Parkinson disease (PD) has no specific neuropsychological scales for assessing the most significant cognitive impairment in PD, which has determined the use of subjective criteria or instruments designed for other diseases, making difficult the comparison between studies or the follow-up of patients. A screening test for dementia in PD (Parkinson's Disease Dementia-Short Screen [PDD-SS]) has recently been validated. To assess the degree of satisfaction of patients and researchers through the use of PDD-SS in clinical practice.
Patients and methodsAn observational, cross-over, multicentre and national study was conducted on 471 patients with PD. The degree of patient satisfaction was measured using a questionnaire in which the items scored from 0 to 10 on a visual analogue scale (0=strongly disagree, 10=completely agree), while the researchers were determined on a 1–5 point Likert scale (1=strongly disagree, 5=completely agree).
ResultsA total of 171 patients (36.3%) patients had dementia associated with PD according to the PDD-SS, of whom 77.3% said they were satisfied with its use. The overall measurement of researcher satisfaction was 3.6±0.6 points. Ninety percent (n=45) of them reported an overall score >3 points in the satisfaction questionnaire. The mean values of perception of applicability, usability and reliability of PDD-SS among researchers was 3.5±0.7, 3.7±0.6 and 3.1±0.5 points, respectively.
ConclusionsPD patients, as well as most of the researchers, were satisfied with the use of PDD-SS in clinical practice.
La enfermedad de Parkinson (EP) carece de escalas neuropsicológicas específicas para valorar las alteraciones cognitivas más relevantes en la EP, lo que ha condicionado la utilización de criterios subjetivos o de instrumentos diseñados para otras patologías, dificultando la comparación entre estudios o el seguimiento de los pacientes. Recientemente se ha validado un test de cribado para la demencia en la EP (Parkinson's Disease Dementia-Short Screen [PDD-SS]). Evaluar el grado de satisfacción de los pacientes e investigadores con el uso del PDD-SS en la práctica clínica habitual.
Pacientes y métodosEstudio observacional, transversal, multicéntrico y nacional en 471 pacientes con EP. El grado de satisfacción de los pacientes se midió mediante un cuestionario cuyos ítems puntuaban según una escala analógica-visual de 0–10 puntos (0=totalmente en desacuerdo, 10=completamente de acuerdo), mientras que en los investigadores se determinó según una escala Likert de 1–5 puntos (1=totalmente en desacuerdo, 5=completamente de acuerdo).
ResultadosEl 36,3% (n=171) de los pacientes presentó demencia asociada a EP según el PDD-SS, considerándose un 77,3% satisfechos con su uso. La satisfacción medida total de los investigadores fue de 3,6±0,6 puntos. El 90% (n=45) de ellos reportó una puntuación global>3 puntos en el cuestionario de satisfacción. La valoración media de la percepción de aplicabilidad, manejabilidad y fiabilidad del PDD-SS entre los investigadores fue de 3,5±0,7; 3,7±0,6 y 3,1±0,5 puntos, respectivamente.
ConclusiónLos pacientes con EP, así como la mayoría de los investigadores se mostraron satisfechos con el uso del PDD-SS en la práctica clínica habitual.
Parkinson's disease (PD) is a neurodegenerative disorder that affects some 3 million patients in Europe and North America and is characterized by a basic phenotype of motor deficits (akinesia, rigidity, tremor, and postural alterations) together with other neurological complications that develop over the long course of the disease.1 Among the most important of these complications are the cognitive, affective and behavioural alterations that can appear even during the early stages of the illness and that become more difficult to deal with than the motor manifestations.2
The prevalence of dementia in PD patients varies between 20 and 40%,3 whereas the risk of developing dementia during the course of the disease is between 4 and 6 times greater than in a control population of the same age.4,5 Moreover, many PD patients without dementia can present varying degrees of cognitive alterations that appear in the early stages of the illness or even in recently diagnosed patients.6 Mild cognitive impairment in Parkinson's disease tends to manifest initially with more or less subtle alterations of attention, memory, visuo-spatial functions and executive functions. A sub-group of patients may present language alterations already from the early stages of disease.7
The lack of an appropriate neuropsychological scale to assess these cognitive alterations in PD has led to a situation in which each expert uses a different methodology and, as a result, the profiles cannot be compared with one another.8
It is worth mentioning that until recently, the tests used to detect cognitive disturbances in PD focused largely on the detection of frontal and subcortical deficits, but were not sensitive to detect cortical dysfunctions.9,10 This is the case of instruments that are not specific for PD, such as the MDRS test (Mattis Dementia Rating Scale) recently validated for application in dementia associated with PD,11 or specific tests for PD, such as the SCOPA-Cog scale.12 In an attempt to overcome this lack, the Parkinson's Disease-Cognitive Rating Scale (PD-CRS) was developed. It is an instrument whose clinicometric development has shown it to be a valid, reliable, and useful test for the accurate diagnosis of cognitive impairment associated with PD.13 This scale has been proven to be useful in discriminating PD patients without cognitive impairment from those who present mild cognitive impairment, and the latter from those with PD-associated dementia. The comparative usefulness of this tool, as well as other instruments used in the cognitive assessment of PD, has recently been reviewed.9
The PD-CRS, which has been independently validated by another group,14 is suitable for both screening and the diagnosis of dementia and treatment trials; however, because of its mean time of application (some 15min), it is not a rapid screening test for dementia in PD, as is the Memory Impairment Screen15 or the Mini-Cog,16 for instance, which is used in Alzheimer's disease.
Given that it is a well-known fact that great time consumption is a limiting factor in the application of neuropsychological scales17 in daily clinical practice, we believe it to be convenient to fill the need for a brief, PD-specific instrument that can be used as a brief screening tool for dementia in patients with cognitive impairment associated with PD. In this sense, the “Parkinson's Disease Dementia-Short Screen” (PDD-SS) has been validated very recently.18 This instrument, the first to be developed to diagnose PD-associated dementia, has demonstrated outstanding diagnostic precision, comparable to that of the MDRS, but with a significantly shorter application time than in its final version: it only takes between 5 and 7min.
As a complement to other clinicometric data in the development of neuropsychological assessment instruments, it is very important to know how they function in ordinary clinical practice. Thus, this “DIFUSIÓN” study was designed with the aim of assessing the applicability and acceptability of the PDD-SS. To this end, a large group of Spanish neurologists used the initial version of the PDD-SS in a sample of 471 PD patients recruited in different regions of Spain considered representative of the PD population.
Patients and methodsA nationwide cross-sectional, multi-centre epidemiological study was conducted with the participation of patients from neurology and Parkinson clinics and out-patient clinics from around Spain. During the 6-month recruitment period, out-patients of both sexes between 40 and 85 years of age and diagnosed with PD according to the Brain Bank of London diagnostic criteria19 were admitted. The lack of changes in patients’ anti-Parkinson medication during the preceding month and the ability to perform a battery of neuropsychological tests, including all the associated motor components were also included in the eligibility criteria.
As measures of cognitive function, all patients underwent the Cognitive Mini-Examination (MEC, for its acronym in Spanish),20 the adapted version of the Mini-Mental State Examination (MMSE),21 and the initial version of the PDD-SS scale. The initial PDD-SS consists of 8 dimensions: delayed verbal memory, phonetic verbal fluency, alternating verbal fluency, a questionnaire comprising five questions with yes/no answers, clock drawing, delayed free recall verbal memory, verbal memory of recoded recognition, and executive functions. The total score on this test was obtained from the sum of the scores obtained on each of the items, with the range of scores on the long version of the test being from 0 to 31 points, with 0 points indicative of the maximum cognitive impairment and 31 points, the absence of cognitive impairment. The cut-off for the screening of dementia was set at 15.5 points; hence, PDD-SS scores of 15.5 or less were deemed indicative of dementia. This cut-off point has a sensitivity rate of 91% and a specificity rate of 85%.18
Patient satisfaction with the use of the PDD-SS was evaluated by means of a 6-item questionnaire (1: I thought the test was easy to do; 2: I thought the test was fast to do; 3: I thought the test was pleasant to do; 4: the support materials are appropriate; 5: the questions make me feel uncomfortable; 6: difficulty in understanding orders), each one assessed using a 10-point visual analogue scale (0: minimum satisfaction; 10: maximum satisfaction). A seventh question asked the patients to point out which section of the test they had had difficulties with. The total score for the questionnaire was obtained by the sum of questions 1, 2, 3, and 5 (in this case, the score was inverted). The maximum score for the test was 40 points; those patients scoring 22 or more (55%) were considered to be satisfied.
With regard to the appraisal of physician satisfaction with the use of the PDD-SS, each researcher participating in the study filled out a 17-item questionnaire about their satisfaction with the PDD-SS. The responses to the questions asked of the researchers were recorded using a Likert-type scale with five choices (1: totally disagree; 2: slightly disagree; 3: indifferent; 4: slightly agree; 5: totally agree). The questionnaire was divided into three blocks evaluating their perception of the PDD-SS's manageability, applicability, and reliability. An average total score was calculated for each investigator based on their answers to the 17 items on the questionnaire. The average overall score was created on the basis of the scores obtained on the satisfaction questionnaire and those obtained on each block of questions.
All the statistical analyses were made using the SAS© System statistical software package for Windows, version 8.2 or later.
The study was approved by a Clinical Research Ethics Review Board and all the patients signed an informed consent form prior to being included in the study.
ResultsOf the 471 patients included in the study, 288 were male (61.2%) and 182 were female (38.6%). The mean age (±SD) was 70.9±9.1 years. As regards the patients’ sociodemographic characteristics, 17.8% stated that they had no studies or that they had not completed primary-school studies (n=84). For their part, 54.8% of the patients stated they had completed primary-school studies (n=258), 17.6%, secondary-school studies (n=83) and 9.1%, university-level studies (n=43).
Of the patients participating in the study, 68.6% (n=323) presented relevant associated clinical history or pathology. The most common clinical finding in the study population was arterial hypertension, present in 38% (n=179) of patients. Next came psychiatric disorders, in 24% of patients (n=113), and dyslipidaemias in 23.1% (n=109) of patients.
The most frequent treatment for Parkinson's disease was the combination of levodopa+carbidopa (70.7%, n=333). This was followed, in order, by: pramipexol (32.5%, n=153); levodopa+carbidopa+entacapone (21.7%, n=102); rasagiline (17%, n=80) and ropirinol (10.4%, n=49).
Of the 52 investigators, 61.5% were male (n=32) and 38.5% were female (n=20). As for their specializations, 36.5% were general hospital neurologists (n=19), 30.8% (n=16) worked in Parkinson units or movement disorder units and, finally, 9.6% (n=5) were general neurologists in non-hospital consultations. Of the investigators included in the analysis, 21.2% (n=11) stated they had more than one specialization.
The total mean score on the Cognitive Mini-Examination in the sample of patients under study was 29.0±5.4 points. Significant differences (p<0.0001) were seen in the proportion of patients with possible cognitive impairment according to age: 19.8% in patients over 65 and 7.1% among patients under 65 years of age. As for PDD-SS, the total mean score obtained was 18.5±6.3 points. A total of 36.3% (n=171) of the patients presented dementia associated with Parkinson's disease according to the score for this test. A high correlation, statistically significant, was found between the scores obtained in the MEC and in the PDD-SS (r=0.73; p<0.0001). The concordance of the results obtained in both tests was 76.86%.
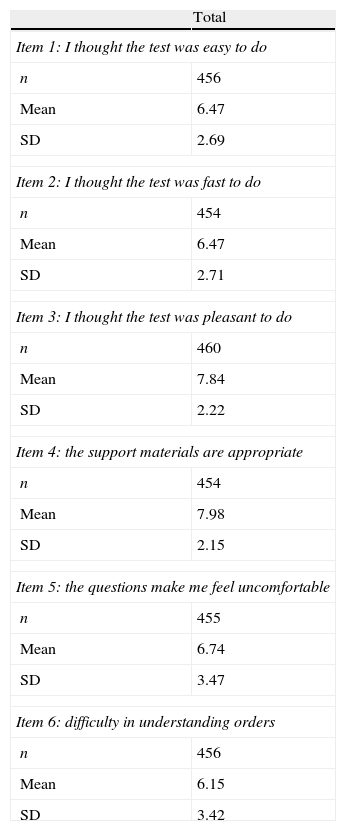
The mean score on the patient satisfaction questionnaire was 27.6±7.4 points. The proportion of patients who said they were satisfied with the use of the PDD-SS amounted to 77.3%. Table 1 shows the assessment of each of the items on the patient satisfaction questionnaire.
Assessment of each of the patient satisfaction questionnaire items (for each item: 0 points=minimum satisfaction; 10 points=maximum satisfaction).
| Total | |
| Item 1: I thought the test was easy to do | |
| n | 456 |
| Mean | 6.47 |
| SD | 2.69 |
| Item 2: I thought the test was fast to do | |
| n | 454 |
| Mean | 6.47 |
| SD | 2.71 |
| Item 3: I thought the test was pleasant to do | |
| n | 460 |
| Mean | 7.84 |
| SD | 2.22 |
| Item 4: the support materials are appropriate | |
| n | 454 |
| Mean | 7.98 |
| SD | 2.15 |
| Item 5: the questions make me feel uncomfortable | |
| n | 455 |
| Mean | 6.74 |
| SD | 3.47 |
| Item 6: difficulty in understanding orders | |
| n | 456 |
| Mean | 6.15 |
| SD | 3.42 |
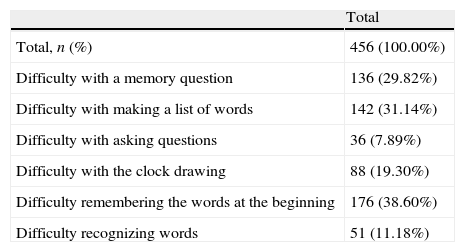
With regard to the section of the test in which patients had difficulties, 37.7% of patients found no difficulty at all in any of the sections of the test; 22.6% encountered difficulties in only one section, and only 1.8% found difficulties in all the sections. A high proportion of patients (38.6%) stated they had had difficulties in the section of the test asking them to recall words learnt earlier (Table 2). Similarly, the sections on memory and word lists were considered difficult by 29.8% and 31.1% of the patients, respectively.
Sections of the PDD-SS patients had some kind of difficulty with.
| Total | |
| Total, n (%) | 456 (100.00%) |
| Difficulty with a memory question | 136 (29.82%) |
| Difficulty with making a list of words | 142 (31.14%) |
| Difficulty with asking questions | 36 (7.89%) |
| Difficulty with the clock drawing | 88 (19.30%) |
| Difficulty remembering the words at the beginning | 176 (38.60%) |
| Difficulty recognizing words | 51 (11.18%) |
Significant differences were seen in the PDD-SS scores between the group of satisfied patients and the group of dissatisfied patients. Thus, the test score was higher in the group of satisfied patients, 19.7±5.9 versus 15.0±5.9 points, respectively (p<0.0001). In consequence, a logistic regression analysis showed two variables to be the explanatory factors for the patient satisfaction/dissatisfaction in the use of the PDD-SS: the score obtained in the test and the difficulty reported by patients. Patients with a higher score on the PDD-SS presented a lower risk of dissatisfaction with the test (OR=0.9); therefore, the lower the patient's degree of dementia, the greater their satisfaction with the PDD-SS. Another factor explaining patient satisfaction/dissatisfaction in the use of the PDD-SS was the difficulty found in completing it: those patients reporting less difficulty in completing the PDD-SS presented a lower risk of dissatisfaction (OR=0.8).
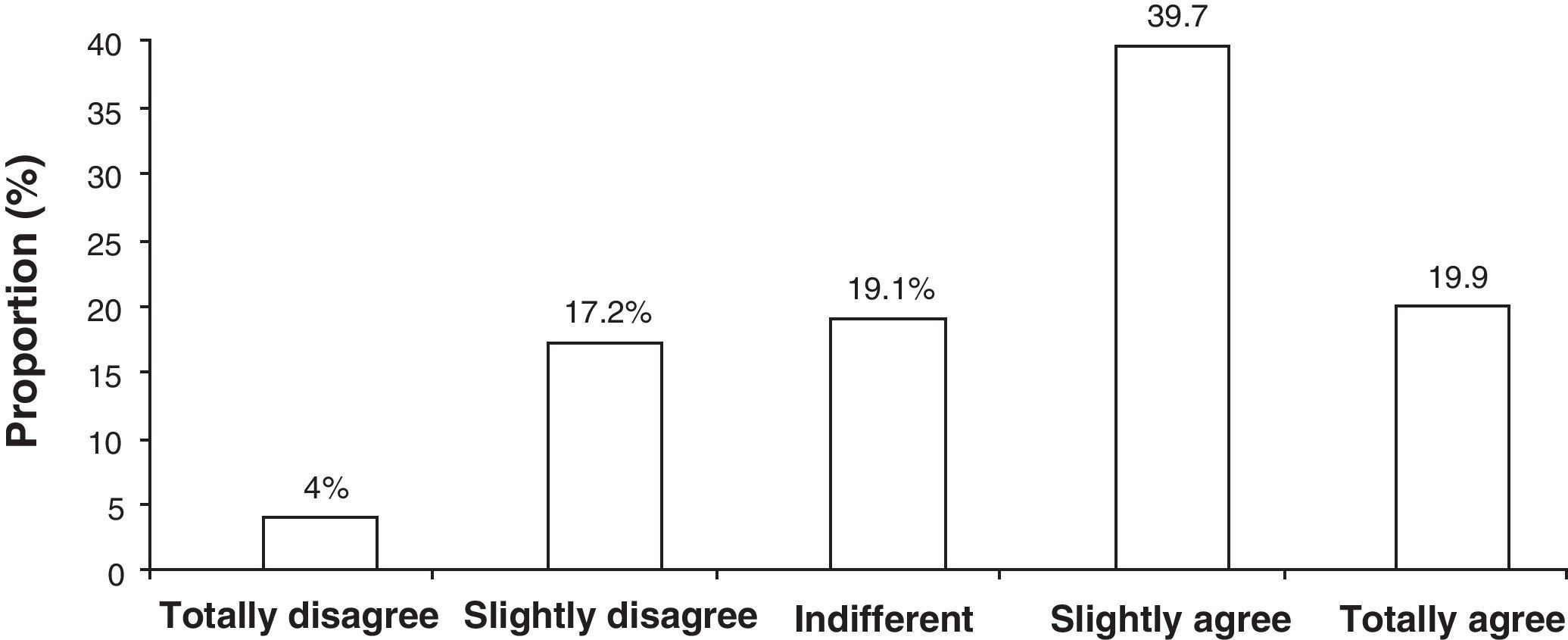
With respect to the assessment of investigators’ satisfaction with the use of the PDD-SS (n=50), all the items on the satisfaction questionnaire gave a mean score of more than three points (out of a maximum of 5), in other words, a positive score with a total mean satisfaction of 3.6±0.6 points. For all responses in total, the most frequent assessment or response was number 4 (“slightly agree”), which represented 39.7% of the assessments, followed by number 5 (“totally agree”) with 19.9% of assessments (Fig. 1).
The vast majority of investigators (90%, n=45) presented a positive overall score (3 or more points) in the satisfaction questionnaire and 26% (n=13) of the investigators presented an assessment of 4 points or more. Only 10% of the investigators (n=5) gave an overall score of less than 3 in the satisfaction questionnaire.
The mean assessment in the perception of the PDD-SS's applicability among the investigators taking part in the study was 3.5±0.7 points. Of these, 78% (n=39) gave a score of more than 3 points in the assessment of the PDD-SS's applicability. With respect to the perception of the test's manageability, the mean assessment among participating investigators was 3.7±0.6 points; 86% (n=43) of the investigators gave a score of more than 3 points in the assessment of the PDD-SS's manageability and convenience. Investigators considered that the test has to be an instrument requiring little support material and it is manageable for the detection of dementia in Parkinson's disease. The mean assessment of the perceived reliability was 3.1±0.5 points, with 70% (n=35) of the investigators considering the PDD-SS as a reliable test for the detection of dementia in Parkinson's disease.
DiscussionThe PDD-SS is a specific tool for detecting cognitive alterations in Parkinson's disease. This test, in view of its simplicity and speed of execution, allows the initial screening of PD patients within specialists’ normal clinical practice, facilitating an early approach to these alterations, desirable from a clinical viewpoint and also economically attractive.22
In the absence of experience with the use of PDD-SS, the present study was designed and conducted with the goal of evaluating certain practical aspects regarding its application, such as the degree of satisfaction among patients and physicians with the use of the PDD-SS in usual clinical practice, thus consolidating its recent validation.18
The results obtained in the present study clearly show patients’ satisfaction with the PDD-SS. It should be noted that the group of patients satisfied with the test obtained a higher score on the PDD-SS. In other words, patients with a lower degree of dementia declare they are more satisfied with the test. The results of this study have shown the applicability of the PDD-SS, which combines the requirements for its application in terms of simplicity, speed, cost, ease of use and interpretation and the use of everyday materials not unfamiliar in the setting of usual clinical practice.22 As for the assessment made by the investigators, this was positive for all items on the questionnaire. Similar values were obtained in the three dimensions assessed: applicability, manageability and reliability.
Furthermore, this study has enabled us to obtain comparative data on the use of the PDD-SS and Lobo's MEC, to date the tool most frequently used by Spanish specialists to screen for dementia in PD patients. It is worth pointing out that, in the present study, the use of the PDD-SS has enabled the detection of a higher percentage of patients with dementia associated with PD than with the use of the MEC. These results suggest that, while both tests are appropriate for the detection of cognitive alterations, the application of the PDD-SS would be the most suitable and specific tool for detecting dementia in PD patients.
The use of questionnaires for assessing patient satisfaction is a well-established methodology frequently used in clinical research23 and, in particular, in patients with PD.24–26 They allow the areas of the patient that are worse to be identified together with the outcomes of care.27 In this study, the questionnaires used have been simple and accurate, necessary requirements for this kind of evaluation.23
The “DIFUSIÓN” study has provided additional details for qualitative interpretation by means of comparative data from a representative sample of the population, as well as by the contextualization of the scores in the clinical conditions of the study population. In short, it can be stated that the data obtained about the PDD-SS in usual medical practice allow us to confirm the usefulness of the test for detecting cognitive alterations in PD patients.
Experimental research on human beingsThe present study has been carried out in accordance with the ethical principles based on the latest version in force of the Declaration of Helsinki and was approved by a Clinical Investigation Review Board.
FundingThis study has received unrestricted financial support from Novartis Farmacéutica, S.A. and Esteve, S.A.
This paper was presented at the Annual Meeting of the SEN in 2008.
Conflict of interestDr. J. Kulisevsky has received financial compensation for his participation as a speaker or on advisory boards from Novartis, Lundbeck, Esteve, Boehringer, Pfizer, GSK, UCB, Merck-Serono, Solvay and Merz. Dr. B. Hernández is an employee of Novartis Farmacéutica S.A. The other authors state they have no conflict of interest to declare.
The financial support and material support for the present investigation have been borne by Novartis Farmacéutica S.A. and Esteve S.A. We are grateful to Marta Muñoz Tudurí of Trial Form Support, Spain, for her collaboration in the preparation of the present manuscript.
E. Agüera Morales, H. R. Sofía; M. Álvarez Saúco, H. Gral. Univ. de Elche; G. Amer Ferrer, H. Son Dureta; F.J. Barrero Hernández, H. Clínico; J.A. Bruguera Hernández, H. La Fe; A. Ares Luque, Complejo Hospitalario; J.J. Asencio Marchante, H. Pto. Real; A. Ávila Ribera, H. General; C. Badia, H. Dr. Peset; J.J. Baiges Octavio, H. Virgen de la Cinta; F. Barriga Hernández, H. Alcorcón; M.J. Catalán Alonso, H. Clínico; A. Castro García, H. Clínico; E. Cebrián, H. Prov. de Pontevedra; E. Cubo Delgado, H. General Yagüe; A. del Olmo, H. Dr. Peset; M.D. Martínez Lozano, H. General; C. Durán Herrera, H. S. Pedro de Alcántara; J. Escudero Torrella, H. General; A. Esquivel López, H. Gregorio Marañón; M. Garcés Redondo, H. Virgen de la Cinta; P. García Ruiz, Fundación J. Díaz; E. Gil Neciga, H. Virgen del Rocío; E. Gómez, Fundación J. Díaz; M. Goñi Imizcoz, H. Divino Vallés; P. Granes Ibáñez, H. Arnau de Lleida; J. Hernández Vara, H. Vall d¿Hebrón; B. Indakoetxea, H. Donostia; P. de Juan Hernández, H. Clínico Univ.; P. Latorre Murillo, H. Can Ruti; E. Lezcano García, H. de Cruces; J.J. López Lozano, H. Puerta de Hierro; S. López Pousa, H. Santa Caterina; J. Marey López, H. Juan Canalejo; M.E. Marta Moreno, H. Miguel Servet; F. Martí, H. Clínico; R. Martín González, H. Sant Joan; C. Martínez Parra, H. Macarena; C. Martínez Rodríguez, H. Cabueñas; J.C. Martínez Castrillo, H. Ramón y Cajal; O. Morsi Hassan, H. Arrixaca; T. Ojea Ortega, H. C. Haya; J. Olazaran Rodríguez, Centro de Especialidades Hermanos Sangro; J. Olivares Romero, H. Torrecadenas; A. Oterino Durán, H. Univ. Valldecilla; B. Pascual Sedano, H. San Pau; L.F. Pascual Millán, H. Clínico; J. Peña Casanova, H. del Mar; N. Rodríguez Fernández, H. General; C. Salvador Aguiar, H. Central de Asturias; F. Sánchez Caballero, H. Valme; V. Serrano Castro, H. Clínico; J. Vaamonde Gamo, H. General de Ciudad Real; A. Villarejo Galende, H. 12 de Octubre; N. Villares, H. Puerta de Hierro y F. Vivancos Matellano, H. La Paz.
La relación de los Miembros del Grupo de Investigadores del estudio DIFUSION se presenta al final del trabajo.
Please cite this article as: Kulisevsky J, et al. Evaluación de la satisfacción médico/paciente con el uso del “Parkinson's Disease Dementia-Short-Screen” (PDD-SS): un test de cribado para la demencia en la enfermedad de Parkinson (estudio DIFUSION). Neurología. 2011;26:461–7.