Multiple sclerosis (MS) is an autoimmune inflammatory disease of the central nervous system. MS is characterised by nerve demyelination that can alter nerve transmission and lead to such symptoms as fatigue, muscle weakness, and impaired motor function. There are 47000 people with MS in Spain. Vibration training can be an effective and complementary alternative to traditional exercise to treat patients with MS. The aim of this study was to analyse the effectiveness of vibration training programmes in patients with MS.
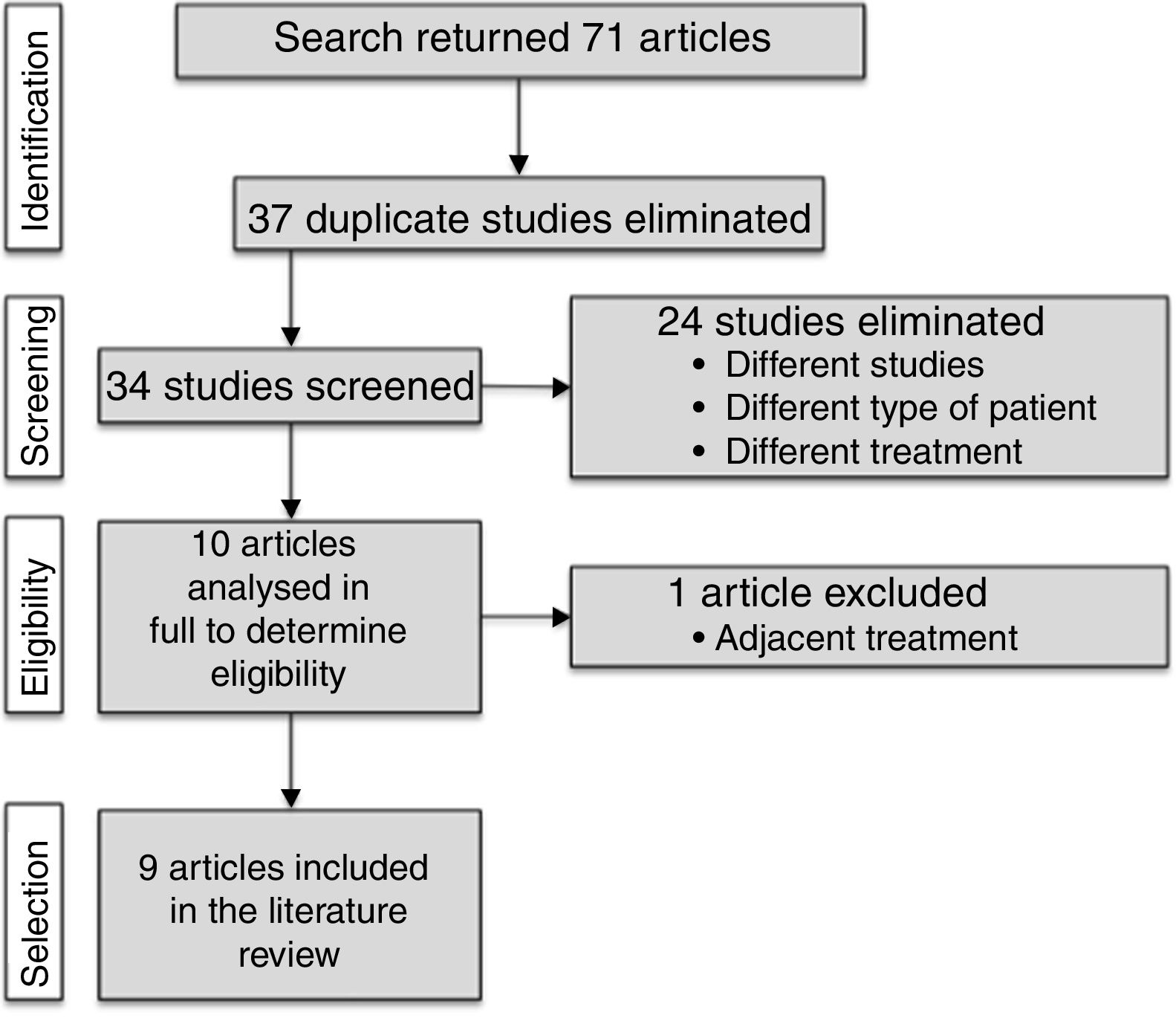
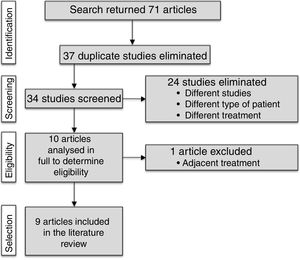
DevelopmentWe searched 5 electronic databases (PubMed, SPORTDiscus, SciELO, Lilacs, IBECS, and ISI Web of Knowledge) in August 2015. By using a set of keywords, we found studies linking vibration training and MS and included randomised controlled trials that applied vibration training to patients with MS. Our search yielded 71 studies. Only 9 of them were included after removing duplicate studies and those which were not relevant according to our selection criteria. These studies obtained different outcomes.
ConclusionsSome studies found improvements in muscle strength, functional capacity, coordination, resistance, balance, and some areas of 88-Item Multiple Sclerosis Spasticity Scale. However, we identified limitations in some of these studies and there are still few publications on vibration training and MS to ensure training effectiveness.
La esclerosis múltiple (EM) es una enfermedad inflamatoria autoinmune del sistema nervioso central. Se caracteriza por la desmielinización del nervio, pudiendo alterar la transmisión nerviosa y conducir a síntomas como fatiga, debilidad muscular y deterioro de la función motora. En España existen 47.000 personas afectadas de EM. El entrenamiento vibratorio puede ser una opción complementaria eficaz al ejercicio tradicional para el tratamiento de la EM. El objetivo fue determinar la efectividad de los programas de entrenamiento vibratorio en los sujetos con EM.
DesarrolloCinco bases de datos electrónicas (PubMed, SPORTDiscus, SciELO, Lilacs, IBECS e ISI Web of Knowledge) fueron consultadas para la búsqueda bibliográfica en agosto del 2015. Un conjunto de términos de búsqueda identificaron estudios que relacionaban el entrenamiento vibratorio y la EM. Se incluyeron ensayos clínicos controlados y aleatorizados que aplicaron un programa de entrenamiento vibratorio dirigido a pacientes con EM. Setenta y un artículos fueron obtenidos tras la búsqueda. Finalmente, se incluyeron 9 de ellos tras descartar los estudios duplicados y aquellos que no fueron relevantes sobre base de los criterios de selección. Se encontraron varios resultados entre los estudios.
ConclusionesAlgunos estudios hallaron mejoras en la fuerza muscular, la capacidad funcional, la coordinación, la resistencia, el equilibrio y algunas áreas del MSSS-88. Sin embargo, detectamos algunas limitaciones entre los estudios y son todavía pocas las publicaciones realizadas hasta la fecha sobre entrenamiento vibratorio y EM para certificar la efectividad de dicho entrenamiento en esta patología.
Multiple sclerosis (MS) is the most frequent neurological disease among young adults, and one of the main causes of disability in this population.1 MS is an autoimmune inflammatory disease of the central nervous system, characterised by demyelination due to inflammation and progressive degeneration of the myelin sheaths enveloping nerves of the eye, brain, periventricular grey matter, brainstem, and spinal cord.2–5 The process can cause the formation of multiple plaques (scleroses) in the white matter of the brain and spinal cord, which can become permanent scars that cause alterations in nerve transmission,6,7 leading to such symptoms as fatigue, muscle weakness, and motor function alterations.3,8
MS prevalence is high in developed countries9; 47000 people are affected in Spain, 600000 in Europe, and over 2000000 in the world.10 The condition is usually diagnosed in patients aged between 20 and 50 years. Women are affected far more frequently than men, and account for approximately two-thirds of cases.9,10 The disease is currently the leading cause of neurological disability in young adults in developed countries, and its incidence rate is increasing.9 The aetiology of MS is unknown, and is usually complex11 and multifactorial, potentially resulting from an interaction between genetic, infectious, and environmental factors.3,9,11–21
The symptoms of MS mean that patients are usually sedentary and generally have lower levels of physical activity than other individuals. This can result in muscle weakness, decreased bone density, poorer cardiovascular health, and higher levels of fatigue.3
Although MS is incurable, symptoms may be addressed with physical exercise, which can help maintain and improve balance, mobility, quality of life, and autonomy in the activities of daily living.3 Physical activity improves impaired bladder and bowel function in patients with MS and can have positive effects on mental health, quality of life,22,23 muscle strength,24 symptomatic fatigue, and other symptoms.25 It may also improve risk factors for cardiovascular or metabolic disorders.26
Patients with MS who partake in aerobic exercise have a lower risk of relapse27 and display improvements in their symptoms.25 Resistance exercise is essential to improving these patients’ functional capacity (mobility, independence, everyday tasks, etc.).3 Strength training increases isometric28 and dynamic strength24 by means of neural adaptation (in the short term) and muscle hypertrophy (in the long term).29 Patients can also achieve functional improvements including greater walking speed30 and muscular endurance,24 decreased symptomatic fatigue,28 and improved balance31 and gait kinematics.32 Finally, flexibility training has been observed to counteract spasticity, reduce or prevent contractures, increase muscle length and range of motion, and to improve posture and balance; all these parameters are affected by MS.3
Of the different training systems studied with patients with MS, vibration training may improve physical function and reduce some signs and symptoms of the disease. This type of training also reduces financial costs and patient fatigue,33 which is of great importance given that fatigue is the main factor limiting physical exercise in this population. Vibration training is based mainly on the transmission of a vibratory stimulus through the body using a vibration platform. This activates a series of sensory receptors, particularly muscle spindles, which are activated by stretching; these receptors cause reflex activation of alpha motor neurons, leading to a tonic reflex which in turn triggers a reflex muscle contraction.34,35 This results in increased muscle strength and improved function, as shown by various recent studies into vibration training in people without MS, which have demonstrated significant gains in strength36,37 and a reduced risk of falling in older people.38 Some studies have also observed increased flexibility of the hamstring muscles in physically active adults after a vibration training programme39; this may have a positive impact on gait. Patients with MS usually display limited mobility, which may reduce their ability to perform the activities of daily living.3 However, evidence of the effectiveness of vibration training on mobility in patients with MS is somewhat limited. This review analyses the available evidence on the effect of vibration training on these patients. We also describe the variables measured and compare the vibration training protocols used in the studies selected.
DevelopmentThe study methodology was developed according to the recommendations of the PRISMA statement.40
SourcesThe literature search was carried out in August 2015 and used the following strategies: (1) (“multiple sclerosis” [Mesh]) AND (“vibration treatment” OR “whole-body vibration” OR “whole body vibration” OR “vibration therapy” OR “vibrotherapy” OR “vibration training” OR “vibration exercise”), and (2) (“multiple sclerosis”) AND (“vibration treatment” OR “whole-body vibration” OR “whole body vibration” OR “vibration therapy” OR “vibrotherapy” OR “vibration training” OR “vibration exercise”).
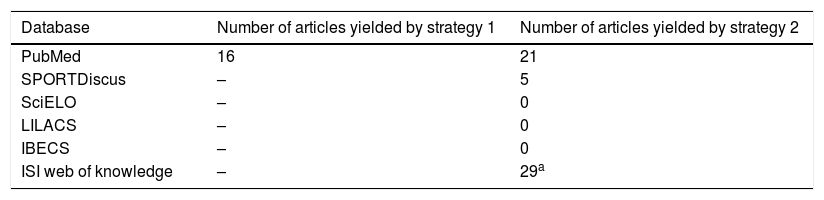
Table 1 shows the databases searched and the number of relevant papers found.
Studies gathered with each search strategy.
| Database | Number of articles yielded by strategy 1 | Number of articles yielded by strategy 2 |
|---|---|---|
| PubMed | 16 | 21 |
| SPORTDiscus | – | 5 |
| SciELO | – | 0 |
| LILACS | – | 0 |
| IBECS | – | 0 |
| ISI web of knowledge | – | 29a |
The studies selected for review were randomised controlled trials (considered the most reliable form of scientific evidence) with samples of patients diagnosed with MS who underwent whole-body vibration (WBV) training. In order to prevent potential biases, we excluded studies in which the participants performed some kind of physical training (aiming to improve a specific basic physical capacity) in addition to vibration training, either as part of the study or on their own initiative.
ResultsThe literature search yielded 71 articles. After reviewing titles and abstracts, we discarded 37 duplicate articles and 24 further studies which did not meet the inclusion criteria. We reviewed the full texts of the remaining 10 articles, excluding one; a final total of 9 articles was therefore included in the systematic review. This selection process is displayed in Fig. 1.
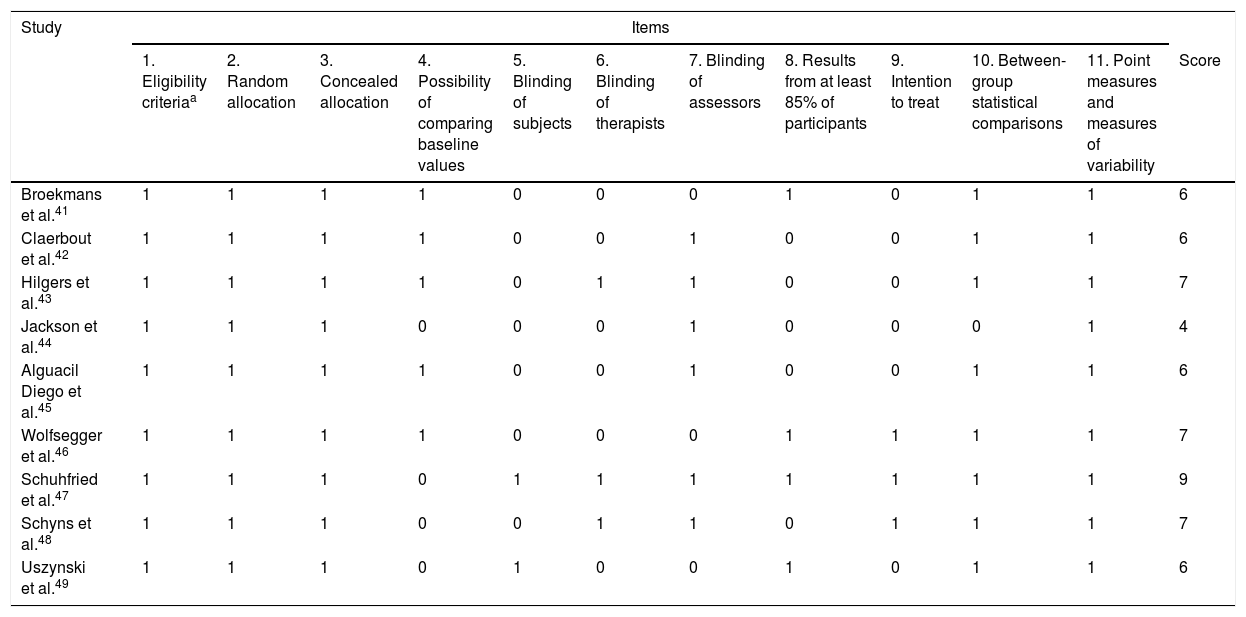
The PEDro scale was used to evaluate the methodological quality of the articles selected. Articles scored one point for each quality indicator met. Table 2 shows the PEDro scale scores of the different studies.
Evaluation of the studies’ methodological quality according to the PEDro scale.
| Study | Items | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Eligibility criteriaa | 2. Random allocation | 3. Concealed allocation | 4. Possibility of comparing baseline values | 5. Blinding of subjects | 6. Blinding of therapists | 7. Blinding of assessors | 8. Results from at least 85% of participants | 9. Intention to treat | 10. Between-group statistical comparisons | 11. Point measures and measures of variability | Score | |
| Broekmans et al.41 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Claerbout et al.42 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Hilgers et al.43 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 7 |
| Jackson et al.44 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 4 |
| Alguacil Diego et al.45 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Wolfsegger et al.46 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Schuhfried et al.47 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Schyns et al.48 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Uszynski et al.49 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
Table 3 summarises the 9 studies selected.41–49 Two studies used general rehabilitation programmes in addition to the vibration training programme, but were included in the review as these programmes were considered not to constitute physical training.42,43
Summary of the studies included in our review article.
| Reference | Sample | Objective | Pre- and post-training variables and measurements | Training protocol | Results | Conclusion |
|---|---|---|---|---|---|---|
| Alguacil Diego et al.45 (2012) | 32 patients with mild to moderate MS (EDSS: 4.1); age: 43±6 years; EG=17 (8 women and 9 men); CG=15 (8 women and 7 men) | To evaluate the effects of WBV on postural control, functionality, and fatigue in patients with MS | TUG1 for dynamic balance and functional mobility; BBS for balance; T10M for walking speed; FSS for fatigue; CDP (SOT1, SOT2, SOT3, SOT4, SOT5, SOT6, MCT-LAT) for postural control and balance; COMP as a measure of global balance; and ST as a measure of postural strategy | Training on 5 consecutive days; 1 daily session comprising 5 sets of 1 minute exercise followed by 1 minute rest; frequency: 6Hz; amplitude: 3mm; total duration of daily intervention: 10minutes | EG displayed significant improvements in SOT1, SOT3, and LAT scores and a trend towards significance for TUG1 score. MCT showed significant differences between groups, with EG showing reduced LAT. No significant differences in SOT, BBS, FSS, TUG1, or T10M results | WBV was useful for balance control but did not affect functionality or fatigue (possibly due to the short duration of the programme) |
| Broekmans et al.41 (2010) | 25 patients with mild to moderate MS (EDSS: 4.3±0.2); age: 47.9±1.9 years; EG=11 (7 women and 4 men); CG=14 (11 women and 3 men) | To determine the effects of a WBV programme on leg muscle performance and function in patients with MS | ID for MIS of knee flexion and extension, MDS, MSE, and knee extension MSM; BBS, TUG1, 2MWT, and T25FW were used to measure functional capacity. Measurements were taken before, during (at 10 weeks), and after training (at 20 weeks) | Five sessions every 2 weeks. Over the 20-week study period, vibration duration systematically increased from 2.5 to 16.5minutes (from 1 to 3 sets per exercise, from 2 to 5 exercises, and from 30 to 60seconds vibration without rest), frequency increased from 20 to 45Hz, and rests between exercises decreased from 120 to 30seconds; vibration amplitude was constant at 2.5mm | No significant intergroup differences were observed in MIS of knee extension and flexion, MDS, MSE, or knee extension MSM. No functional capacity improvements were detected in BBS, TUG1, 2MWT, or T25FW. However, CG displayed significantly lower MIS at the end of the programme | WBV is safe but probably does not improve leg muscle function or performance |
| Claerbout et al.42 (2012) | 55 patients with MS (EDSS: 5.5); EG1 (WBV-full)=20 (6 women and 14 men) (age: 39.1±8.2 years); EG2 (WBV-light)=18 (4 women and 14 men) (age: 43.8±12.6 years); CG=17 (11 women and 6 men) (age: 47.6±8.3 years) | To evaluate the effects of an exercise programme performed on a vibration platform on strength and functional mobility in patients with MS undergoing a multidisciplinary rehabilitation programme | Handheld dynamometer to measure maximal strength of the tibialis anterior, quadriceps, hamstrings, and gluteus medius; 3MWT, TUG1, and BBS to measure functional mobility | Training programme comprising 10 sessions over 3 weeks, with exercise duration increasing from 30 to 60seconds, rest between exercises increasing from 30 to 60seconds, and frequency increasing from 30 to 40Hz; amplitude was constant at 1.6mm. Damping mats of 2 and 10cm thickness were used as the contact surface for the WBV-full and WBV-light groups, respectively. Total training duration: 7-13minutes | Significant increases in strength were observed in all muscles analysed. Only the WBV-full group displayed significant strength improvements in the quadriceps and hamstrings, with a trend towards significance for improvements in gluteus medius strength. Significant increases were observed in scores for all functional tests, but no significant interaction effects were attributable to the programme | A 3-week programme of exercises performed on a vibration platform significantly improved muscle strength but not functionality in patients with MS |
| Hilgers et al.43 (2013) | 60 patients with mild to moderate MS (EDSS: 3.3±1.5); age: 43.3±8.3 years; EG=30 (22 women and 8 men); CG=30 (23 women and 7 men) | To determine the effects of WBV on physical function and walking capacity in patients with MS undergoing a standard rehabilitation programme | SST to measure strength; TUG2 for coordination; T10M for walking speed; 6MWT for endurance | WBV 3 times per week for 3 weeks; 3 sets per session of 60seconds exercise with rests of 30seconds (first 3 sessions) or 5seconds (all other sessions) between sets; frequency of 30Hz; amplitude of 1mm (first 6 sessions) or 2mm (last 3 sessions). CG followed the same protocol with the platform disconnected. Maximum session duration: 12minutes | The only variable related to walking capacity in which a significant improvement was observed was the 6MWT, in which EG patients could walk 4.5 times further than those in the CG. Both groups displayed significant improvements in walking capacity in the SST, TUG2, T10M, and 6MWT | Vibration training improves endurance-related determinants of walking capacity in patients with MS |
| Jackson et al.44 (2008) | 15 patients with MS (EDSS: 4.2±2.3); age: 54.6±9.6 years; 12 women and 3 men | To determine the acute effects of high- and low-frequency WBV on quadriceps and hamstring muscle performance | ID to measure MIT of the quadriceps and hamstring muscles after 1, 10, and 20minutes of vibration | Participants were randomly allocated to receive either 2-Hz or 26-Hz vibration in the first session, and received the other frequency in the second session. Vibration duration was 30seconds; amplitude was 6mm | No statistically significant differences were observed between MIT scores at baseline and at 1, 10, or 20minutes after WBV, for either frequency or muscle group. A significant increase was observed in quadriceps MIT between minutes 1 and 10 after WBV at both frequencies. The higher frequency caused higher torque responses at all time points, with a non-significant increase in torque production for at least 20minutes after WBV | It is as yet unclear whether WBV is a viable option as a neuromuscular warm-up or as a training exercise for patients with MS |
| Wolfsegger et al.46 (2014) | 17 patients with MS. EG=9 (8 women and 1 man); EDSS: 2.5±1.0; age: 43.0±13.4 years. CG=8 (7 women and 1 man); EDSS: 2.4±0.8; age: 39.3±10.6 years. 1 dropout | To investigate the effect of vibration training on gait function in patients with MS | TUG1 to evaluate functional mobility; plantar pressure measurement system to assess walking speed, stride length, double support phase, and step variability under 4 different conditions: comfortable overground gait, comfortable gait on treadmill, comfortable gait (reduced velocity) on treadmill, and comfortable gait (increased velocity) on treadmill. Measurements were taken before and after WBV and 2 weeks after the exercise programme ended | 3-week WBV programme; 1 session per week; gradual increase of volume and intensity from 5 to 7 sets per session and 45-60seconds per set; frequency increasing from 2.5 to 5Hz; rest duration decreasing from 60 to 30seconds | No significant improvements were observed in the gait variables studied or in functional mobility | This vibration training protocol had no effect on gait function or functional capacity in patients with moderate MS |
| Schuhfried et al.47 (2005) | 12 patients with MS. EG=6 (5 women and 1 man); EDSS: 3.9±0.8; age: 49.3±13.3 years. CG=6 (4 women and 2 men); EDSS: 3.7±0.8; age: 46±12.7 years | To test the effectiveness of vibration training for improving postural control, balance, and mobility in patients with MS | Dynamic posturography using the SOT to measure postural control; TUG1 to assess functional mobility; Functional Reach Test to measure standing balance. Measurements were taken before and 15minutes, 1 week, and 2 weeks after training | Single session of 9minutes; amplitude of 3mm; frequency of 2-4.4Hz (increasing according to patient tolerance). 5 sets of 1 minute exercise followed by 1-minute rest. CG performed the same exercise on the platform, but received transcutaneous electrical nerve stimulation simulating vibration | EG displayed significant improvements over CG in TUG1 scores 1 week after treatment | Vibration training may have a positive effect on postural control and mobility in patients with MS |
| Schyns et al.48 (2009) | 16 patients with MS (HAI: 1-6). Group 1=8 (5 women and 3 men); age: 45.8±8.4 years. Group 2=8 (7 women and 1 man); age: 49.5±6.14 years. 4 dropouts | To examine the effectiveness of vibration training on muscle strength and tone, sensitivity, and functional performance in patients with MS | MAS to assess muscle tone of the adductors, quadriceps, hamstrings, and calf muscles; MSSS-88 for self-perceived impact of MS on abnormal muscle tone; handheld dynamometer for isometric strength production of the hip flexors, extensors, adductors, and abductors and the quadriceps, hamstrings, and ankle dorsiflexors; NSA for knee, ankle, and foot sensitivity and proprioception; T10M for gait performance; TUG1 for functional balance; MSIS-29 for health-related quality of life. Measurements were taken after each 4-week training period and each 2-week rest period. | Group 1 underwent 3 weekly sessions of vibration training for 4 weeks, a 2-week rest period, 4 weeks of the same exercise protocol but without vibration, then a final 2-week rest period. Vibration frequency of 40Hz; exercise duration of 30seconds. Group 2 followed the same programme in reverse order. | Group 1 displayed significant improvements in the pain section of the MSSS-88. Group 2 displayed no improvements in the muscle spasms area of the MSSS-88. However, comparison between groups using the Wilcoxon signed-ranks test did reveal an improvement, with a lower score for spasms in patients receiving vibration plus exercise vs patients receiving exercise alone. | Exercise may be beneficial for patients with MS, although evidence is limited as to whether the addition of vibration training leads to additional improvements |
| Uszynski et al.49 (2015) | 27 patients with MS (GNDS: 0-3). EG=14 (10 women and 4 men); age: 45.5 years. CG=13 (100% women); age: 54 years. 3 dropouts (analysis: 12 per group) | To obtain data and to study the feasibility of a larger randomised controlled trial to determine whether vibration training is more effective than standard exercise of the same duration and intensity | ID to assess isokinetic strength in knee flexion and extension at 90, 180, and 300°/seconds; neurotensiometer for vibration perception threshold in the big toe, the first and fifth metatarsophalangeal joints, and the heel; VAS for self-assessed sensation of numbness, cramps, or tingling or burning sensation in the hands and feet; TUG1 for dynamic balance; 6MWT for walking endurance; Mini-Balance Evaluation Systems Test for balance; MSIS-29 to assess the physical and psychological impact of MS; MFIS to assess the effect of fatigue on physical, cognitive, and psychosocial function | 3 sessions per week for 12 weeks. Warm-ups and cool-downs; 3 sets of exercise at a frequency of 40Hz with 10seconds of rest between sets; amplitude of 0-10mm. The overload principle was used to progress the exercise. CG performed the same exercises without vibration | Statistically significant improvements were seen in the vibration perception threshold in the fifth metatarsal joint and heel in the CG (perhaps due to higher baseline values in the EG) | The protocol is viable; no adverse effects were observed. A clinical trial into the effect of adding vibration training to another therapy is feasible. A sample of 120 patients would be necessary to detect functional benefits; 400 participants would be necessary to detect effects on muscle strength |
2MWT: 2-Minute Walk Test; 3MWT: 3-Minute Walk Test; 6MWT: 6-Minute Walk Test; BBS: Berg Balance Scale; CDP: computerised dynamic posturography; CG: control group; COMP: SOT composite score; EDSS: Expanded Disability Status Scale; EG: experimental group; FSS: Krupp Fatigue Severity Scale; GNDS: Guys Neurological Disability Scale; HAI: Hauser Ambulation Index; ID: isokinetic dynamometer; LAT: latency; MAS: Modified Ashworth Scale; MCT: motor control test; MDS: maximal dynamic strength; MFIS: Modified Fatigue Impact Scale; MIS: maximal isometric strength; MIT: maximal isometric torque; MS: multiple sclerosis; MSE: maximal strength endurance; MSIS-29: Multiple Sclerosis Impact Scale; MSM: maximum speed of movement; MSSS-88: 88-Item Multiple Sclerosis Spasticity Scale; NSA: Nottingham Sensory Assessment; SOT: sensory organisation test; SOT1: SOT condition 1 (eyes open, fixed surface and visual surround); SOT2: SOT condition 2 (eyes closed, fixed surface); SOT3: SOT condition 3 (eyes open, fixed surface, sway-referenced visual surround); SOT4: SOT condition 4 (eyes open, sway-referenced surface, fixed visual surround); SOT5: SOT condition 5 (eyes closed, sway-referenced surface); SOT6: SOT condition 6 (eyes open, sway-referenced surface and visual surround); SST: Sit-to-Stand Test; ST: SOT strategy analysis score; T10M: Timed 10-Metre Walk Test; T25FW: Timed 25-Foot Walk test; TUG1: Timed Up and Go test (stand, walk 3m in a straight line, and sit down); TUG2: Timed Up and Go test (stand and walk 5m); VAS: Verbal Analogue Scales; WBV: whole-body vibration.
Table 3 also shows the number, sex, age, and level of disability of the patients included in each study. The selected studies explore the effects of vibration training in people diagnosed with MS.41–45 The majority of studies use the Expanded Disability Status Scale to measure patients’ level of disability.41–47 This scale scores patients’ level of disability from 0 (no disability) to 10 (death due to MS). Depending on the study, mean scores vary between 2.5 and 5.5, indicating minimal to severe disability.41–46 Sample sizes range from as few as 12 patients47 to a maximum of 60 patients.43 Patients’ ages ranged from 39.1 to 54.6 years.42,44 The majority of patients completed the exercise programmes; however, patient dropout was reported in some studies.41,42,45,46,48,49 Alguacil Diego et al.45 report 2 patients dropping out of the programme: one from the control group and another from the experimental group. Two controls in the study by Broekmans et al.41 did not complete the programme. Wolfsegger et al.,46 Schyns et al.,48 and Uszynski et al.49 note 1, 4, and 3 dropouts in their exercise programmes, respectively. Finally, Claerbout et al.42 report 8 dropouts: 4 in the WBV-full group and 4 in the WBV-light group.
Six studies measured participants’ strength.41–44,48,49 Isokinetic dynamometers were used in 3 cases.41,44,49 Broekmans et al.41 measured knee flexion and extension maximal isometric torque (at 45° and 90°), maximal dynamic torque (at a velocity of 60°/second), maximal strength endurance (180°/second), and maximal speed of movement of knee extension. Jackson et al.44 measured quadriceps and hamstring maximal isometric torque (60° of knee flexion). Uszynski et al.49 measured isokinetic muscle strength for knee flexion and extension at 90, 180, and 300°/second. Claerbout et al.42 measured muscle strength of the tibialis anterior, quadriceps, hamstrings, and gluteus medius using a handheld dynamometer. Schyns et al.48 used the same instrument to measure the isometric strength of the hip flexors, extensors, adductors, and abductors; the quadriceps; the hamstrings; and the ankle dorsiflexors. Finally, Hilgers et al.43 measured strength using the Sit-to-Stand Test.
Five studies assessed participants’ functional mobility.41,42,45–47 Alguacil Diego et al.,45 Schuhfried et al.,47 and Wolfsegger et al.46 used the Timed Up and Go1 (TUG1) test; Broekmans et al.41 used the Berg Balance Scale (BBS), the TUG1, the Two-Minute Walk Test, and the Timed-25-Foot Walk; and Claerbout et al.42 used the TUG1, the Three-Minute Walk Test, and the BBS.
Various studies specifically measured parameters related to dynamic balance and postural control.45,48,49 Uszynski et al.49 and Schyns et al.48 assessed balance using the TUG1 and Alguacil Diego et al.45 measured balance and postural control with the BBS and with computerised dynamic posturography (using the Sensory Organisation Test), in addition to using the TUG1 to measure dynamic balance. Schuhfried et al.47 also used posturography (Sensory Organisation Test) to measure postural control, as well as measuring standing balance with the Functional Reach Test. Uszynski et al.49 used the Mini-Balance Evaluation Systems Test to measure balance.
Alguacil Diego et al.,45 Hilgers et al.,43 and Schyns et al.48 assessed walking speed and performance using the 10-Metre Walk test. Wolfsegger et al.46 used a mobile plantar pressure measurement system to assess walking speed, stride length, the double support phase, and step variability under 4 different conditions: comfortable overground gait, comfortable gait on treadmill, comfortable gait (reduced velocity) on treadmill, and comfortable gait (increased velocity) on treadmill.
Alguacil Diego et al.45 were the only researchers to evaluate fatigue using the Krupp Fatigue Severity Scale. Only 2 studies assessed patients’ coordination.43,45 Hilgers et al.43 measured this variable with the Timed Up and Go2 (TUG2) test, whereas Alguacil Diego et al.45 performed posturographic analysis using the Motor Control Test.
Hilgers et al.43 and Uszynski et al.49 used the Six-Minute Walk Test to evaluate endurance. Other important parameters were also assessed. Schyns et al.48 measured the self-perceived impact of MS on muscle tone alterations using the 88-Item Multiple Sclerosis Spasticity Scale (MSSS-88). The researchers also measured the muscle tone of the adductors, quadriceps, hamstrings, and calf muscles using the Modified Ashworth Scale, as well as knee, ankle, and foot sensitivity and proprioception. Uszynski et al.49 and Schyns et al.48 assessed the physical and psychological impact of MS and health-related quality of life using the Multiple Sclerosis Impact Scale. Uszynski et al.49 used a neurotensiometer to measure the vibration perception threshold in the big toe, first and fifth metatarsophalangeal joints, and the heel. They also used verbal analogue scales to assess patients’ subjective sensation of reduced sensitivity, cramps, and burning or tingling sensations in the hands and feet, and the Modified Impact Scale to measure the effect of fatigue on physical, cognitive, and psychosocial function.
One variable affecting vibration dose is the type of exercise performed on the platform. Each study used a different exercise programme.41–49 In the study by Alguacil Diego et al.,45 patients were asked to hold a semi-squat position on the vibration platform. The training used in the study by Hilgers et al.43 consisted in maintaining a moderate squat position on the platform. Wolfsegger et al.46 and Schuhfried et al.47 asked patients to stand on the platform in a squat position with slight flexion of the hip, knee, and ankle joints. Jackson et al.44 used a similar exercise, asking participants to stand almost completely straight on the vibration platform with their feet separated by 13cm and a knee flexion angle of approximately 25°, with body weight resting mainly on the balls of the feet, without raising the heels (to minimise vibration of the head). The purpose of the standardised distance between the feet was to ensure that each patient received the same vibration amplitude; this is determined by the separation of the feet when a rotational vibration unit is used. Broekmans et al.41 used a training programme including high and deep squats, wide stance squats, lunges, and heel rises. These exercises were performed statically, dynamically, and unilaterally. Similarly, Claerbout et al.42 developed an exercise programme including squats: static left- and right-leg unipodal and bipodal squats, dynamic squats, toe-stands, and lunges. Schyns et al.48 and Uszynski et al.49 used a range of exercises on the vibration platform: static and dynamic squats, dynamic calf raises, static lunges, one-leg standing, and steps up and down. Table 3 shows training intensity and volume.
DiscussionThe aim of this review was to analyse the content of the most relevant studies into vibration training and its effects on patients with MS. Claerbout et al.42 observe significant time-related strength improvements in all the muscles analysed, although the main finding attributable to the exercise programme was the significant improvement in quadriceps and hamstring muscle strength: these improvements were observed only in the group that trained with a 2-cm damping mat covering the vibration platform. They also report a trend towards significance in the gluteus medius improvements. Jackson et al.44 did not find significant differences for maximal isometric torque between baseline values and measurements taken 1, 10, or 20minutes after vibration training for either frequency (2 or 26Hz) or muscle group. However, there was a significant time-related improvement in quadriceps maximal isometric torque, which increased significantly between minutes 1 and 10 after vibration training at both frequencies. Broekmans et al.41 also report no significant intergroup differences in maximal isometric strength in knee extensions or flexions; neither did the vibration training programme improve any of the values related to maximal dynamic strength, maximal strength endurance, or maximal speed of movement of knee extension. However, the control group displayed significantly lower maximal isometric strength in knee flexions at the end of the programme. Hilgers et al.43 found significant time-related strength improvements in the Sit-to-Stand Test, but no group-time interaction attributable to the training. Schyns et al.48 found no significant isometric strength improvements in the hip flexors, extensors, adductors, or abductors; the quadriceps; the hamstrings; or the ankle dorsiflexors. Uszynski et al.49 do not report any knee flexion or extension isokinetic strength improvements attributable to the programme.
The study by Schuhfried et al.47 is the only one reporting significant differences between groups in functional capacity, measured with the TUG1 one week after intervention. However, Alguacil Diego et al.45 found only a trend towards statistical significance for improvements in TUG1 scores attributable to the programme. Wolfsegger et al.46 report no improvements in functional mobility, measured with the same test. Likewise, Broekmans et al.41 detected no improvements in functional capacity in the BBS, TUG1, Two-Minute Walk Test, or Timed 25-Foot Walk Test. Claerbout et al.42 report significant increases for all functional tests, but no interaction effects attributable to the programme.
Alguacil Diego et al.45 found no significant improvements attributable to the programme in the Sensory Organisation Test, the TUG1, or the BBS for balance and postural control. However, they did find significant intergroup differences in the Motor Control Test: the experimental group displayed significant reductions in latency, which had a positive effect on balance control. The experimental group also displayed significant time-related improvements in Sensory Organisation Test conditions 1 (fixed platform with open eyes) and 3 (fixed platform with sway-referenced vision) and latency. Uszynski et al.49 did not find any significant improvements in TUG1 scores for dynamic balance. Schyns et al.48 used the same test, finding no significant improvements in functional balance.
The only evidence of a significant change in walking endurance was reported by Hilgers et al.43 in the Six-Minute Walk Test results; these researchers found that members of the experimental group were able to walk 4.5 times further than controls. Significant improvements over baseline values were observed in both groups in this test.
No improvements were recorded for walking speed. Although Hilgers et al.43 found significant improvements in the 10-Metre Walk Test in both groups, no significant intergroup differences were observed; Alguacil Diego et al.45 report similar findings. Finally, Alguacil Diego et al.45 report significant improvements in scores in the Krupp Fatigue Severity Scale; this finding is noteworthy as fatigue is an important determinant of functional capacity for patients with MS.
Claerbout et al.42 conclude that a 3-week programme of exercise on a vibration platform is associated with a significant improvement in muscle strength. Broekmans et al.,41 however, state that while the therapy is safe, it probably does not improve leg muscle performance. Similarly, Jackson et al.44 conclude that it is as yet unclear whether vibration training is a viable option as a neuromuscular warm-up or as a training exercise for patients with MS. Regarding functionality, Schuhfried et al.47 achieved significant improvements in functional capacity. However, Alguacil Diego et al.,45 Broekmans et al.,41 and Claerbout et al.42 conclude that vibration training does not affect functionality in people with MS, although it is beneficial for coordination and balance control45 and can improve endurance-related determinants of walking ability.43 One study argues that vibration training does not influence fatigue, possibly due to the programme's short duration.45 Finally, Schyns et al.48 report significant improvements in the pain component of the MSSS-88 in patients receiving 4 weeks of WBV plus exercise 3 times per week, 2 weeks of no intervention, then 4 weeks of exercise alone 3 times per week. The muscle spasms component of the MSSS-88 showed no evidence of improvement for another experimental group receiving the same treatments in the reverse order. However, comparison between groups, using the Wilcoxon signed-ranks test, did reveal an improvement, with patients receiving vibration plus exercise scoring lower for muscle spasms than patients receiving exercise alone. Research into the effect of vibration training on patients with MS continues to be scarce. Future research should explore the effectiveness of this training, the most effective combination of vibration dose parameters (type of exercise, duration, density, and intensity), and its long-term effect on these individuals. Table 4 summarises the protocols used and the beneficial effects found in the studies selected for review.
Summary of the protocols obtaining favourable results.
| Reference | Protocol | Benefit |
|---|---|---|
| Claerbout et al.42 | Static unipodal and bipedal squats, dynamic squats, toe-stands, and lunges on a vibration platform. 10 sessions over 3 weeks; exercise duration of 30-60seconds; rest of 30-60seconds between exercises; frequency of 30-60Hz; amplitude of 1.6mm; 2cm damping mat | Strength |
| Alguacil Diego et al.45 | Semi-squat on the vibration platform. 5 consecutive days; 5 sets of 1minute exercise followed by 1minute rest; frequency of 6Hz; amplitude of 3mm | Coordination and balance |
| Hilgers et al.43 | Static moderate squat position on the vibration platform. 3 weekly sessions for 3 weeks; 3 sets of 60seconds exercise followed by 5-30seconds rest; frequency of 30Hz; amplitude of 1mm (first 6 sessions) or 2mm (last 3 sessions) | Endurance |
| Schuhfried et al.47 | Squat position with slight flexion of the hip, knee, and ankle joints. Single 9-minute session; 5 sets of 1minute exercise followed by 1-minute rest; frequency of 2-4.4Hz (increasing according to endurance); amplitude of 3mm | Functional mobility |
| Schyns et al.48 | 10 lower limb strength exercises and stretches. 3 weekly sessions over 4 weeks; exercise duration of 30seconds; frequency of 40Hz. After a 2-week rest period, participants received a further 4 weeks on the same exercise protocol without vibration, followed by a final 2-week rest period | Pain and spasms |
We would emphasise that some studies examine the effect of vibration on other diseases that have an effect on the respiratory system.50–52 In patients with MS, positive effects have been observed in such areas as strength, coordination, balance, endurance, functional mobility, and pain and spasms. The studies reporting positive results vary greatly in terms of the training programmes used. Nonetheless, the following recommendations can be made: 3-5 weekly training sessions for 3-5 weeks; exercises mainly focusing on the lower body (squats and/or lunges); exercises lasting 30-60seconds with breaks of 30-60seconds (fatigue should always be monitored during training); amplitude of 1-2mm; frequencies of 2-6Hz to improve mobility and coordination and 30-40Hz to improve strength and endurance and to reduce pain.
ConclusionsWBV is beneficial for muscle strength, functional capacity, coordination, endurance, balance, and some areas of the MSSS-88. Strength and functional mobility and capacity were the variables most commonly addressed in the studies reviewed. The next most frequently measured variables were walking speed and coordination. Finally, some studies addressed balance and postural control, endurance, and fatigue. The different studies employ different protocols with different vibration doses. We have identified some limitations in the protocol designs of some studies, including the absence of long-term follow-up, relatively unsophisticated measurements, and the short duration of training programmes.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Bueno IC, Ramos-Campo DJ, Rubio-Arias JA. Efectos del entrenamiento vibratorio de cuerpo completo en pacientes con esclerosis múltiple: una revisión sistemática. Neurología. 2018;33:534–548.