Many studies have demonstrated that iron deficiency modifies the normal function of the central nervous system (CNS) and alters cognitive abilities. When cellular damage occurs in the CNS, neuroprotective mechanisms, such as the production of neurotrophic factors, are essential in order for nervous tissue to function correctly. Insulin-like growth factor II (IGF-II) is a neurotrophic factor that was recently shown to be involved in the normal functioning of cognitive processes in animal models. However, the impact of iron deficiency on the expression and function of this molecule has not yet been clarified.
MethodsMixed primary cell cultures from the CNS were collected to simulate iron deficiency using deferoxamine. The expression of IGF-I, IGF-II, IGF-IR, and IGF-IIR was determined with the Western blot test.
ResultsWe observed increased expression of IGF-II, along with a corresponding decrease in the expression of IGF-IIR, in iron-deficient (DFe) mixed primary cell cultures. We did not observe alterations in the expression of these proteins in isolated microglia or neuronal cultures under the same conditions. We did not detect differences in the expression of IGF-I and IGF-IR in DFe cultures.
ConclusionsIn vitro iron deficiency increases the expression of IGF-II in mixed glial cell cultures, which may have a beneficial effect on brain tissue homeostasis in a situation in which iron availability is decreased.
Muchos estudios han demostrado que la deficiencia de hierro modifica el funcionamiento normal del sistema nervioso central, alterando las habilidades cognitivas. Ante una situación de daño celular en el sistema nervioso central existen mecanismos neuroprotectores, como la producción de factores neurotróficos, los cuales son esenciales para un funcionamiento adecuado del tejido nervioso. El factor de crecimiento de insulina tipo II (IGF-II) es un factor neurotrófico que recientemente se ha involucrado en el funcionamiento normal de los procesos cognitivos en modelos animales; sin embargo, el impacto de la deficiencia de hierro sobre la expresión y funcionamiento de esta molécula aún no ha sido determinado.
MétodosSe emplearon cultivos primarios mixtos de células del sistema nervioso central, en los que se simuló la deficiencia de hierro empleando deferoxamina y se determinó la expresión de IGF-I, IGF-II, IGF-IR e IGF-IIR por medio de western-blot.
ResultadosSe observó un incremento en la expresión de IGF-II y una disminución en la expresión de IGF-IIR en cultivos primarios mixtos deficientes en hierro. No se observaron cambios en la expresión de dichas proteínas en cultivos individuales de microglía o neuronas en las mismas condiciones. No se encontraron diferencias en la expresión de IGF-I e IGF-IR en condiciones de deficiencia de hierro.
ConclusionesLa deficiencia de hierro in vitro induce un incremento en la expresión de IGF-II en cultivos mixtos de células gliales, lo que puede favorecer la homeostasis del tejido cerebral en situaciones de disminución en la disponibilidad de hierro.
Iron is a micronutrient essential to the development and functioning of the central nervous system (CNS). However, dietary iron deficiency and its consequences still constitute a global health problem.1 UNICEF calculates that nearly 2 billion people worldwide have iron deficiency, including 20% to 25% of the paediatric population.2 Iron is crucial to the CNS because it participates in the cellular migration and differentiation processes, myelination, synaptogenesis, gliogenesis, neurogenesis, and in neurotransmitter synthesis. Together, these processes enable different cerebral regions to function correctly.3–5 Iron deficiency has a negative effect on these processes and provokes changes in neurological and cognitive function in people of all ages6,7 since it affects such key brain structures as the hippocampus and cerebral cortex.8
Throughout the development of the CNS, there should be a state of balance between external factors (such as dietary iron) and internal factors. The latter include growth factors, which are molecules that control cell proliferation, differentiation, and survival processes,9,10 activate signalling pathways to modulate gene transcription, and promote the transduction of extracellular signals.11,12 Recent studies in animal models have shown that insulin-like growth factor II (IGF-II) exerts a protective effect against neural or glial damage. It also contributes to inducing the generation and differentiation of new cells, which improves cognitive processes.13 IGF-II is distributed among multiple CNS structures which include the hippocampus, hypothalamus, striatum, cerebral cortex, and cerebellum.14,15 Its specific activator (IGF-IIR) must be activated in order to promote the factor's biological effects.16,17 However, despite the importance of IGF-II as a neurotrophic factor and the known negative effects of iron deficiency on cognitive processes, the effect of iron deficiency on the expression of IGF-II and of its receptor remains unknown.
The purpose of this study was to identify changes in IGF-II and IGF-IIR expression in CNS primary cell cultures under conditions of iron deficiency. This will provide a better understanding of the molecular changes that promote cognitive deficits arising due to that deficiency.
Subjects, materials, and methodsExperiments were performed in the Neurochemistry Laboratory at the Faculty of Medicine, Universidad Autónoma del Estado de México, between August 2012 and July 2013.
BALB/c miceBreeding pairs of BALB/c mice were kept in standard conditions during 3 weeks (ad libitum access to food and purified water; 12:12hour light/dark cycle; mean temperature of 20°C). Pregnant females were separated from their mates and kept under observation in the same conditions until their litters were born. Newborn mouse pups (<24hours old) were used to initiate primary cell cultures.
Dissection of brain tissue to obtain neural and glial cellsFour to eight newborn mouse pups (<24hours old) were killed by decapitation on chilled plates. Researchers placed brain tissue in a digestion medium (DMEM+0.25% trypsin/1mM EDTA) and incubated it for 1hour in standard conditions (37°C, 5% CO2) to degrade the connective tissue. The digestion process was halted using a complete culture medium (DMEM+FBS 10%) and the cells obtained were centrifuged at 1200rpm for 10minutes.
Mixed culture of CNS cellsPurified cells were cultivated to a density of 1 to 1.2×105cells/cm2 in the growth area and then incubated under standard conditions (37°C, 5% CO2) for 10 to 15 days until they had achieved a confluency of ≥80%.
Neuron cell cultureAfter 24hours, the mixed glial cell culture supernatant was recovered and centrifuged to obtain non-adherent cells. These cells were then cultivated to a density of 1 to 1.2×105cells/cm2 in B27-supplemented neurobasal medium with 5% FBS, using Petri dishes previously coated with poly-l-lysine. The culture was kept under standard incubation conditions (37°C, 5% CO2) for 10 to 15 days.
Microglial cell cultureWhen the mixed culture had reached the desired confluency, researchers added 0.25% trypsin/1mM EDTA over 30 to 45minutes to induce astrocyte separation. Once cells had been separated, we removed and centrifuged the supernatant at 1200rpm at 21°C for 5minutes. Cells were cultivated to a density of 1 to 1.2×105cells/cm2 in the growth area. We added complete culture medium (DMEM+FBS 8%) and kept the sample under standard conditions (37°C, 5% CO2) until confluency reached 80%.
Initiating iron-deficient (DFe) cell culturesSelected cell cultures were treated with deferoxamine (100μM) for over 24hours to create conditions of iron deficiency in the medium. The normal iron cultures were not treated.
Protein extraction and quantificationProteins were extracted from cell cultures by adding 50 to 80μL lysis buffer supplemented with protease and phosphatase inhibitors (+20μL PIC 50×+100μL NaF 50mM+20μL PMSF 1mM+20μL Na3VO4 2mM) in each well. Cells were then lysed by mechanical shearing and collected in 1.5mL tubes and shaken on ice for 45minutes Researchers then centrifuged cells at 13000rpm at 4°C for 20minutes to extract the proteins from the supernatant. The cell pellet was discarded. Proteins were quantified using the Bradford method previously described in the Quick Start™ Bradford Protein Assay by BIO-RAD.
Analysis of protein expression using Western blotResearchers prepared a 10× gel running buffer to detect proteins of interest. Before using quantified proteins, we measured actin expression as a control for the sample volume employed, using monoclonal mouse anti-actin antibody (Sigma–Aldrich) at a concentration of 1:2000. Electrophoresis gels were subsequently run for control samples (with sufficient iron) and test samples (with iron deficiency), using 60μg of protein for each sample. After running the gel, proteins were transferred to a PVDF membrane. After protein transfer, the membrane was washed and blocked with a 5% milk solution for 1 hour. IGF-IIR was detected using rabbit polyclonal primary anti-mouse antibody (Santa Cruz Biotechnology) diluted to 1:250. IGF-II was detected with rabbit polyclonal anti-mouse antibody (Abcam) diluted to 1:1500 and agitated overnight at 4°C. The secondary antibody was mouse polyclonal anti-rabbit (Thermo) diluted to 1:2000 for IGF-IIR and IGF-II and incubated for 90minutes at room temperature. Lastly, we developed the membrane using the colorimetric method with diaminobenzidine+hydrogen peroxide in PBS, agitated for 15 to 30minutes.
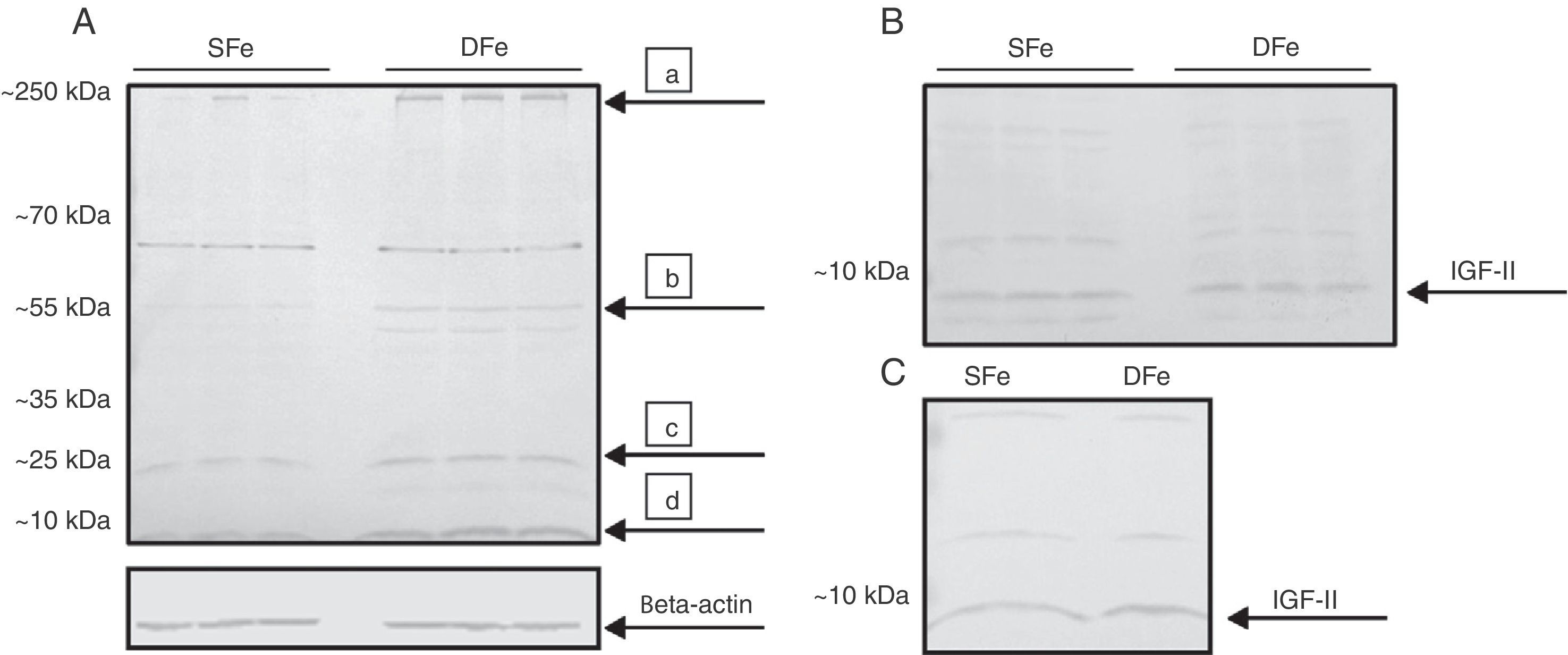
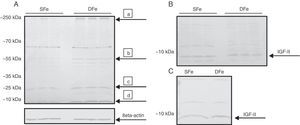
ResultsExpression of IGF-II under conditions of iron deficiencyTo determine the effect of iron deficiency on CNS cells, we examined differences in IGF-II expression in primary cultures of mixed glial cells in DFe and control samples. We detected a band with an approximate molecular weight of <10kDa corresponding to the molecular weight of 7.5kDa that can be expected for a mature IGF-II molecule (Fig. 1). Expression of this band was found to be higher in DFe cultures than in controls. In addition, we consistently observed bands with molecular weights between 20 and 70kDa, as well as bands with a molecular weight greater than 250kDa (Fig. 1). These proteins behaved similarly to IGF-II and their expression was also higher in DFe cultures than in controls, except for the band with a weight of ∼70kDa which did not exhibit differences between the samples.
Western blot analysis of IGF-II expression in CNS cell cultures from BALB/c mice. (A) Mixed cultures in iron-sufficient (SFe) or iron-deficient (DFe) conditions. Arrows indicate the main proteins found: (a) IGFBP-3, (b) IGFBP-2, (c) IGFB-4, (d) IGF-II. (B) Microglial cell cultures, SFe and DFe. (C) Neuronal cell cultures, SFe and DFe. Beta-actin was used as the loading control.
Expression of IGF-II was analysed in isolated microglia and neuron cultures in order to determine the cell population responsible for increased IGF-II expression in mixed cultures. However, these isolated cultures did not display any changes in IGF-II expression in samples with an iron deficiency. As in mixed cultures, we detected bands weighing ∼20 and 55kDa for both cell types, as well as other bands weighing ∼35 and 70kDa for neuron cultures only. Similarly, these bands exhibited no changes in expression in samples with iron deficiency.
Based on these results, we observe that iron deficiency induces an increase in IGF-II expression in mixed CNS cell cultures, but not in cultures of microglia or neurons alone.
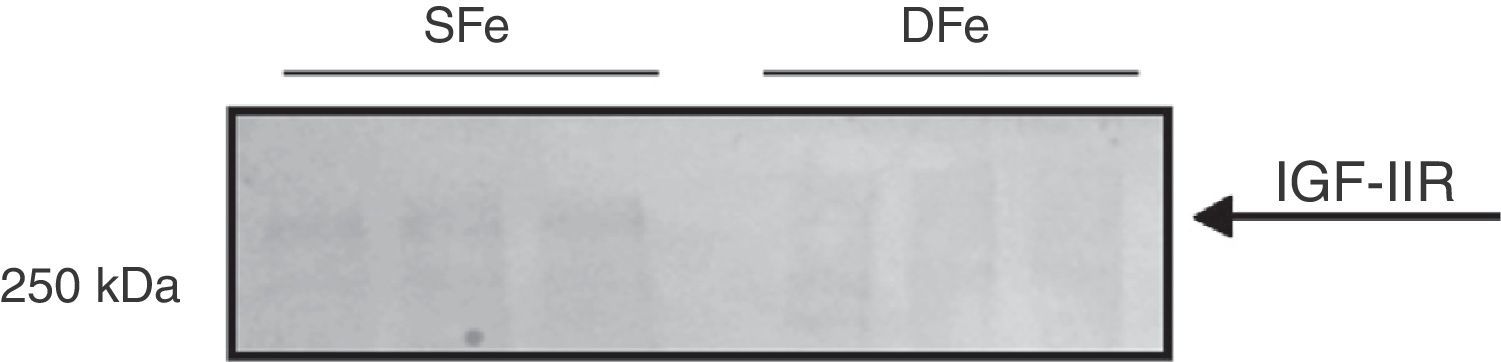
Expression of IGF-II receptor in mixed glial cell culturesOnce changes in IGF-II expression had been detected, we aimed to determine whether this effect could also be found for the IGF-II receptor. Analysis of the expression of IGF-II receptor in mixed cultures of glial cells revealed a band with a molecular weight greater than 250kDa, possibly corresponding to IGF-IIR (∼300kDa), in cells cultured with normal iron levels. On the other hand, expression of this molecule is diminished, and almost non-existent, when iron deficiency is present (Fig. 2). In addition, a band weighing ∼70kDa was consistently present and showed no changes in expression in the cultures with iron deficiency. This band does not correspond to the molecular weight reported for the molecule in question. These results show that iron deficiency decreases the expression of IGF-IIR in mixed CNS cell cultures.
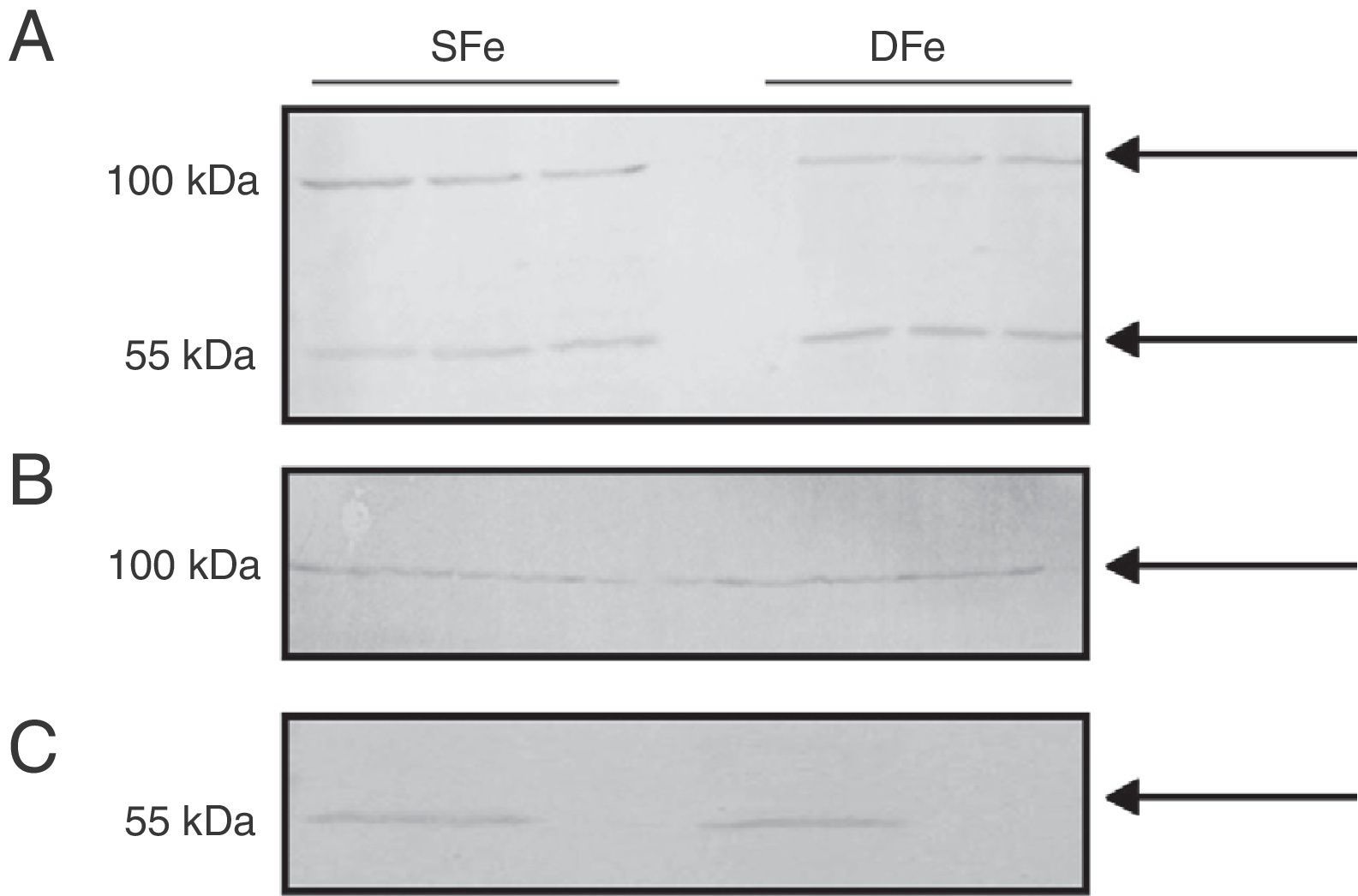
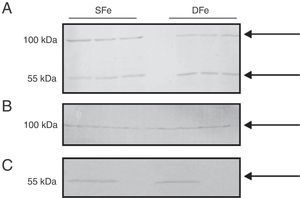
Expression of insulin-like growth factor I and its specific receptor in mixed glial cell cultures and populations of microglia or neurons onlyTo compare expression of both IGF-II and its receptor to the expression of the other closely related growth factor, we examined IGF-I and IGF-IR in cultured CNS cells. There were no observable differences in expression of IGF-I between DFe cell cultures and controls because the bands identified had molecular weights of ∼50 and ∼100kDa, figures which do not correspond to the normal molecular weight for IGF-I (7.6kDa) (Fig. 3). In addition, we examined expression in cell populations consisting of only microglia or only neurons, and the anticipated band weighing less than 10kDa was not found here either. There were no changes in the expression of proteins weighing 50 or 100kDa in samples with iron deficiency.
Western blot analysis of IGF-I expression in CNS cell cultures from BALB/c mice. (A) Mixed glial cell cultures, (B) Microglial cell cultures, (C) Neuron cell cultures. Cultures in iron-sufficient (SFe) or iron-deficient (DFe) conditions. Arrows indicate the bands detected by the antibody.
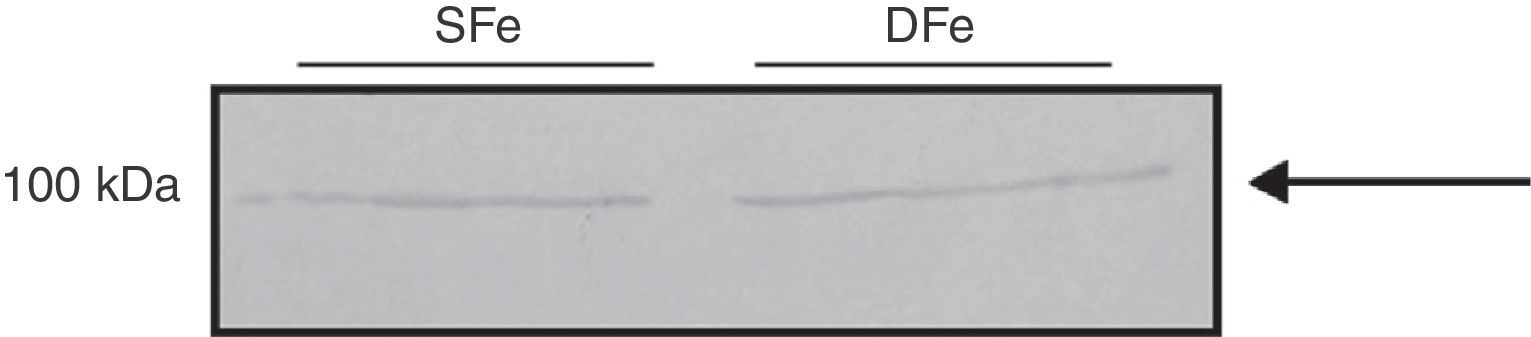
We did detect a band of ∼100kDa in mixed cell cultures, and this might correspond to IGF-IRβ which has a molecular weight of 97kDa. A band weighing ∼50kDa was also detected. We observed no differences in expression of this protein in the DFe samples (Fig. 4).
DiscussionProper diet in the early stages of development is crucial to promote CNS growth and development.18 Evidence supports the association between dietary micronutrients and the function of neurotrophic factors.19–22 Vitamin A deficiency decreases the expression of brain-derived neurotropic factor (BDNF) and nerve growth factor in the CNS.19,23 There is also a link between vitamin B and increased levels of BDNF in the CNS.24,25 Antioxidants like vitamins E and C may promote the protective effects of BDNF and ciliary neurotrophic factor in nervous tissue.22,26,27 Nevertheless, there is little evidence as to the relationship between iron deficiency and the expression of specific neurotrophic factors other than BDNF.
Studies have shown that iron deficiency may modify IGF-I expression in mice by altering the mTOR signalling pathway, which is regulated by the Akt pathway.28,29 This process decreases signalling mediated by IGF-I and affects neuron proliferation, survival, and myelination.30 When the deficiency is corrected using iron supplements, IGF-I levels may normalise, but this does not occur with IGF-II.30,31 Even though they belong to the same family, they may exhibit different responses ranging from genetic regulation to their effects on cellular homeostasis.
This study shows that iron deficiency increases the expression of IGF-II in primary CNS cell cultures. It should be noted that other studies have demonstrated that BDNF expression decreases when iron deficiency is present,32 which suggests that IGF-II expression may be stimulated when brain tissue experiences stress due to lack of the micronutrient. This promotes a neuroprotective effect medicated by that neurotrophic factor. The increase in IGF-II expression due to iron deficiency was mainly observed in mixed cell cultures, that is, cultures constituted by neurons, astrocytes, microglial cells, oligodendrocytes, and even endothelial cells. This may mean that the different cell populations interact to create a favourable environment in which IGF-II expression can increase in the presence of iron deficiency. The phenomenon is demonstrated by the fact that researchers examining cultures of isolated microglia or neurons were not able to observe iron deficiency-related differences in IGF-II expression. Furthermore, given that most cells obtained from mixed cultures are astrocytes, the increase in IGF-II expression observed in DFe conditions may be due to the activity of this cell population. This possibility is currently under study.
Additional bands identified by the Western blot test, with molecular weights of ∼20, 50, and 70kDa, resemble those described by Walter et al.33; for this reason, they may correspond to IGF-II bound to IGF-binding proteins (IGFBP). Since IGF transport and activity are modulated temporally and locally by these binding proteins,34 the proteins may form complexes with IGFs to regulate their renal clearance, transport the IGFs in vascular compartments, and modulate their interaction with cell-surface receptors.34,35 Some of the most concentrated proteins found in the intact CNS, especially in the choroid plexus and meninges, include IGFBP-2, -4, -5, and -6.36,37 In pathological conditions, however, IGFBP-2, -3, and -6 have been found in cerebrospinal fluid, and they have been linked to IGF-II transport.38,39 This being the case, the protein band with a molecular weight of ∼20kDa may correspond to IGFBP-4, which has been isolated in 2 forms with weights of 24 and 29kDa.40 In the CNS, this protein has been observed in the ependyma, choroid plexus, meninges, and myelinated nerve fibres; if lesions are present, they may also be found in neurons, astrocytes, microglia, and macrophages.41 In turn, the bands identified as weighing about 50kDa or less may correspond to IGFBP-2 or IGFBP-3, which have molecular weights of 32 to 34kDa and 53kDa, respectively. These proteins are present in cerebrospinal fluid,42,43 choroid plexus, cortical nerve fibres, ependyma, and the meninges. Lesions that affect cerebral tissue may result in increased expression of these proteins in neurons, astrocytes, and macrophages.41 IGFBP-2 has a greater affinity for IGF-II than for IGF-I,44 whereas IGFBP-3 has a greater affinity for IGF-I.45 It should be noted that the band with a molecular weight of 70kDa may also correspond to IGFBP-3, since an isoform of that protein with a higher molecular weight also exists.46
Lastly, researchers identified a band with a molecular weight >250kDa; this may correspond to IGF-II bound to its receptor, which has a molecular weight of ∼300kDa.17 In general, additional bands showed increased expression in DFe conditions, except for the 70kDa band whose expression did not vary. If these findings correspond to IGFBP, they may underscore the important roles of these proteins in regulating and modulating IGF functions under both physiological and pathological conditions.
Scientists know that the multiple functions of IGF-II are mainly mediated by 2 types of receptors, IGF-IR and IGF-IIR, which have different affinities.47 IGF-II shows the closest affinity for its specific receptor IGF-IIR, which participates in IGF-II signalling to mediate metabolic response in neurons.16,48 IGF-IIR plays an important role because improving the cognitive functions that depend on IGF-II mainly requires IGF-IIR.13 In contrast to the increase in IGF-II expression observed under DFe conditions, we observed decreased expression of IGF-IIR under the same conditions. Studies have demonstrated that adding IGF-II in vitro induces expression of IGF-IIR on the cell surface of neuron cultures.49,50 This suggests that IGF-II levels modulate the expression of their receptor in CNS cells such that if concentrations of IGF-II drop, concentrations of its specific receptor will increase, and vice versa. In this way, IGF-IIR can compensate for increases or decreases in IGF-II and activate signalling pathways that promote the cell processes involved in CNS growth and development in pathological situations, such as iron deficiency. These results do not coincide with those from other studies of IGF-I and its receptor finding that iron deficiency decreases the expression of IGF-I without altering IGF-IR expression.51
Similarly to Fushimi et al.,49 we detected additional bands with molecular weights of ∼70 and 80kDa. Expression of these proteins did not vary under DFe conditions. However, we did observe an increase in the ∼60kDa band with iron deficiency. Bands with a lower molecular weight may be explained by fragmenting of the receptor during cell lysis, or else lack of specificity of the polyclonal antibody employed in this study.
Lastly, we analysed expression of IGF-I and IGF-IR in mixed glial cell cultures, a decision partially based on the fact that functional binding of IGF-II to cells could be modified by either IGFBP or IGF-IR.47,52 We also compared the response of IGF-II to that of IGF-I (and their respective receptors) under DFe conditions. Like IGF-II, IGF-I is known to promote neuron growth and development and it even displays a protective effect under conditions of hypoxia and hypoglycaemia.53 However, the distributions of these proteins are somewhat different54 even though both are widely distributed in brain tissue. Some reports state that iron deficiency decreases IGF-I levels in plasma.55 Other studies report that this condition affects signalling mediated by IGF-I and therefore has an impact on cell populations,30 and that iron deficiency can even increase expression of this molecule and of its receptor.41 However, our study was unable to record differences in expression of this molecule under DFe conditions; the antibody we used does not enable detection of the band in question, which is expected to have a molecular weight of <10kDa. Analysis of IGF-I expression detected additional bands, with molecular weights of ∼100 and 55kDa, in the mixed culture: ∼100kDa in glial cells and ∼55kDa in neurons. Although these bands may correspond to IGF-I bound to IGFBP, or IGF-I to IGF-IR,45,56 there are no results to confirm this. In any case, no differences in the expression of these proteins were observed between DFe and normal samples.
The ∼100kDa band detected in the analysis of IGF-IR expression may correspond to the IGF-IR beta subunit, which has a molecular weight of 97kDa. We also found another band with a molecular weight of ∼55kDa, which may be the result of the receptor being fragmented during the lysis process. There were no differences in the expression of this protein given DFe and iron-sufficient conditions. It should be noted that results for IGF-I and IGF-IR expression in our study do not coincide with those reported by Tran et al.,30 whose team did observe changes in the expression of these molecules in the presence of iron deficiency.
We should point out that other factors not examined in this study, such as duration of iron deficiency, also have an effect. Iron deficiency is known to be a chronic process that affects the CNS from the earliest stages of development and may still be present in adulthood.51,57 Previous studies have observed that expression of IGF-II, IGF-IIR, and even IGFBP display different responses during acute and chronic phases of the deficiency when a brain lesion is present. During the acute phase, levels of IGF-II, and also of IGFBP-2, -3, and -6, increase in cerebrospinal fluid, whereas their concentrations will drop during the chronic phase.33 Since this study analysed the effect of acute deficiency of this micronutrient on specific cell populations, researchers must now determine the effect of chronic iron deficiency in in vivo studies as well as in cell cultures.
Results from our study indicate that iron deficiency in an in vitro model causes increased expression of IGF-II in primary mixed cultures of CNS cells. The increase is accompanied by decreased expression of the receptor IGF-IIR. The increased expression of this molecule under conditions of iron deficiency may exert a neuroprotective effect and promote homeostasis in nervous tissue under pathological conditions.
FundingThis project was funded by PROMEP, the programme for continuing professor education run by the Mexican Secretariat of Public Education (SEP). EMG received a postgraduate grant from the Seoul National University of Science and Technology (CONACYT).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Morales González E. Efecto de la deficiencia de hierro sobre la expresión de factor de crecimiento de insulina tipo II y su receptor en células neuronales y gliales. Neurología. 2014;29:408–415.
Preliminary results from this study were presented in poster format at the 2013 Experimental Biology Meeting.