update and improve understanding of medication overuse headache management.
La cefalea con uso excesivo de medicación es una cefalea secundaria en la que el uso regular o frecuente de medicación analgésica produce un aumento de la frecuencia de una cefalea de base, pasando de episódica a crónica. La prevalencia de esta entidad está en torno al 1-2%, siendo más frecuente en mujeres entre 30 y 50 años con comorbilidades psiquiátricas como depresión o ansiedad y otros procesos de dolor crónico. Es importante conocer el manejo de esta entidad. Por este motivo, el Grupo de Estudios de Cefaleas de la Sociedad Española de Neurología ha pretendido realizar este documento de consenso sobre esta patología.
DesarrolloEsta guía ha sido redactada por un grupo de expertos a partir de la revisión de la evidencia científica publicada y estableciendo recomendaciones prácticas para su adecuado manejo y tratamiento. El tratamiento de la cefalea con uso excesivo de medicación tiene varios pilares fundamentales y suele ser complejo: información y educación sobre el desarrollo de la cefalea con uso excesivo de medicación, tratamiento preventivo, suspensión del fármaco de uso frecuente y tratamiento de deshabituación. Es importante el seguimiento de pacientes con riesgo de recurrencias.
ConclusionesEsperamos que este documento resulte de utilidad y permita su aplicación práctica en la consulta diaria y que sirva para actualizar y mejorar el conocimiento del manejo de esta patología.
The third edition of the International Classification of Headache Disorders (ICHD-3)1 defines medication-overuse headache (MOH) as headache occurring on 15 or more days per month in a patient with a pre-existing primary headache and developing as a consequence of regular overuse of acute or symptomatic headache medication for more than 3 months. According to the ICHD-3, MOH usually, but not invariably, resolves with the withdrawal of the overused substance. The current classification enables the diagnosis of both MOH and a pre-existing headache (typically migraine or tension-type headache) in the same patient. At present, there is no consensus on the most appropriate strategy for managing MOH.
The Spanish Society of Neurology’s Headache Study Group has drafted a consensus document on MOH based on the results of a literature review and our own clinical experience. We aimed to provide updated information on this entity and to establish a series of practical recommendations for its treatment.
Epidemiology and risk factorsA systematic review of population-based studies conducted after the publication of the second edition of the International Classification of Headache Disorders2 estimated the prevalence of MOH at 0.5% to 7.2%.3 The disparity between prevalence rates may be attributed to methodological or geographical differences. Among the studies reviewed, the 2 including the largest populations were conducted in Sweden,4 in a population of 50 000 individuals and finding an estimated prevalence rate of 1.8% (2.5% in women), and in Norway,5 in 30 000 individuals and reporting a prevalence rate of 1.7% (2.2% in women). A Spanish study included a population of nearly 10 000 individuals from the region of Cantabria, and reported a prevalence rate of 1.4% (2.6% in women).6 From these studies, we may conclude that MOH mainly affects individuals aged 30 to 50 years3 and is more frequent among women (female-to-male ratio, 4:1).7
Although the prevalence of MOH is not very high in the general population, the condition represents a significant problem for specialised care. In fact, MOH has been estimated to affect 30%-50% of patients attending headache units.8
The risk of MOH largely depends on the type of drug being overused. Thus, for MOH to appear, non-opioid analgesics and anti-inflammatory drugs must be used on at least 15 days per month, whereas triptans, ergotamine, opioids, and combination analgesic treatment must be used on a minimum of 10 days per month.1 A systematic review including 29 studies found that overuse of opioids, barbiturates, or combination analgesics is more likely to cause MOH than triptan or ergotamine overuse.8,9
The risk of MOH is also influenced by the type of the pre-existing headache. In most cases, the pre-existing headache is migraine6; MOH is less frequent in patients with tension-type headache or cluster headache.8
The involvement of psychological factors has also been analysed. Certain behavioural patterns may predispose individuals to medication overuse, increasing their likelihood of developing MOH. To detect these behavioural profiles, a dependence scale may be administered to determine whether the patient feels that he or she is in control of their treatment, and whether medication use is associated with anxiety or worry.10 Other studies have evaluated whether specific traits or personality disorders may predispose to MOH. A recent study showed that patients with MOH are more likely to present obsessive and dysphoric traits.11
Other factors associated with MOH include lower socioeconomic status and level of education4 and presence of multiple comorbidities.12
A prospective longitudinal study evaluating a population of over 25 000 individuals with episodic headache over 11 years identified the following risk factors for MOH: use of tranquillisers, combination of musculoskeletal or gastrointestinal symptoms, anxiety, depression, sedentary lifestyle, smoking, and migraine rather than non-migraine headache. Age below 50 years, female sex, and low education level were identified as non-modifiable risk factors.13 In another prospective study including 131 patients with migraine from a single centre, headache intensity and frequency and the associated disability were found to be predictors of MOH. However, no association was observed between personality traits or level of physical activity and incidence of MOH.14
Genetics of medication-overuse headacheMOH is a complex entity. Although epidemiological data suggest a genetic predisposition, the currently available scientific evidence and statistical data are inconclusive. Few studies have been performed, with those published including small samples from selected countries (mainly Italy and Japan); where associations have been found between MOH and a given polymorphism, the results have not been replicated. The following epidemiological data support the involvement of genetic factors in the pathogenesis of MOH:
- •
Patients with no predisposition to primary headache and no family history of migraine have nearly no risk of developing MOH in the context of analgesic overuse due to other conditions.15,16
- •
The risk of developing MOH is greater in patients with family history of MOH or substance abuse.17 In fact, MOH and substance dependence have common pathophysiological mechanisms. Both entities have been associated with alterations to the frontostriatal circuit, which may cause impulsive, compulsive, and addictive behaviours, and in the reward circuitry.18
- •
Approximately 40% of patients with MOH who respond to withdrawal therapy relapse within a year of withdrawal.19
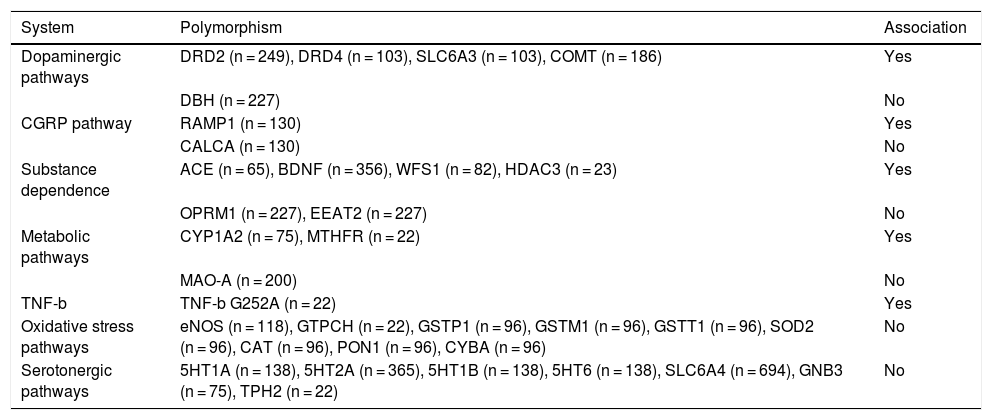
To date, 17 human studies (12 case-control studies and 5 case series) have been published, analysing 50 polymorphisms in 33 candidate genes for MOH. All of these were conducted in Italian or Japanese populations. Not all studies specify the type of primary headache affecting the participating patients; furthermore, all studies included triptan overuse among the inclusion criteria, but they did not consider overuse of such other drugs as analgesics, non-steroidal anti-inflammatory drugs (NSAID), or opioids.20
Ten of the 17 studies reported an association between MOH and some of the polymorphisms analysed. A total of 12 polymorphisms have been associated with MOH (Table 1); these are located in genes involved in the dopaminergic system or associated with substance dependence. All 10 studies include very small samples. Of the 17 studies analysing the association between polymorphisms and MOH, only 7 included more than 100 patients. Furthermore, the exact number of patients tested is difficult to determine, since some authors used the same samples for different studies. According to our calculations, these studies have analysed approximately 1000 patients with MOH, 300 with episodic migraine, 50 with chronic migraine, and 600 healthy controls. Furthermore, the results from these studies have not been replicated.
Polymorphisms associated with medication-overuse headache.
| System | Polymorphism | Association |
|---|---|---|
| Dopaminergic pathways | DRD2 (n = 249), DRD4 (n = 103), SLC6A3 (n = 103), COMT (n = 186) | Yes |
| DBH (n = 227) | No | |
| CGRP pathway | RAMP1 (n = 130) | Yes |
| CALCA (n = 130) | No | |
| Substance dependence | ACE (n = 65), BDNF (n = 356), WFS1 (n = 82), HDAC3 (n = 23) | Yes |
| OPRM1 (n = 227), EEAT2 (n = 227) | No | |
| Metabolic pathways | CYP1A2 (n = 75), MTHFR (n = 22) | Yes |
| MAO-A (n = 200) | No | |
| TNF-b | TNF-b G252A (n = 22) | Yes |
| Oxidative stress pathways | eNOS (n = 118), GTPCH (n = 22), GSTP1 (n = 96), GSTM1 (n = 96), GSTT1 (n = 96), SOD2 (n = 96), CAT (n = 96), PON1 (n = 96), CYBA (n = 96) | No |
| Serotonergic pathways | 5HT1A (n = 138), 5HT2A (n = 365), 5HT1B (n = 138), 5HT6 (n = 138), SLC6A4 (n = 694), GNB3 (n = 75), TPH2 (n = 22) | No |
ACE: angiotensin-converting enzyme; BDNF: brain-derived neurotrophic factor; CALCA: calcitonin-related polypeptide alpha; CAT: catalase; CGRP: calcitonin gene–related peptide; COMT: catechol-O-methyltransferase; CYBA: cytochrome B-245 alpha chain; CYP1A2: cytochrome P450 1A2; DBH: dopamine beta-hydroxylase; DRD2: dopamine D2 receptor; DRD4: dopamine D4 receptor; EEAT2: excitatory amino acid transporter 2; eNOS: endothelial nitric oxide synthase; GNB3: G protein subunit beta 3; GSTM1: glutathione S-transferase mu 1;GSTP1: glutathione S-transferase pi 1; GSTT1: glutathione S-transferase theta 1; GTPCH: guanosine triphosphate cyclohydrolase; HDAC3: histone deacetylase 3; 5HT1A: 5-hydroxytryptamine 1A; 5HT2A: 5-hydroxytryptamine 2A; 5HT1B: 5-hydroxytryptamine 1B; 5HT6: 5-hydroxytryptamine 6; MAO-A: monoamine oxidase A; MTHFR: methylenetetrahydrofolate reductase; OPRM1: opioid receptor mu 1; PON1: paraoxonase 1; RAMP1: receptor activity modifying protein 1; SLC6A3: solute carrier family 6 member 3; SLC6A4: solute carrier family 6 member 4; SOD2: superoxide dismutase 2; TPH2: tryptophan hydroxylase 2; WFS1: Wolfram syndrome 1.
Only one pharmacogenomic study has evaluated response to withdrawal therapy, in a sample of 33 patients with chronic migraine (19 with and 14 without MOH).21 Around 57% of patients presented a unique genomic expression pattern associated with treatment response, although different tissues and multiple metabolic pathways were involved. Associations were found with genes involved in soluble NSF attachment protein receptor (SNARE) interactions in vesicular transport, apoptosis, neurodegenerative disease, adipocyte function, lymphocytic cell signalling, and nicotinoid and insulin metabolism. The authors postulate that this diversity reflects the great complexity of MOH.
No family linkage studies, genome-wide association studies, or proteomics studies of MOH have been conducted to date.
In the light of the above, we may conclude that none of the polymorphisms analysed has shown a clinically relevant association with MOH. Multicentre studies with large samples are needed, and new genetic, epigenetic, and pharmacogenomic techniques should be applied to research into MOH.
Pathophysiology of medication-overuse headacheAs is the case with many types of headache, the pathophysiology of MOH is poorly understood. By definition, intake of analgesics plays a crucial role, although the available data suggest that some individuals with pre-existing primary headache are genetically predisposed to addictive behaviours, resulting in frequent, continuous analgesic drug use. This ultimately increases the excitability of the cerebral cortex and the trigeminovascular system, favouring central and peripheral sensitisation, as occurs in chronic migraine.22
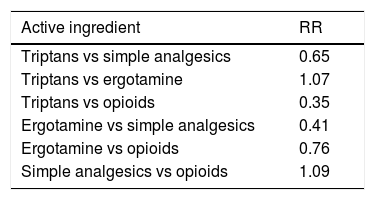
AnalgesicsClinical experience suggests that any analgesic drug may lead to MOH. However, some types of drug require lower doses and shorter exposure times. A systematic literature review including 29 studies used prevalence data to estimate the relative risk of MOH associated with different types of analgesics.9Table 2 shows weighted averages for the adjusted relative risk of MOH for different types of analgesics. The results suggest that use of opioids and combination analgesics is associated with a greater risk of MOH than triptan and ergotamine use, probably due to differences in the mechanisms involved. We should bear in mind the accessibility of simple analgesics, as compared to prescription-only analgesics.
Weighted average of the adjusted relative risk of medication-overuse headache for different analgesics.4
| Active ingredient | RR |
|---|---|
| Triptans vs simple analgesics | 0.65 |
| Triptans vs ergotamine | 1.07 |
| Triptans vs opioids | 0.35 |
| Ergotamine vs simple analgesics | 0.41 |
| Ergotamine vs opioids | 0.76 |
| Simple analgesics vs opioids | 1.09 |
RR: relative risk.
MOH and substance use disorder present several common features. Some patients with MOH develop tolerance to analgesics and present characteristics of substance use disorder, such as withdrawal symptoms, loss of control, use of the drug at higher doses or for longer than recommended, inability to reduce consumption, and high relapse rates.
A study conducted in Norway used the Severity of Dependence Scale to analyse the degree of analgesic dependence associated with MOH.23 The study included 25 patients with MOH, 15 patients with chronic headache but not medication overuse, and 25 controls. Diagnosis was based on the criteria established in the ICHD-3 and the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). A total of 62% of patients with MOH presented overuse of simple analgesics, with 38% overusing central analgesics (codeine, opioids, triptans). Around 50% of patients with MOH were diagnosed with substance use disorder according to the DSM-IV. Frequent intake of central analgesics and high scores on the Severity of Dependence Scale were associated with higher levels of dependence, whereas lower scores were associated with successful detoxification.
Central sensitisationCentral sensitisation is one of the possible mechanisms involved in medication-induced pain chronification. Experimental studies with rodents have shown the ability of analgesics to induce pain system hyperexcitability. Exposure of laboratory rats to simple analgesics and triptans for 7 days resulted in persistent changes in dural afferent fibres, including increased expression of calcitonin gene–related peptide (CGRP) and nitric oxide synthase.24 Furthermore, in rats with triptan-induced latent sensitisation, stress or exposure to nitric oxide donors promotes the appearance of allodynia, increased Fos expression in the trigeminal nucleus caudalis, and increased plasma CGRP concentration, and lowers the sensory threshold for cortical spreading depression.25
Electrophysiological studies of patients with MOH have shown neural hyperexcitability associated with increased pain response and habituation deficits for different modalities (cortical, somatosensory, and CO2 laser evoked potentials). Pain system hyperexcitability is reversible with analgesic withdrawal, although detoxification may take up to a year, hence the importance of preventing relapses in these patients.26
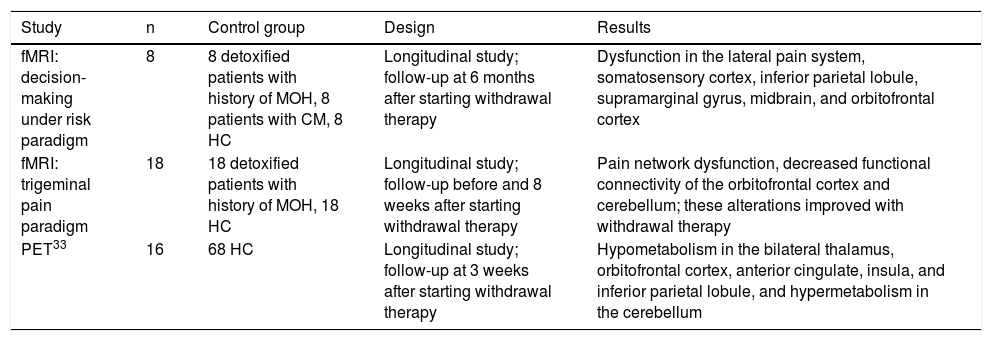
Neuroimaging findings in medication-overuse headacheNeuroimaging studies have shown structural and functional changes in the brains of patients with MOH.22 These changes mainly affect the pain system, particularly regions involved in pain discrimination and cognitive, attentional, and emotional processing (Table 3). Although MOH is diagnosed clinically, neuroimaging studies may shed light on key aspects of its pathophysiology. However, few studies have addressed this subject, with very few including a control group; some studies have reported contradictory findings.27
Studies using functional magnetic resonance imaging of patients with medication-overuse headache.
| Study | n | Control group | Design | Results |
|---|---|---|---|---|
| fMRI: decision-making under risk paradigm | 8 | 8 detoxified patients with history of MOH, 8 patients with CM, 8 HC | Longitudinal study; follow-up at 6 months after starting withdrawal therapy | Dysfunction in the lateral pain system, somatosensory cortex, inferior parietal lobule, supramarginal gyrus, midbrain, and orbitofrontal cortex |
| fMRI: trigeminal pain paradigm | 18 | 18 detoxified patients with history of MOH, 18 HC | Longitudinal study; follow-up before and 8 weeks after starting withdrawal therapy | Pain network dysfunction, decreased functional connectivity of the orbitofrontal cortex and cerebellum; these alterations improved with withdrawal therapy |
| PET33 | 16 | 68 HC | Longitudinal study; follow-up at 3 weeks after starting withdrawal therapy | Hypometabolism in the bilateral thalamus, orbitofrontal cortex, anterior cingulate, insula, and inferior parietal lobule, and hypermetabolism in the cerebellum |
CM: chronic migraine; fMRI: functional magnetic resonance imaging; HC: healthy controls; MOH: medication-overuse headache; PET: positron emission tomography.
From an anatomical viewpoint,28 patients with MOH display reduced grey matter volume (GMV) in pain matrix structures (orbitofrontal cortex, anterior cingulate cortex, insula, precuneus) and increased GMV in the periaqueductal area, thalamus, and ventral striatum. In a second study conducted a year later, the researchers found a correlation between decreased GMV in the orbitofrontal cortex at baseline and poorer response to withdrawal therapy.29 Subsequent studies27 have failed to replicate these results. No conclusive data are available on whether patients with MOH present alterations in white matter volume.28
Data from studies using resting-state fMRI have revealed functional alterations in networks involved in pain modulation and cognitive/behavioural responses to pain (mainly the orbitofrontal region).30 Based on the hypothesis that MOH may be an addiction disorder and that the mesocorticolimbic dopaminergic system may play a role in its pathogenesis,31 some authors have focused on the study of both accumbens nuclei (ventral striatum) and the rostral and dorsocaudal putamen (dorsal striatum). Their results have enabled discrimination between patients with and without medication overuse. Alterations in reward system connectivity and different patterns of GMV in the striatum seem to play a prominent role in the diagnosis of this entity. The results of a study using fMRI during the execution of a decision-making under risk task also support this view.32
Finally, a study with positron emission tomography, which included patients with chronic migraine and MOH and a matched control group detected hypometabolism in the thalamus, orbitofrontal cortex, anterior cingulate cortex, insula, striatum, and right inferior parietal lobule, and hypermetabolism in the cerebellar vermis. Orbitofrontal hypometabolism persisted 3 weeks after medication withdrawal.33
In conclusion, the currently available neuroimaging data suggest that patients with MOH present functional and structural alterations in areas involved in pain modulation. These changes have been correlated (albeit not consistently) with such clinical parameters as duration of medication overuse, headache frequency and intensity, and, in the case of the orbitofrontal cortex, response to withdrawal therapy.30 These changes, and particularly changes in GMV, are frequently reversible and must therefore be considered an effect of the disease rather than a pathogenic mechanism. Lastly, the prominent role of the mesocorticolimbic dopaminergic areas (striatum, ventral tegmental area, orbitofrontal cortex) is compatible with the hypothesis of an addictive behaviour in patients with MOH.
Symptoms of medication-overuse headacheDiagnostic criteria. Classification by type of medicationMOH presents in patients with history of primary headache; in the context of medication overuse, these patients may develop another type of headache or present worsening of the existing headache. MOH is a secondary headache from group 8 of the ICHD-3 (headache attributed to a substance or its withdrawal). Table 4 lists the ICHD-3 diagnostic criteria for MOH.
Diagnostic criteria for medication-overuse headache.
| Medication-overuse headache |
| Headache occurring on ≥ 15 days/month in a patient with a pre-existing headache disorder |
| Regular overuse for > 3 months of one or more drugs that can be taken for acute and/or symptomatic treatment of headache |
| Not better accounted for by another ICHD-3 diagnosis |
| Classification of headache according to the overused drug |
| Ergotamine-overuse headache: headache occurring on ≥ 15 days/month as a consequence of regular use of ergotamine on ≥ 10 days/month for > 3 months |
| Triptan-overuse headache: headache occurring on ≥ 15 days/month as a consequence of regular use of triptans on ≥ 10 days/month for > 3 months |
| Non-opioid analgesic-overuse headache: headache occurring on ≥ 15 days/month as a consequence of regular use of paracetamol, NSAIDs, acetylsalicylic acid, or other non-opioid analgesics on ≥ 15 days/month for > 3 months |
| Opioid-overuse headache: headache occurring on ≥ 15 days/month as a consequence of regular use of opioids on ≥ 10 days/moth for > 3 months |
| Combination analgesic–overuse headache: headache occurring on ≥ 15 days/month as a consequence of regular use of combination analgesics on ≥ 10 days/moth for > 3 months. The term “combination analgesics” refers to formulations combining analgesic drugs from different classes (for example, paracetamol and codeine) or analgesics with drugs acting as adjuvants (for example, caffeine). |
| Medication-overuse headache attributed to multiple drug classes not individually overused: headache occurring on ≥ 15 days/month as a consequence of regular intake of any combination of ergotamine, triptans, non-opioid analgesics, or opioids on a total of ≥ 10 days/moth for > 3 months, without overuse of any single drug or drug class alone |
| Medication-overuse headache attributed to unspecified or unverified overuse of multiple drug classes: headache occurring on ≥ 15 days/month as a consequence of regular intake of any combination of ergotamine, triptans, non-opioid analgesics, and/or opioids on ≥ 10 days/month for > 3 months (the identity, quantity and/or pattern of use or overuse of these classes of drug cannot be reliably established) |
| Medication-overuse headache attributed to other medication: headache occurring on ≥ 15 days/month as a consequence of regular intake of one or more medications other than those described above on 10 days/month for > 3 months |
ICHD-3: International Classification of Headache Disorders, 3rd edition; NSAID: non-steroidal anti-inflammatory drug.
The specific symptoms and the minimum dose and number of days needed for MOH to appear depend on the type of medication. Patients may present MOH after 1.7 years taking triptans, 2.7 years for ergotamine, and 4.8 years for analgesics. The minimum dose necessary to develop MOH is lower for triptans (18 single doses/month) than for ergotamine (37 single doses/month) or analgesics (114 single doses/month).7,34
Clinical characteristics of medication-overuse headacheDiagnosis of MOH is based on clinical history and physical examination results. Headache must be drug-resistant and present daily or almost daily; the type, intensity, and location of pain may vary over time. The characteristics of MOH depend on the pre-existing primary headache. Any physical or mental effort may trigger an attack, since the pain threshold is usually low. Patients may present withdrawal symptoms if analgesics are discontinued abruptly. Symptoms improve several days after drug withdrawal. Diagnosis is more complex when migraine and MOH co-present.34
MOH may be associated with asthenia, gastrointestinal symptoms, irritability, anxiety, restless limbs, depression, memory alterations, and difficulty concentrating. Several comorbidities have been described, including depression, arterial hypertension, and obesity, and to a lesser extent allergies (16%), digestive disorders (6%), and musculoskeletal disorders.12
Patients with migraine and overuse of triptans usually present daily attacks of unilateral, pulsating migraine-like headache, and autonomic symptoms. However, some patients do not present these symptoms, further hindering diagnosis.34 Ergotamine overuse has distinct characteristics, including bradycardia or tachycardia, paraesthesia, irritable bowel syndrome, arterial hypertension, dizziness, and limb weakness.35
Potential complications of medication overuseMedication overuse may cause a wide range of complications. An in-depth analysis of all possible adverse effects of rescue medication is beyond the scope of this article. However, some of these complications are worth mentioning, with ergot derivatives being the drugs most frequently associated with severe complications.
Long-term ergotamine use has been associated with sensory neuropathy, cognitive slowing,36 and structural and functional changes in cerebral blood vessels.37 Other complications include intermittent claudication, acrocyanosis, rectal ulcers, persistent nausea, rebound headache, myocardial ischaemia, and fibrotic disorders (peritoneal, pleural, or myocardial).
Kidney disease has only been clearly associated with phenacetin-containing analgesics, although acute use or combination with other drugs may increase their nephrotoxic effects under certain circumstances.38 Long-term NSAID use has also been associated with chronic gastritis and malabsorption of such nutrients as vitamin B12, causing complications associated with B12 deficiency. We should also mention the complications associated with withdrawal syndrome, which will be discussed in a later section.
Lastly, long-term triptan use has been associated with ischaemic colitis.39
Special considerations for children and adolescentsThe prevalence of medication overuse in children and adolescents ranges from 0.3% to 0.5%,40 with combination analgesics (including caffeine in many cases) being the most frequently used drugs.41 Prevalence seems to be greater among female patients, with a female-to-male ratio of approximately 4:1.
Children also benefit from withdrawal therapy, although few data are available on the most beneficial approach in this population. According to some studies, withdrawal therapy achieves a 90% decrease in the number of headache days per month in at least 53% of patients in this age range, regardless of whether preventive treatment is started.42 Duration of medication overuse of over 2 years has been identified as a predictor of poor prognosis in children and adolescents.
Prevention and treatment of medication-overuse headacheAs mentioned previously, MOH is most common in patients with migraine. Treatment is complex, and requires a comprehensive approach. Patient habits and lifestyle, other comorbidities, current treatments, and psychological and affective factors are all key considerations.
Prevention of medication-overuse headacheHealthcare authorities play a pivotal role in primary prevention; the report issued by the Spanish Agency of Medicines and Medical Devices43 on 21 February 2017 called attention to the increase in the use of opioids. Primary care centres and pharmacies are the parties most likely to identify patients with primary headache at risk of developing MOH, enabling the implementation of primary prevention strategies, such as controlling the use of analgesics, triptans, ergotamine (the latter is available over-the-counter), tranquillisers, and opioids. In Spain, tramadol is indicated for any type of pain, including migraine and tension-type headache; consumption of this drug, in combination with paracetamol, has trebled in 2008-2015.29
Primary care physicians should inform patients about how to use these drugs correctly, warn them about the risks associated with medication overuse, instruct them to keep a headache diary to record the number of headache days and use of acute medication, prescribe the drugs recommended in clinical practice guidelines for migraine and other headache disorders, and refer patients to specialist departments if headache frequency increases.
Campaigns should be developed to warn the population about the risk of MOH if analgesic drugs are overused, as occurs with smoking prevention. A campaign run in Denmark, which reached over 10% of the population, resulted in a significant increase in public awareness of MOH (from 31% to 38%).44
Treatment of medication-overuse headacheManagement of MOH is based on the following pillars:
- •
Patient education about the pathogenic mechanisms of MOH
- •
Pharmacological and non-pharmacological preventive treatment
- •
Detoxification and discontinuation of the overused drugs
- •
Follow-up of patients at risk of recurrence.
These strategies are frequently used in combination in clinical practice. Drug withdrawal is frequently combined with treatment of withdrawal symptoms, which is addressed in a later section.
Patient educationPatients must be informed about the pathogenic mechanisms of MOH and the drugs that cause this type of headache in each case. Physicians should advise patients to reduce the use of acute medication for headache (fewer than 15 days/month for simple analgesics and NSAIDs and fewer than 10 days/month for other drugs), discourage the use of medications before symptoms appear, avoid prescribing high-risk drugs (ergotamine, opioids, fixed combinations of analgesics), and promote the use of headache diaries to monitor headache and medication.
Treatment of MOH is based on the discontinuation of the drug causing the symptoms. Patients must be informed about the risk of rebound headache and understand that medication overuse can be a key factor in headache chronification.45
A study conducted in Italy showed that in patients with comorbidities and low medical needs, counselling alone was as effective as outpatient and inpatient detoxification programmes,46 with a success rate of over 70% at 2 months. However, in a subsequent study by the same research group, which included patients with more complex forms of MOH (recurrent forms, opioid or barbiturate overuse, comorbidities, psychological disorders, previous treatment failure), in-hospital detoxification showed greater efficacy (success rate of 87%, vs 60% in the other 2 groups).47
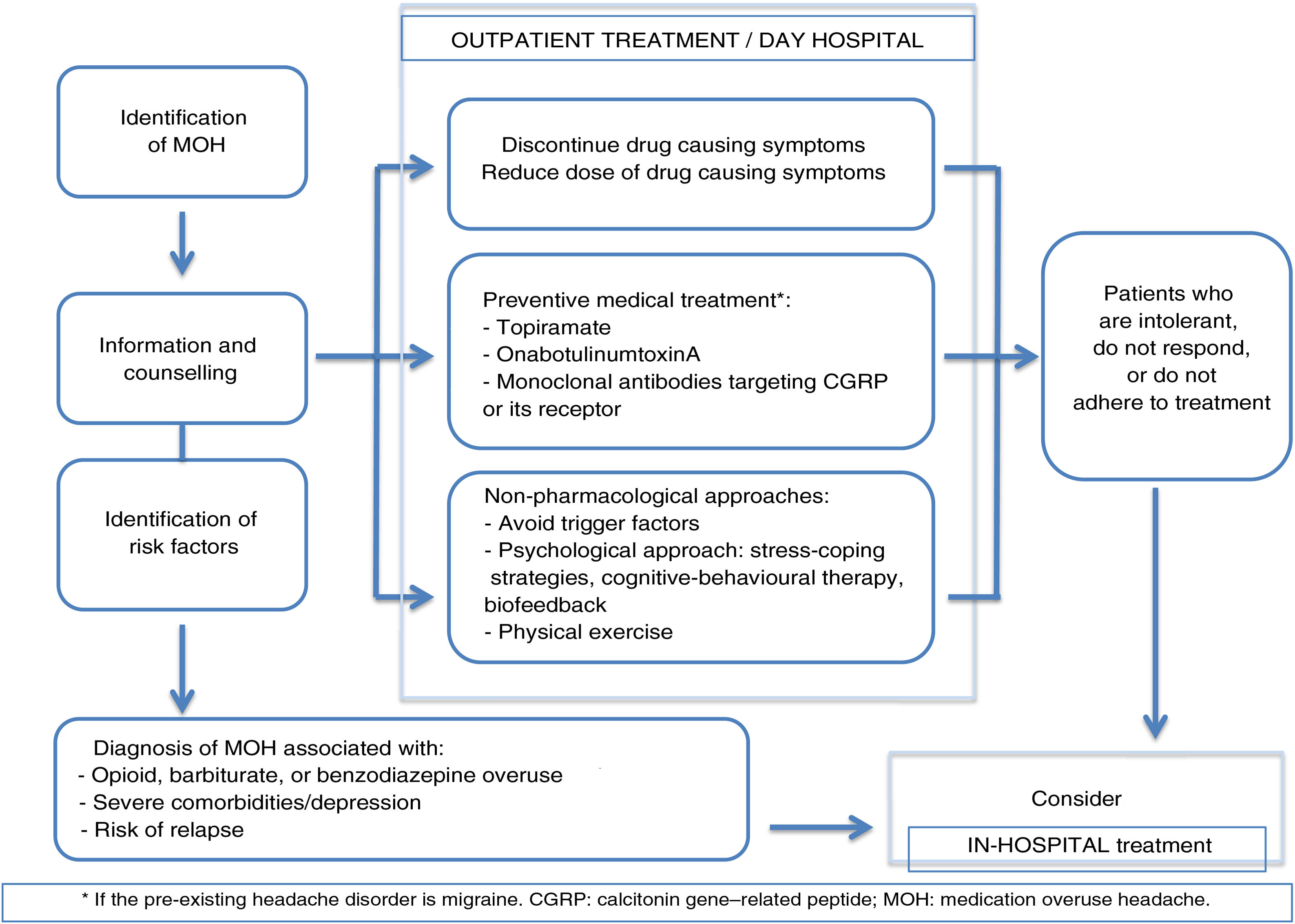
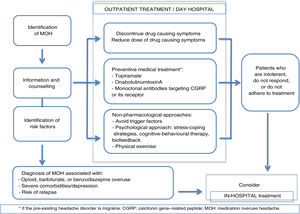
The usefulness of patient education and counselling for preventing MOH has been confirmed in other studies48; this approach should therefore be implemented in daily practice at primary care centres. More complex cases (overuse of several drugs, recurrence, or severe psychiatric comorbidities) should be managed in specialist headache units.35 Hospitalisation should be considered in case of opioid or barbiturate overuse, complex medical or psychiatric comorbidities, or failure of outpatient withdrawal therapy on at least 2 occasions (Fig. 1).
Pharmacological and non-pharmacological preventive treatmentNon-pharmacological treatment is based on a number of approaches, including biofeedback, behavioural therapy, stress coping strategies, and regular physical exercise.22
Several clinical trials support the efficacy of topiramate,49 onabotulinumtoxinA (OnabotA),50–52 and CGRP monoclonal antibodies (erenumab, fremanezumab, and galcanezumab)53–55 for the treatment of chronic migraine associated with medication overuse in patients with no history of withdrawal therapy. However, this evidence is from post hoc analysis. Subsequent studies with topiramate and OnabotA have failed to confirm this effect.56,57 Such other treatments as valproic acid, cannabinoids, pregabalin, greater occipital nerve stimulation, and acupuncture have been evaluated in trials with low statistical power and poor methodological quality; these studies therefore provide poor-quality results.58
In the only randomised clinical trial comparing preventive treatment from study onset in patients without prior withdrawal therapy, withdrawal therapy without initial preventive treatment, or no specific treatment, the group of patients receiving preventive treatment presented fewer headache days and fewer migraine days per month, with 53% of patients showing a significant decrease (> 50%) in the number of headache days per month.59 Furthermore, a systematic review by Chiang et al.58 concluded that combining preventive treatment with early discontinuation of the drug causing MOH was more effective than drug discontinuation alone.
Detoxification and withdrawal of the overused drugsDrug discontinuation may cause withdrawal symptoms, whose duration depends on the type of drug involved (shorter duration for triptans than for ergotamine, NSAIDs, or opioids).60 Withdrawal syndrome rarely lasts longer than 2 weeks. The most common withdrawal symptom is worsening of headache, although patients may also present nausea, vomiting, sleep disorders, and anxiety.61,62
Treatment of withdrawal headacheDifferent treatment strategies have been developed for headache secondary to analgesic drug withdrawal, and focus on discontinuing the drug causing the problem.61,62
Some drugs, including steroids, antipsychotics, sodium valproate, and tricyclic antidepressants have classically been considered to reduce withdrawal symptoms, although this hypothesis is not supported by data from placebo-controlled trials.61,62
SteroidsOpen-label studies have shown that prednisone facilitates analgesic withdrawal as it improves withdrawal symptoms with very few adverse effects.63,64 A small placebo-controlled trial showed the benefits of oral prednisone65; however, subsequent studies failed to replicate these results.66,67
AntipsychoticsUse of such antipsychotics as intravenous prochlorperazine was found to be potentially effective in a retrospective, non-controlled study including 135 patients with chronic daily headache, many of whom also had MOH.68 However, prochlorperazine doses were highly variable and many patients also received other preventive drugs; therefore, the effectiveness of the drug cannot be clearly established.
Sodium valproateIntravenous valproate has been used in small series of patients with MOH, with a loading dose of 15 mg/kg for 30 minutes and a maintenance dose of 5 mg/kg every 8 hours, infused over 15 minutes, for 12-48 hours; the study showed discreet efficacy for the treatment of withdrawal headache.69 However, it may also be administered orally for the preventive treatment of migraine in selected cases.
Other drugsSome researchers have suggested that naproxen dosed at 550 mg70 or sumatriptan,71,72 administered for several days, may improve withdrawal headache; however, this evidence is from case series, rather than placebo-controlled trials.
Treatment of other withdrawal symptomsNSAIDs may improve not only headache but also withdrawal symptoms. A study including a series of 22 patients evaluated the effect of naproxen for the treatment of withdrawal headache following hospitalisation for ergotamine detoxification.70 Ten patients received symptomatic treatment (antiemetics, analgesics, and hydration), whereas the remaining 12 were treated with naproxen (500 mg twice daily). On day 8 after ergotamine withdrawal, patients treated with naproxen presented less pain, nausea, vomiting, and restlessness.
The results of an open-label study suggest that intravenous hydration and combination therapy with dexamethasone, metoclopramide, and benzodiazepines for 7-15 days may help control these symptoms.73 It has also been suggested that amitriptyline74 and intravenous clomipramine75 may be useful, given their sedative and pain modulation effects.
Follow-up of patients at risk of recurrenceMOH is associated with a high risk of recurrence (25%-35%), particularly in patients with depressive symptoms, given that drug withdrawal or dose reduction may reduce the frequency of primary headache but cannot resolve it. Patients must therefore be evaluated periodically. Regular follow-up after treatment reduces the risk of relapse.22
ConclusionsBased on our review of the available evidence and our own clinical experience, we propose the following recommendations for the treatment of MOH and withdrawal symptoms:
- -
Patients taking symptomatic drugs not recommended for the treatment of headache (particularly ergotamine, opioids, and drug combinations) should be closely monitored. NSAIDs rarely cause MOH, and management of triptan overuse is frequently easier.
- -
Discontinuation of the overused drug is essential. However, in the light of the available evidence, it is even more important, particularly in patients with migraine, to implement early preventive treatment with the following drugs: topiramate, OnabotA, or monoclonal antibodies binding to the CGRP ligand or the CGRP receptor.
- -
Such non-pharmacological approaches as behavioural therapy, stress-coping strategies, and regular exercise may help in the management of MOH.
- -
Patients with MOH should be managed at headache units.
- -
Although outpatient detoxification is preferred, hospitalisation may be necessary in some cases (opioid or barbiturate overuse, recurrence, certain comorbidities).
The authors have no conflicts of interest to declare.
Please cite this article as: González-Oria C, Belvís R, Cuadrado ML, Díaz-Insa S, Guerrero-Peral AL, Huerta M, et al. Documento de revisión y actualización de la cefalea por uso excesivo de medicación (CUEM). Neurología. 2021;36:229–240.