Symptomatic intraparenchymal haemorrhages are an infrequent complication of fibrinolytic treatment (appearing in 1.7%-4.6% of cases), with a mortality rate of 54%-75%.1–3 Up to 55% of these haemorrhages (3.3% of all patients treated) involve a remote location with regards to the area of infarction.3,4
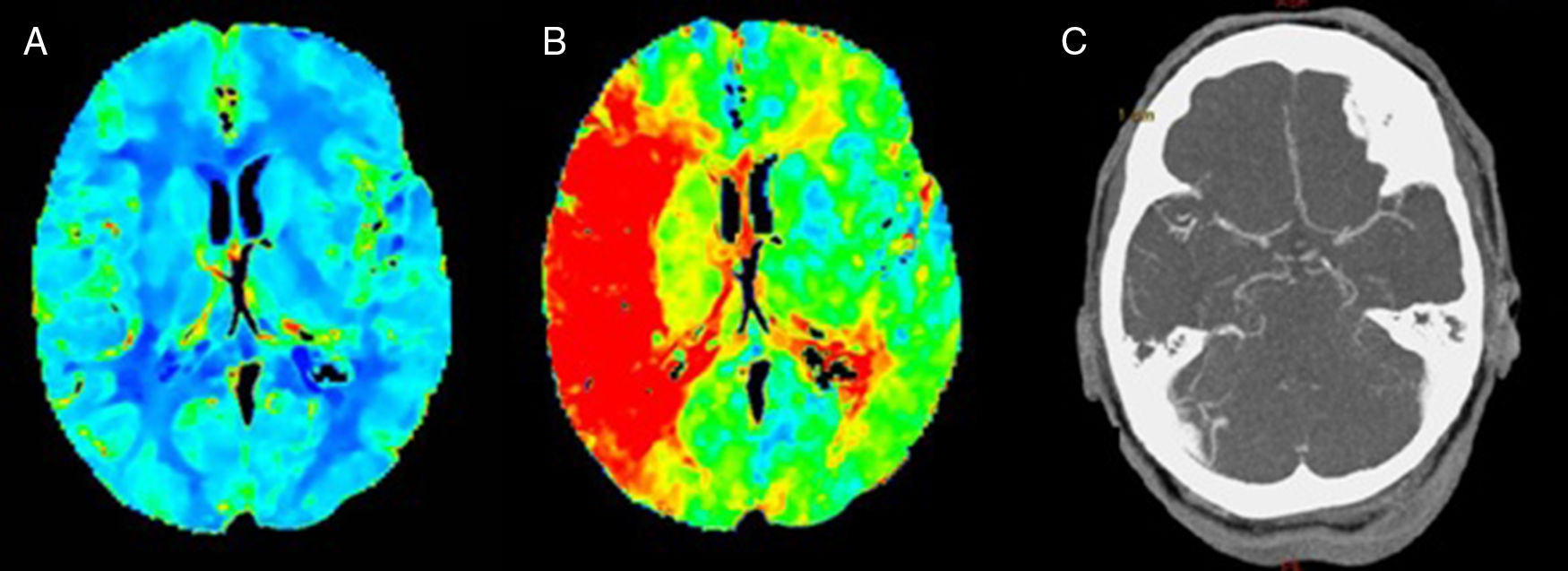
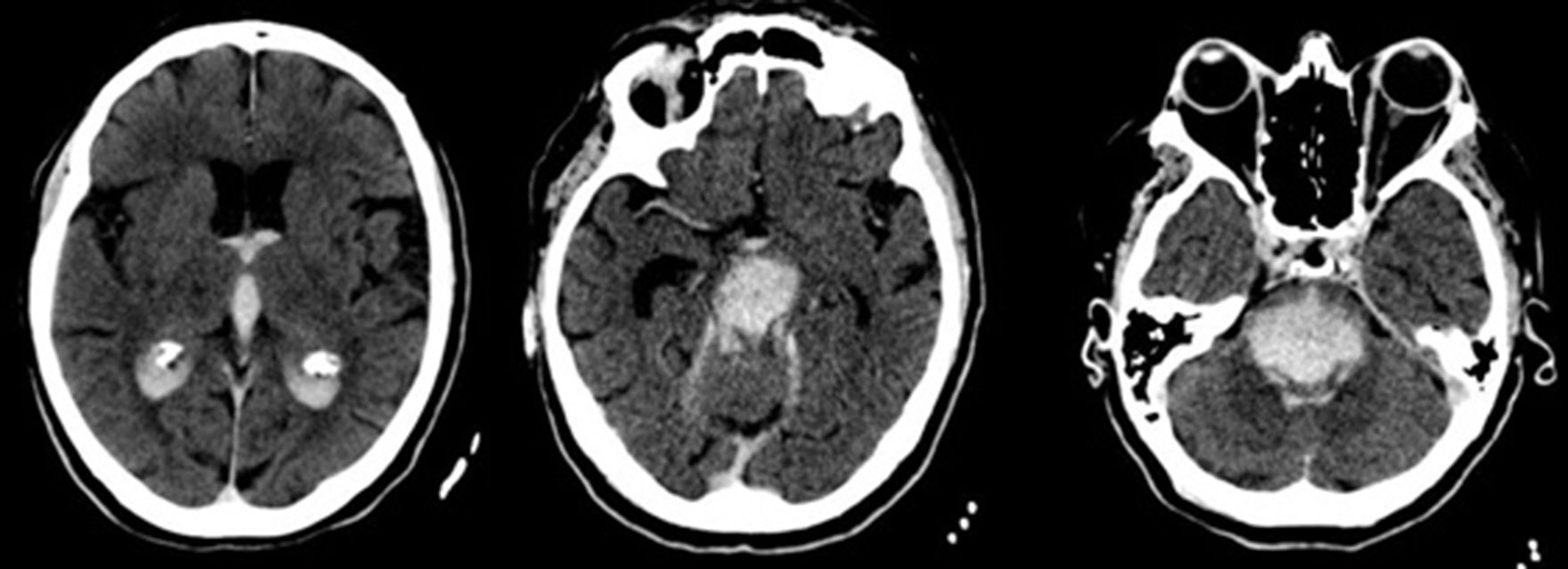
We present the case of a patient who died after a massive brainstem haemorrhage, possibly secondary to a transtentorial herniation (Duret haemorrhage) during treatment of a proximal occlusion of the right middle cerebral artery (MCA) with intravenous recombinant tissue plasminogen activator (rt-PA). Our patient was a 59-year-old Romanian woman with obesity and arterial hypertension of unknown progression time, treated with angiotensin-converting enzyme (ACE) inhibitors; the patient presented sudden-onset left hemiparesis and dysarthria. On the way to hospital, unknown atrial fibrillation was detected, with controlled ventricular response and arterial pressure of 200/100mmHg, subsequently decreasing without administration of antihypertensive treatment. Upon arrival, we observed right hemispheric syndrome (NIHSS=13). The emergency blood analysis showed macrocytosis without anaemia (Hb 13.9g/dL, MCV 106.5fL), platelets and leucocytes within normal ranges, and INR values at the higher threshold of normality (1.28) with normal thromboplastin time. A multiparametric brain CT scan (baseline brain CT scan, CT perfusion, and CT-angiography) revealed occlusion of the right distal M1 segment, with no established infarct on the baseline brain CT scan, and symmetrical cerebral blood volume maps with longer time-to-peak in all cortical territories of the right MCA (Fig. 1). After confirming with family members that there were no contraindications, fibrinolytic treatment with intravenous rt-PA was started 2 hours and 45 minutes after symptom onset. During this procedure, we observed a slight exacerbation of motor symptoms (NIHSS=15), with arterial pressure remaining within the established limits (160/70mmHg). At 45 minutes of starting perfusion, level of consciousness suddenly decreased, with no verbal or eye response, and the patient displayed decerebrate rigidity, conjugate gaze palsy, nonreactive mydriatric pupils, and bradycardia (30bpm). We suspended fibrinolytic treatment and performed orotracheal intubation. An emergency brain CT scan revealed a transtentorial herniation and extensive pontine haematoma extending to the midbrain, diencephalon, third and fourth ventricles, and lateral ventricles (Fig. 2). The patient was admitted to the intensive care unit and died at 96 hours.
Henri Duret, a 19th-century French surgeon, reported haemorrhages in the brainstem after increasing intracranial pressure by injecting petroleum jelly or water into the skulls of experimental dogs. Since then, the eponym “Duret haemorrhage” is used to describe brainstem haemorrhages secondary to transtentorial herniation of any aetiology.5,6 The most common causes are haematomas (subdural, epidural, or intraparenchymal) and acute brain oedema, observed in up to 15% of cerebral infarctions (29% in cases of large vessel occlusion),7 which may be explained by the rapid increase in intracranial pressure.8 Other frequently observed factors are sudden changes in intracranial pressure secondary to lumbar punctures or surgical evacuation of subdural haematomas.9–11
Since the introduction of fibrinolytic treatment in both heart and brain diseases, intracerebral haemorrhages have been considered a relatively frequent complication (<10%1). Brainstem haemorrhages secondary to transtentorial herniation have also been reported to a lesser extent (4.6% of all intraparenchymal haemorrhages12).13 Brain herniation has recently been reported to be the only radiological sign showing a statistically significant association with clinical exacerbation after intravenous rt-PA administration.14 Published data on the time lapsed until the manifestation of this complication are scarce; a period of 24 hours after the procedure is usually described. Before rt-PA was introduced, 2 cases were reported of this complication appearing during treatment with urokinase.15 Since the introduction of the drug, we have found only one other published case of Duret haemorrhage during perfusion, with a volume higher than that typically found in this disease (probably due to the fibrinolytic treatment itself) and with the same poor outcome,4 due to the location and severity of bleeding, which are important predictors of mortality in brain haemorrhage. Age is another important prognostic predictor in brain haemorrhage, with a mortality rate of 50% in patients older than 85 vs 27.9% in younger patients16; both our patient and the previously reported case were younger (59 and 75 years, respectively).
We do not know the cause of the early manifestation of this complication in our patient, since she did not present a history of factors that would increase the risk of haemorrhage, or show findings suggestive of this complication in the baseline CT scan. Duret haemorrhage is a rare complication of fibrinolytic treatment for cerebral infarction. Today, more than a century after its initial description, understanding of its pathophysiological mechanism and treatment continues to be limited and prognosis is poor in most cases. We therefore deem it very useful to expand our knowledge of the radiological characteristics of this entity and its possible manifestation during fibrinolytic therapy, especially in patients who may be eligible for another revascularisation treatment.
Please cite this article as: Quintas S, Palmí Cortés I, Zapata-Wainberg G, López Ruiz R, Vivancos J. Hemorragia de Duret durante la perfusión de tratamiento fibrinolítico. Neurología. 2019;34:340–342.