A considerable percentage of events initially diagnosed as ischaemic stroke have non-cerebrovascular causes; these are called stroke mimics (SM). Currently available evidence about these events is heterogeneous and comes from studies with small samples.
ObjectiveThe purpose of our study is to identify conditions that may present as SM, define their epidemiological and clinical characteristics, and determine the percentage of cases of SM treated with intravenous fibrinolysis.
MethodsProspective study including all patients admitted to a tertiary university hospital between June 2005 and April 2015 with a diagnosis of acute stroke. We analysed demographic data, cardiovascular risk factors, time from code stroke activation to admission, stroke severity (NIHSS), final destination after discharge, degree of disability (mRS), and treatment. We compared SM and ischaemic strokes. We ruled out patients with intracranial haemorrhage, subarachnoid haemorrhage, or other causes of SM that may be detected on the baseline CT scan.
ResultsFour hundred four of the 4570 included patients (8.8%) were found to have SM. Patients with SM were younger (70.3% vs 74, P<.0001), less likely to exhibit cardiovascular risk factors and atrial fibrillation (13% vs 34%, P<.0001), scored lower on the NIHSS at baseline (2 vs 4, P<.0001), and included fewer cases of aphasia (9.4% vs 19.6%, P<.02) and dysphagia (1.2% vs 17%, P<.0001) than patients with stroke. SM caused fewer code stroke activations (28% vs 40%, P<.0001). Patients with SM required shorter hospital stays (4.9 vs 7.8 days, P<.0001), were less frequently admitted to the stroke unit (47% vs 60%, P<.0001) and more frequently discharged home (95% vs 62%, P<.0001), and had better outcomes (mRS scores 0-2; 76% vs 54%, P<.0001). Intravenous fibrinolysis was administered to 4.7% of these patients. Epileptic seizures were the most frequent cause of SM (26%).
ConclusionsIn our sample, 8.8% of all diagnoses of ischaemic stroke were SM. These events have different demographic, clinical, and prognostic characteristics; epilepsy is the most common aetiology. Despite receiving specialised emergency care, 19 patients with SM (4.7%) were treated with intravenous fibrinolysis.
Un porcentaje de casos diagnosticados inicialmente como infartos isquémicos son de causa no cerebrovascular o stroke mimics (SM). Los datos publicados al respecto son heterogéneos y, generalmente, con cohortes pequeñas.
ObjetivoNuestro objetivo es establecer qué enfermedades cursan como SM, definir sus características epidemiológicas y clínicas e identificar el porcentaje de casos tratados con fibrinólisis.
MétodosRegistro prospectivo de los eventos considerados cerebrovasculares desde junio del 2005 a abril del 2015, analizando datos demográficos, factores de riesgo cardiovascular, activación de Código Ictus e ingreso, severidad (NIHSS), destino al alta, morbilidad (mRS) y tratamiento recibido. Se han comparado los ictus isquémicos con los SM. Se excluyeron las hemorragias intracraneales, subaracnoideas y las causas de SM detectables en la TC inicial.
ResultadosSobre 4.570 casos, 404 (8,8%) son SM. Los pacientes con SM son más jóvenes (70,3 vs. 74 años, p<0,0001), tienen menos factores de riesgo cardiovascular y fibrilación auricular (13 vs. 34%, p<0,0001), una menor puntuación en NIHSS (2 vs. 4, p<0,0001) y menos afasia (9,4 vs. 19,6%, p<0,02) y disfagia (1,2 vs. 17%, p<0,0001). En los SM se activan menos códigos ictus (28 vs. 40%, p<0,0001) y requieren menos días de ingreso (4,9 vs. 7,8; p<0,0001) y menos ingresos en la unidad de ictus (47 vs. 60%, p<0,0001). Los SM son dados de alta a domicilio con mayor frecuencia (95 vs. 62%, p<0,0001) y con menor discapacidad (mRS 0-2; 76 vs. 54%, p<0,0001). Un 4,7% de los SM recibieron fibrinólisis. La primera causa de SM fueron las crisis epilépticas (26%).
ConclusionesLos SM supusieron el 8,8% de los ingresos con diagnóstico inicial de ictus isquémico. Los SM tienen características demográficas, clínicas y pronósticas diferentes, siendo la epilepsia la etiología más frecuente. Pese a recibir atención urgente especializada, 19 pacientes (4,7%) fueron trataron con fibrinólisis.
Cerebrovascular disease constitutes the second most frequent cause of death in Spain, the leading cause of death among women, and the main cause of disability in adults. The recent introduction of hospital stroke units and the wide range of new measures for early diagnosis and treatment of cerebrovascular diseases has enabled faster, specialised management of these patients. Despite the great efforts made, a considerable percentage of patients are initially diagnosed with stroke, but subsequently classified as having stroke mimics after further testing and follow-up.
Given that computed tomography (CT) has only 40% sensitivity for detecting acute ischaemic stroke,1 a diagnosis of ischaemic stroke is inevitable in patients with acute neurological symptoms and normal CT images. In fact, 1.4% to 16.7% of patients with stroke mimics receive intravenous fibrinolysis2; this percentage may increase due to efforts to reduce door-to-needle times in tertiary hospitals.3
According to Gibson and Whiteley,4 26% (95% CI, 17-44) of cases of initially suspected stroke are stroke mimics. In their literature review, epileptic seizures constituted the most frequent cause of stroke mimics. Although these tend to occur in younger patients, women, and individuals with a history of stroke and cognitive impairment,4 no specific clinical characteristics have consistently been identified in patients with stroke mimics.
Findings vary greatly between studies due to the heterogeneity of study designs; results are therefore difficult to replicate. The main problems are the lack of a standard definition of stroke mimics and the heterogeneity in the comparison group. The frequency, aetiology, and clinical characteristics of stroke mimics may vary significantly depending on whether studies include cases where stroke is suspected by pre-hospital services (primary care centres, emergency services) and non-specialised hospital services4 or cases identified after a neurological examination, admitted to stroke units, or treated for stroke.2,5,6
Our study describes the rate of stroke mimics in a sample of patients who underwent a thorough neurovascular study and a neurological examination by stroke specialists. We define clinical and epidemiological characteristics and evaluate cases of patients with stroke mimics who received fibrinolysis.
Patients and methodsAll patients were prospectively included from June 2005 to April 2015 in the BASICMAR database, a continuous record of patients with acute stroke at the neurology department at Hospital del Mar.
Hospital del Mar is the main centre at Parc de Salut Mar, a healthcare institution providing care to 3 of the 10 districts of Barcelona, serving a population of 305000, and offering tertiary care for acute stroke.
In our centre, all patients with suspected stroke are assessed by the on-call neurologists, either at admission (patients transported to the emergency department following pre-hospital or in-hospital code stroke activation, or detection of focal neurological signs by pre-hospital services or by hospital triage in patients not eligible for acute stroke treatment) or during interconsultations (patients evaluated by a non-specialised team and with suspected stroke).
Suspected stroke is based on the World Health Organization definition of stroke: “a clinical syndrome typified by rapidly developing signs of focal or global disturbance of cerebral functions, lasting more than 24hours or leading to death, with no apparent causes other than of vascular origin.”7
After evaluation, patients undergo an emergency study including a blood analysis (electrolyte study, glucose levels, complete blood count, coagulation test, and d-dimer and fibrinogen determination), an electrocardiography study, chest X-ray, and a head CT scan.
If the process is confirmed to be of cerebrovascular origin, or this aetiology continues to be suspected after an emergency head CT scan, patients are recorded in our database as stroke cases.
Patients whose symptoms are compatible with ischaemic stroke of less than 4.5hours’ progression are treated with intravenous tissue plasminogen activator (tPA), according to the exclusion criteria of the SITS-MOST protocol.8
Patients are either admitted to the stroke unit or to the neurology ward, stay at the emergency department, transferred to another department or hospital, or discharged. All patients undergo a vascular study with brain CT-angiography or Doppler/duplex ultrasound of the intracranial and supra-aortic vessels.
Complementary tests may be performed depending on the patient and clinical and aetiological suspicion; these include a complete blood count, a follow-up head CT scan or MRI scan (depending on the symptoms), a coagulation study, transthoracic or transoesophageal echocardiography, or Holter ECG monitoring, either during hospitalisation or on an outpatient basis.
All the patients with a definitive diagnosis of stroke are followed up 3 months after the event by a stroke specialist, on an outpatient basis. The neurologist reassesses the case, examines the results of complementary tests performed during admission or after discharge, and evaluates the patient in search of new symptoms or processes that may have appeared after hospitalisation. Patients displaying symptoms initially attributable to stroke but subsequently determined not to be of cerebrovascular origin are recorded in our database as stroke mimic cases; where possible, the new diagnosis is also recorded.
Our study excluded all patients showing abnormalities on the baseline head CT scan (intraparenchymal and subarachnoid haemorrhages, subdural haematomas, intracranial tumours, and other space-occupying lesions).
We therefore only included patients displaying no pathological findings on the baseline head CT scan (except for ischaemic lesions), for whom differential diagnosis of ischaemic stroke or transient ischaemic attack vs stroke mimic is necessary. We also excluded patients with symptoms of vascular origin (fistulas, vasculitis, retinal vein thrombosis), given that the strict definition of stroke mimics excludes all cerebrovascular alterations.
We considered stroke mimics to be those cases initially regarded as ischaemic strokes and associated with no signs of other lesions on the baseline head CT scan, and subsequently determined to be non-vascular in origin based on the results of the study conducted in the acute or subacute phase, the complementary tests performed by stroke specialists, and a 3-month follow-up assessment.
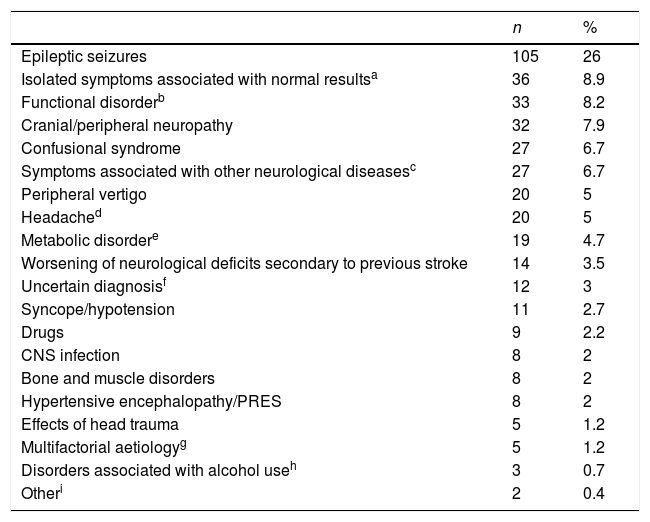
Stroke mimics were classified by the final diagnosis established after admission or at the 3-month follow-up consultation (Table 1).
Aetiological classification of stroke mimics (n=404).
| n | % | |
|---|---|---|
| Epileptic seizures | 105 | 26 |
| Isolated symptoms associated with normal resultsa | 36 | 8.9 |
| Functional disorderb | 33 | 8.2 |
| Cranial/peripheral neuropathy | 32 | 7.9 |
| Confusional syndrome | 27 | 6.7 |
| Symptoms associated with other neurological diseasesc | 27 | 6.7 |
| Peripheral vertigo | 20 | 5 |
| Headached | 20 | 5 |
| Metabolic disordere | 19 | 4.7 |
| Worsening of neurological deficits secondary to previous stroke | 14 | 3.5 |
| Uncertain diagnosisf | 12 | 3 |
| Syncope/hypotension | 11 | 2.7 |
| Drugs | 9 | 2.2 |
| CNS infection | 8 | 2 |
| Bone and muscle disorders | 8 | 2 |
| Hypertensive encephalopathy/PRES | 8 | 2 |
| Effects of head trauma | 5 | 1.2 |
| Multifactorial aetiologyg | 5 | 1.2 |
| Disorders associated with alcohol useh | 3 | 0.7 |
| Otheri | 2 | 0.4 |
CNS: central nervous system; PRES: posterior reversible encephalopathy syndrome.
Symptoms of stroke that cannot be classified into any other category since they do not meet any other set of diagnostic criteria or which have no organic explanation but are not clearly associated with a psychiatric disorder.
Organic disorders are ruled out; symptoms may be attributed to psychiatric disorders (anxiety or conversion disorders in most cases).
This category includes multiple sclerosis and other demyelinating diseases, Parkinson's disease and parkinsonism, cognitive impairment, transient global amnesia, limbic encephalitis, amyotrophic lateral sclerosis, neurosarcoidosis, and meningeal carcinomatosis.
This category includes electrolyte imbalances, hypo- and hyperglycaemia, uraemic encephalopathy, hyperammonaemia, and hepatic encephalopathy.
Patients diagnosed with stroke mimics but managed as cases of stroke due to uncertainties in diagnosis.
We gathered data on demographic variables (age and sex) and previous functional status according to the modified Rankin Scale (mRS).9 Data on cardiovascular risk factors were obtained by interviewing patients, relatives, and caregivers, and from patients’ medical records, and defined according to international guidelines. The questionnaire included the following risk factors: smoking (active smoker, former smoker for less than one year, or former smoker for more than one year), alcohol consumption >60g/day (active drinker, former drinker for less than one year, or former drinker for more than one year; we also recorded alcohol intake in grams per day), hyperlipidaemia (medical diagnosis, use of medication, or serum cholesterol concentrations >220mg/dL, LDL >130mg/dL, or triglycerides >150mg/dL), arterial hypertension (evidence of at least 2 readings of systolic blood pressure >140mmHg or diastolic blood pressure >90mmHg recorded on different days before stroke), diabetes (medical diagnosis or use of medication), atrial fibrillation confirmed by ECG performed before stroke or during hospitalisation, ischaemic heart disease (history of angina or myocardial infarction), peripheral artery disease (medical diagnosis of intermittent claudication or an ankle-brachial index <0.90), and history of stroke or transient ischaemic attack.
We also gathered such other clinical characteristics as stroke severity according to the National Institutes of Health Stroke Scale (NIHSS), aphasia (both isolated and combined with other symptoms), and dysphagia. We recorded pre-hospital or in-hospital code stroke activations, administration of intravenous tPA, patient location following admission (stroke unit, neurology ward, other unit/department), days of hospitalisation, and patient location after discharge (home, residential centre, rehabilitation centre, palliative care centre, or another hospital). The level of functional independence was determined at discharge using the mRS; scores ≤2 indicate independence and scores ≥3 indicate dependence.
Statistical analysisData are presented as means±SD or medians with interquartile ranges for continuous variables, depending on whether data follow a normal distribution, and as frequencies and percentages for categorical variables. The t test and the chi-square test were used to evaluate differences between means for continuous variables and between percentages for dichotomous variables, respectively. The non-parametric Mann–Whitney U test was used for continuous, non-normally distributed variables. To evaluate the predictive factors of stroke mimics, we performed a multivariate analysis using logistic regression, which included the variables with P-values <.1 in the univariate analysis. Statistical analysis was performed with SPSS 19.0.
ResultsWe gathered a total of 5726 patients initially diagnosed with stroke between June 2005 and April 2015. We excluded 678 (11.84%) patients with intraparenchymal haemorrhages and 402 (7.02%) with subarachnoid haemorrhages. We also excluded patients with evident signs of disease on head CT images, such as subdural haemorrhage (12 patients) and intracranial tumours (21), patients with stroke mimics of vascular origin (19), and those whose medical histories lacked data (24); this left a total of 4570 patients. Of these, 4166 (91.2%) received a final diagnosis of ischaemic stroke; the remaining 404 (8.8%) had stroke mimics.
Tables 2 and 3 summarise the demographic and clinical characteristics analysed and the differences in diagnosis, management, progression, and prognosis.
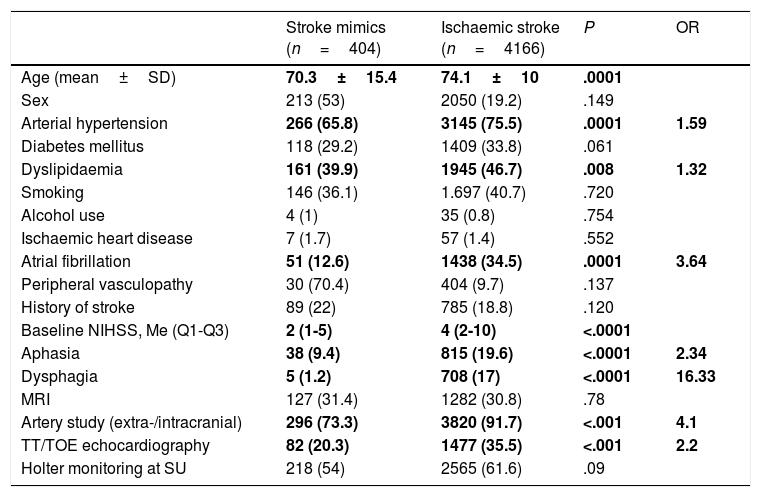
Demographic and clinical characteristics and diagnostic tests for stroke mimics and ischaemic stroke.
| Stroke mimics (n=404) | Ischaemic stroke (n=4166) | P | OR | |
|---|---|---|---|---|
| Age (mean±SD) | 70.3±15.4 | 74.1±10 | .0001 | |
| Sex | 213 (53) | 2050 (19.2) | .149 | |
| Arterial hypertension | 266 (65.8) | 3145 (75.5) | .0001 | 1.59 |
| Diabetes mellitus | 118 (29.2) | 1409 (33.8) | .061 | |
| Dyslipidaemia | 161 (39.9) | 1945 (46.7) | .008 | 1.32 |
| Smoking | 146 (36.1) | 1.697 (40.7) | .720 | |
| Alcohol use | 4 (1) | 35 (0.8) | .754 | |
| Ischaemic heart disease | 7 (1.7) | 57 (1.4) | .552 | |
| Atrial fibrillation | 51 (12.6) | 1438 (34.5) | .0001 | 3.64 |
| Peripheral vasculopathy | 30 (70.4) | 404 (9.7) | .137 | |
| History of stroke | 89 (22) | 785 (18.8) | .120 | |
| Baseline NIHSS, Me (Q1-Q3) | 2 (1-5) | 4 (2-10) | <.0001 | |
| Aphasia | 38 (9.4) | 815 (19.6) | <.0001 | 2.34 |
| Dysphagia | 5 (1.2) | 708 (17) | <.0001 | 16.33 |
| MRI | 127 (31.4) | 1282 (30.8) | .78 | |
| Artery study (extra-/intracranial) | 296 (73.3) | 3820 (91.7) | <.001 | 4.1 |
| TT/TOE echocardiography | 82 (20.3) | 1477 (35.5) | <.001 | 2.2 |
| Holter monitoring at SU | 218 (54) | 2565 (61.6) | .09 |
Me (Q1-Q3): median (interquartile range); MRI: magnetic resonance imaging; NIHSS: National Institutes of Health Stroke Scale; OR: odds ratio; SD: standard deviation; SU: stroke unit; TOE: transoesophageal; TT: transthoracic.
Data are expressed as n (%), except otherwise indicated. Statistically significant values are shown in bold.
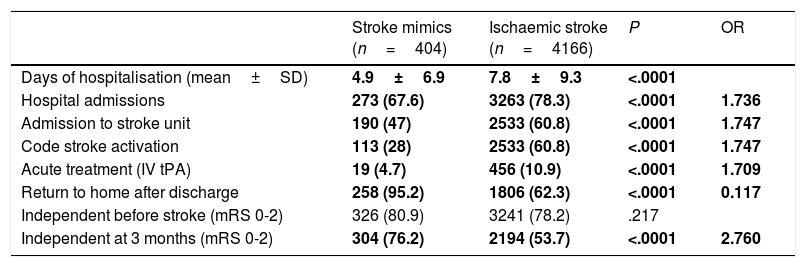
Differences in management, progression, and prognosis between stroke mimics and ischaemic stroke.
| Stroke mimics (n=404) | Ischaemic stroke (n=4166) | P | OR | |
|---|---|---|---|---|
| Days of hospitalisation (mean±SD) | 4.9±6.9 | 7.8±9.3 | <.0001 | |
| Hospital admissions | 273 (67.6) | 3263 (78.3) | <.0001 | 1.736 |
| Admission to stroke unit | 190 (47) | 2533 (60.8) | <.0001 | 1.747 |
| Code stroke activation | 113 (28) | 2533 (60.8) | <.0001 | 1.747 |
| Acute treatment (IV tPA) | 19 (4.7) | 456 (10.9) | <.0001 | 1.709 |
| Return to home after discharge | 258 (95.2) | 1806 (62.3) | <.0001 | 0.117 |
| Independent before stroke (mRS 0-2) | 326 (80.9) | 3241 (78.2) | .217 | |
| Independent at 3 months (mRS 0-2) | 304 (76.2) | 2194 (53.7) | <.0001 | 2.760 |
IV: intravenous; mRS: modified Rankin Scale score; OR: odds ratio; SD: standard deviation; tPA: tissue plasminogen activator.
Data are expressed as n (%), except otherwise indicated. Statistically significant values are shown in bold.
Compared to the patients diagnosed with ischaemic stroke, those with a final diagnosis of stroke mimic were younger (70.6±15.4 years vs 74.1±10 years; P<.0001) and had fewer vascular risk factors (arterial hypertension, 65.8% vs 75.5%; P<.0001; dyslipidaemia, 39.9% vs 46.7%; P<.008; atrial fibrillation, 12.8% vs 34.5%; P<.0001). They also showed lower baseline NIHSS scores (2 [1-5]; P<.0001) and were less likely to have aphasia (9.4% vs 19.6%; P<.0001) and dysphagia (1.2% vs 17%; P<.0001). No association was found between stroke mimics and sex, other cardiovascular risk factors, alcohol or tobacco use, or history of ischaemic heart disease, peripheral artery disease, or stroke.
Stroke mimics resulted in fewer hospital admissions (67.6% vs 78.3% of ischaemic strokes; P<.0001), fewer admissions to the stroke unit (47% vs 60.8%; P<.0001), and shorter hospital stays (4.9±6.9 days vs 7.8±9.3 days; P<.0001). Following discharge, patients with stroke mimics are more frequently able to return to their homes than those with ischaemic stroke (93.5% vs 32.3%); the latter patients are usually transferred to other centres or require institutionalisation. Although no differences were observed in the level of independence before admission to hospital, patients with stroke mimics were more frequently independent at 3 months (75.2% vs 53.9%; P<.0001). The cases for which data were unavailable on the latter 3 variables correspond mainly to patients with stroke.
A total of 19 patients (4.7%) were treated with intravenous tPA and showed no evidence of symptomatic or asymptomatic intracranial haemorrhage after treatment. After the neurovascular study and the 3-month follow-up evaluation, epileptic seizures were the most frequent final diagnosis among patients with stroke mimics (105 patients, 26%). Table 1 lists the final diagnoses of all patients with stroke mimics.
DiscussionIn our single-centre study, 8.8% of patients were found to have stroke mimics after a complete neurovascular study. A wide range of non-cerebrovascular diseases present similar symptoms and form of onset to those of stroke. The proportion of patients with stroke mimics has classically been estimated at 19%.10 A more recent review of 25 studies published since 2012, analysing patients with initial suspicion of stroke, estimates the frequency of stroke mimics at 26% (95% CI, 17-44).4 These studies were conducted in different settings; suspicion of stroke was therefore established by a wide range of healthcare professionals, including primary care physicians, emergency services staff, emergency department physicians not specialising in neurology, and neurologists. Data gathering is heterogeneous in these studies (patients admitted to the emergency department with suspected stroke, patients admitted to stroke units, etc.), which may explain the variability in the frequency of stroke mimics between articles.
The articles cited and other published studies on the topic do not distinguish between ischaemic stroke and subarachnoid or intraparenchymal haemorrhage, and include subdural haemorrhages and intracranial tumours in the category of stroke mimics. To adapt the study to clinical practice, we aimed to approach our literature review from a different perspective; to our knowledge, this is the first article to use this methodology. Our approach reflects the reality of modern acute stroke management in tertiary hospitals, where neurologists are responsible for the diagnosis and management of stroke patients. In this case, diagnosis is uncertain when focal neurological signs are not accompanied by pathological findings on the baseline CT scan, which may lead to the administration of recanalisation therapy (intravenous tPA) during the acute phase in patients not meeting exclusion criteria for treatment. Therefore, only ischaemic stroke would be included, given that intraparenchymal and subarachnoid haemorrhages are easily detectable by CT.
With this methodology, 91.2% (4166) of the cases of suspected ischaemic stroke were finally confirmed, whereas the remaining 8.8% (404) were stroke mimics. The percentage of stroke mimics increases to 11.2% if we include subdural haemorrhages and intracranial tumours.
As in most published studies, the most frequent cause of stroke mimics was epilepsy (26%).2,4,5 Although percentages vary depending on the study design and the classification used, the most frequent differential diagnoses are the same: epileptic seizures, headache, space-occupying lesions, toxic metabolic disorders, confusional syndrome, syncope, functional disorders, neuropathy, peripheral vertigo, and symptoms of uncertain origin. In our series, the second most frequent final diagnosis of stroke mimics (8.9%) was isolated neurological symptoms associated with normal study results, where symptoms were not clearly vascular in origin but were not classified into any other category. Nine of these patients had isolated sensory symptoms; vertebrobasilar aetiology was suspected in 15 but could not be confirmed by neuroimaging.
Numerous studies have attempted to identify the clinical and demographic predictors of stroke mimics. According to Hand et al.,11 the following 8 factors can independently predict a final diagnosis of stroke: ability to identify the exact time of symptom onset, focal symptoms, abnormal vascular findings, neurological signs, symptom lateralisation in either brain hemisphere, and possibility of classifying symptoms into one of the TOAST categories.12 On the other hand, cognitive impairment and alterations in other systems are predictors of stroke mimics.
The study by Tobin et al.13 aims to develop a prediction model for identifying stroke mimics. In their sample, 22% of the 196 patients with suspected stroke were finally diagnosed with stroke mimics. The authors conclude that lack of lateralisation of signs, low diastolic blood pressure, and history of stroke or transient ischaemic attack are strong predictors of non-vascular events.
A systematic review by Nguyen and Chang,2 including one study with a sample of 8187 patients, shows that patients with stroke mimics tend to have fewer cardiovascular risk factors (arterial hypertension, diabetes mellitus, and dyslipidaemia). According to these researchers’ results, stroke mimics are more frequent in women, in younger patients, and in patients with lower baseline NIHSS scores than is stroke, although they did not observe a consistent association between these variables and the risk of stroke mimics.
In our study, stroke mimics were associated with younger age, arterial hypertension, dyslipidaemia, and atrial fibrillation. From a clinical viewpoint, patients with stroke mimics scored lower on the NIHSS at baseline and were less likely to have aphasia and dysphagia; we were unable to analyse other clinical characteristics due to insufficient data.
The multivariate analysis detected no predictors of stroke mimics.
We also observed differences in management, progression, and prognosis: patients with stroke mimics are less frequently admitted to hospital, and when they are hospitalised, they are less likely to be admitted to the stroke unit, have shorter stays, are more frequently able to return to their homes after discharge, and show better functional prognosis at 3 months.
Between 1.4% and 16.7% of patients with stroke mimics are treated with tPA during the acute phase, with only 2 reported cases of symptomatic intracranial haemorrhage; fibrinolysis is therefore a safe procedure for these patients, who experience a lower rate of complications than patients with stroke (although no data are available for asymptomatic intracranial haemorrhage).2,14 Results should be interpreted with caution given the heterogeneity in the definition of stroke mimics and the comparison group. Furthermore, the literature on the topic may have a publication bias, with “negative” results being underrepresented. Stroke mimics may be underdiagnosed given that normal neuroimaging results after the episode are frequently considered to indicate “aborted stroke.”15 This concept is increasingly challenged due to the high sensitivity of multimodal neuroimaging for detecting acute ischaemia.
In our series, 19 patients with stroke mimics received fibrinolysis (4.7%); final diagnoses were epileptic seizures (8 patients), headache (4), CNS infection (2), functional disorders (2), drug adverse reactions (2), and peripheral vertigo (1). None of our patients had symptomatic or asymptomatic intracranial haemorrhages or any other treatment-related complication.
Future studies will probably focus on evaluating whether the number of treated stroke mimics increases with the implementation of measures for reducing door-to-needle times. These cases are expected to become more frequent mainly due to a decrease in door-to-needle times; this phenomenon has already been observed in other studies.3 In the light of this, developing an assessment protocol for detecting stroke mimics is even more crucial.
This study presents a practical approach to the true problem of stroke mimics: differential diagnosis with ischaemic stroke, which entails a high risk of treatment errors.
Despite the lack of definitive predictive clinical patterns for differentiating stroke mimics from stroke based on the patient's medical history and physical examination, we may conclude that patients with stroke mimics are usually younger and less likely to have a history of arterial hypertension and atrial fibrillation, and display less severe symptoms and more favourable progression and prognosis than patients with stroke.
FundingThe study was funded by the Spanish Ministry of Health, the Institute of Health Carlos III, and the European Regional Development Fund (RD12/0042/0020 and RD12/0042/0061).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Avellaneda-Gómez C, Rodríguez Campello A, Giralt Steinhauer E, Gómez González A, Serra Martínez M, de Ceballos Cerrajería P, et al. Estudio descriptivo de los stroke mimics después de un estudio neurovascular completo. Neurología. 2019;34:7–13.
This study was presented as an oral communication at the 20th Annual Meeting of the Catalan Society of Neurology, held in Barcelona on 25–27 May 2016.