The Fototest and Mini-Cog include all the domains that are necessary in a cognitive assessment. This study aims to evaluate the diagnostic accuracy of the combined use of both instruments for detecting cognitive impairment.
MethodsWe performed a phase III diagnostic accuracy study with 2 independent samples: STUDY, which included 448 participants randomly allocated to 2 datasets (BASE [80%] and TEST [20%]); and EXTERNAL, which included 61 participants. The index test was consecutive administration of the Fototest and Mini-Cog, and the reference test was formal cognitive assessment. We evaluated the diagnostic accuracy of two-step vs consecutive application of the tests and simple (Comb-Simple), logistic regression (Comb-LR), and random decision tree (Comb-RDT) models of their combined use for detecting cognitive impairment (Global Deterioration Scale score ≥ 3). We performed an exploratory analysis of the BASE dataset, selecting criteria that maximise accuracy; a pre-specified analysis was used to evaluate the selected criteria in the TEST and EXTERNAL datasets.
ResultsThe diagnostic accuracy (95% confidence interval) of the combined models in the BASE dataset (Comb-Simple: 88.3 [88.5−91.4]; Comb-LR: 91.6 [88.2−94.3]; Comb-RDT 95.2 [92.5−97.2]) was significantly higher than the individual values observed for the Mini-Cog and Fototest (81.6 [77.1−85.4] and 84.9 [80.8−88.5], respectively). These results were replicated in the TEST (Comb-Simple: 88.9; Comb-LR: 95.6; Comb-RDT: 92.2) and EXTERNAL datasets (Comb-Simple: 91.8; Comb-LR: 90.2; Comb-RDT: 88.5). Two-step application had the same diagnostic accuracy than consecutive application but required less time (mean [SD] of 197.3 s [56.7] vs 233.9 s [45.2]; P < .0001).
ConclusionsCombined application of the Fototest and Mini-Cog takes less than 4 minutes and improves the diagnostic accuracy of both instruments. Two-step application is more efficient as it requires less time while maintaining the same diagnostic accuracy.
El Fototest y el Mini-Cog incluyen todos los dominios que debieran formar parte de una evaluación cognitiva. Nuestro objetivo es evaluar la utilidad diagnóstica del uso conjunto de ambos instrumentos para el diagnóstico de deterioro cognitivo (DC).
MétodosEstudio Fase III de evaluación de pruebas diagnósticas con dos muestras independientes, ESTUDIO (448 sujetos), dividida aleatoriamente en dos dataset (BASE 80%, TEST 20%), y EXTERNA (61 sujetos). Prueba index: Fototest y Mini-Cog aplicados consecutivamente; prueba de referencia: evaluación cognitiva formal. Se evalúa la UD del uso combinado y escalonado de los modelos Simple (Comb-Simple), Regresión Logística (Comb-RL) y Árbol Aleatorio (Comb-AA) para identificar DC (GDS ≥ 3). Se realiza un análisis exploratorio en BASE seleccionando los criterios que maximizan la Exactitud; la evaluación se realiza en las muestras TEST y EXTERNA mediante un análisis preespecificado con los criterios seleccionados.
ResultadosLa UD de los modelos combinados en BASE (Comb-Simple 88.3 (88.5−91.4) [Exactitud (LI95%-LS95%)], Comb-RL 91.6 (88.2−94.3) y Comb-AA 95.2 (92.5−97.2)) es significativamente superior a la de Mini-Cog y Fototest (81.6 (77.1−85.4) y 84.9 (80.8−88.5) respectivamente); estos resultados son replicados en TEST (Comb-Simple 88.9 (Exactitud), Comb-RL 95.6 y Comb-AA 92.2) y EXTERNA (Comb-Simple 91.8, Comb-RL 90.2 y Comb-AA 88.5). La aplicación escalonada mantiene la misma UD pero requiere menos tiempo (197.3 ± 56.7 vs 233.9 ± 45.2, p < 0.0001).
ConclusionesEl uso conjunto del Fototest y el Mini-Cog requiere menos de cuatro minutos y mejora la UD de ambos instrumentos. El uso escalonado es más eficiente porque manteniendo la misma UD requiere menos tiempo de aplicación.
The purpose of cognitive assessment is to detect and measure impairment in any cognitive domain (attention, memory, language, executive function, praxis, visuospatial skills), using these findings in the diagnostic and treatment process. In some cases (for instance in consultations due to cognitive complaints, which are increasingly frequent1) this assessment is a fundamental part of neurological examination.2
Cognitive assessment may be informal: during medical history taking, the physician may detect disorientation, impaired language expression, marked memory problems,3 the “don’t know” sign,4 or the head turning sign,5 all of which suggest cognitive impairment (CI). However, assessment is usually performed with validated instruments for structured evaluation of the patient’s cognitive function. Like such other instruments as the ophthalmoscope or the reflex hammer, these cognitive instruments and tests must meet certain technical requirements, and the physician must be proficient in their use and the interpretation of results. In clinical practice, given the unavoidable limitations on consultation times, these instruments must necessarily be quick and simple to administer. Furthermore, these instruments should evaluate all the cognitive domains currently considered in the diagnostic criteria for different constructs of CI (attention, memory and learning, language, executive function, praxis, and visuospatial skills)6–8 and, needless to say, present good psychometric properties, like any other diagnostic tool.9 These multi-domain brief cognitive tests (BCT) should be validated specifically for the diagnosis of CI (and not only for dementia); otherwise, the tool would be unable to detect cognitive problems at a stage when the appropriate intervention may either revert the problem or delay or halt dementia progression.10 Normative data applicable to the population of interest should also be available for these BCTs, and these tools should ideally be available free of charge.11
Among the multi-domain BCTs validated in Spain for CI (Supplementary material, Table 1), only the MoCA12,13 and the ACE-III14,15 evaluate all cognitive domains. However, these tests require 10–15 and 15–20 minutes to administer, respectively; these administration times are excessive for general neurology consultations,16 and impracticable in primary care.17 Although the Mini-Mental State Examination,18 Eurotest,19 Fototest,20 Mini-Cog,21 and Clock Drawing Test22 present shorter administration times, they do not assess all cognitive domains. Furthermore, the Mini-Mental State Examination presents many other limitations that have led to a decline in its use in recent years.23
Physicians frequently administer several BCTs to evaluate more cognitive domains, increasing the sensitivity and diagnostic accuracy of these tests in cases of CI. The most widely known examples are the 7 Minute Screen, which combines an orientation task, a cued recall task, a semantic verbal fluency task, and the Clock Drawing Test24; and the Mini-Cog, which includes a delayed recall task and the Clock Drawing Test.21
The Fototest20 and the Mini-Cog21 are 2 very short tests (administration times of 3 and 2 minutes, respectively) that are widely used in Spain and have been validated specifically for CI. The former evaluates language, executive function, and episodic visual memory with free and cued recall, whereas the latter assesses attention, verbal memory, and visuospatial skills and visuoconstructive praxis. This study explores the hypothesis that the combination of the Fototest and the Mini-Cog, which together evaluate all the cognitive domains that should be included in a cognitive assessment, improves the diagnostic accuracy of either test alone while requiring very little time to administer (< 5 minutes), a particularly interesting feature in our setting.
Our objective was to evaluate the diagnostic accuracy and predictive validity of the combination of Fototest plus Mini-Cog in detecting CI in everyday clinical practice.
MethodsDesignWe conducted a phase III study for the evaluation of diagnostic tests,25 with a cross-sectional, prospective, naturalistic, pragmatic design.26,27 The study included new patients attended between 21 February 2018 and 25 September 2018 (inclusive) at a neurology consultation specialising in cognitive-behavioural neurology, at Hospital Universitario Virgen de las Nieves, a tertiary-level hospital in Granada, Spain (study sample).
For the purposes of external validation, we also included an independent sample of patients attended due to cognitive complaints at a cognitive-behavioural neurology clinic at a private hospital in Malaga, Spain, from February 2019 (external sample).
In both instances, recruitment was consecutive and systematic, only excluding individuals who had not completed primary education, since previous studies have shown that the Mini-Cog is not recommended for this population.28,29
Cognitive assessmentAll individuals, regardless of their age, the reason for consultation, or the sample in which they were included, underwent a brief cognitive assessment (index assessment) consisting of the administration of the Fototest20 and the Spanish-language version of the Mini-Cog.29 The tests were administered consecutively, and in that order, by a neurologist (CCP, IRG, RCC, or ICM). The administration time for both BCTs was measured with a digital stopwatch, with a precision of one-hundredth of a second. Times were rounded up to the next whole number.
All individuals attended due to cognitive complaints or behavioural alterations, or with Fototest scores equal to or lower than the 10th percentile,30 also underwent a formal cognitive assessment (reference assessment), including tests of orientation (time, space, and person), attention (forward and backward digit span, Trail Making Test A [TMT-A]31), memory (learning, free recall, and recognition of the CERAD word list,32 for patients from Granada, or the Free and Cued Selective Reminding Test [FCSRT]33 for patients from Malaga), language (short version of the Boston Naming Test,34 semantic verbal fluency,35 and understanding of commands), motor praxis (gesture imitation from the EULA test36), executive function (short-form version of the WAIS similarities subtest, coin test from the Eurotest,19 semantic verbal fluency,35 and TMT-B31), and visuospatial function (copy and drawing tasks from the CERAD32). Scores equal to or below the 5th percentile, with z-score = –1.5, or scaled scores ≤ 6, depending on the norms for each test, were considered pathological. This assessment was performed by a researcher (SLA, MFS, or MEG) blinded to the results of the BCTs. These individuals also underwent functional assessment (Barthel Index37 and Lawton-Brody IADL scale38). The reference assessment and the BCTs were administered on the same day in both samples, except in 9 individuals from the external sample, who underwent these tests within a maximum of 8 days.
DiagnosisRegardless of the reason for consultation and the final diagnosis, all individuals were classified according to their cognitive and functional status using the Global Deterioration Scale39 (Supplementary material, Table 2); scores 1 and 2 were classified as “no CI” and scores ≥ 3 as “CI.” This diagnosis was established by a neurologist with experience in cognitive and behavioural neurology (CCP), based solely on the results from the formal cognitive assessment.
Models evaluatedWe calculated diagnostic accuracy for the Fototest and Mini-Cog independently, and for the following combinations of both instruments.
Combined use.
- -
Simple (Comb-simple): single score resulting from the sum of the Fototest score and the Mini-Cog score.
- -
Logistic regression model (Comb-LR): includes Fototest and Mini-Cog scores as predictors, with sex, age, and level of education as covariates.
- -
Random decision tree (Comb-DT): we developed a classification model using a machine learning technique, applying the algorithm “classification and regression tree” (CART) for supervised learning with automatic Bayesian optimisation of accuracy (percentage of correct predictions) using the OptiML feature40 of the free-access BigML platform.41 Models were trained with a total of 9 predictors, including sociodemographic variables (age, sex, and level of education), Fototest scores (total score and scores for naming, free recall, total recall, and naming fluency), and Mini-Cog scores (total score and scores for recall and the clock-drawing task); the response variable was “CI.”
- -
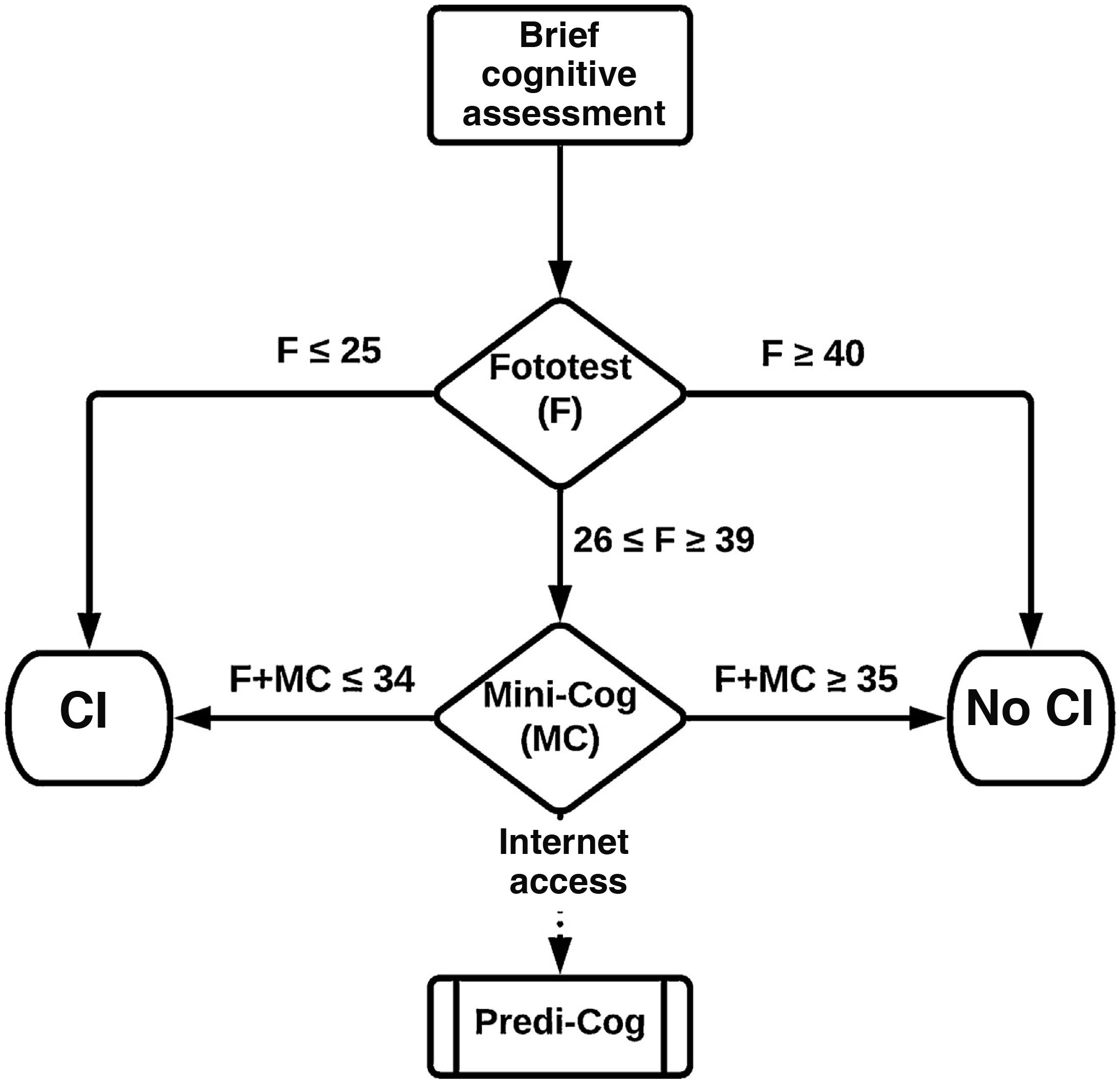
Stepped use: 2-step evaluation. In the first step, we considered 2 cut-off points for the Fototest. The lower cut-off point (Fototest ≤ 25) maximises the positive predictive value or precision, minimising or even eliminating false positives. The higher cut-off point (Fototest ≥ 40) maximises the negative predictive value, minimising or eliminating false negatives. Individuals scoring ≤ 25 on the Fototest were classified as CI and those scoring ≥ 40 as no CI, without the Mini-Cog being administered. The second step considers Mini-Cog results, only in patients with Fototest scores 26–39, applying the previously mentioned combined models (Step-simple, Step-LR, Step-DT).
We conducted a descriptive study of sociodemographic variables and results; comparisons were performed using the t test and the chi-square test, according to whether variables were continuous or categorical.
The analysis was performed in the study sample; to prevent overadjustment and, consequently, overestimation of diagnostic accuracy, we randomly divided the study sample into 2 datasets. The first (base dataset) included 80% of participants and was used to create the different models and to perform an exploratory analysis enabling selection of the optimal cut-off points and criteria, whereas the second (test dataset), including the remaining 20%, was used for independent validation of the different models and criteria selected in the base dataset.
The diagnostic accuracy of the different models in the base dataset was evaluated with the area under the curve (AUC) and the corresponding standard error (SE) for CI vs no CI; AUCs were compared according to the method proposed by Hanley and McNeil.42 The optimal cut-off point was that which maximised the accuracy of the test (percentage of correct classifications). To select the Comb-DT model, given that many of the 98 models created presented similar accuracy values, we decided to maximise the phi coefficient (Matthew’s correlation coefficient) and, according to the principle of parsimony, to select the model with the smallest number of nodes.
The assessment and internal validation of the models and criteria selected in the base dataset were performed independently on the test dataset, and the external validation was performed on the external sample, using a pre-specified analysis of diagnostic accuracy with the parameters sensitivity, precision, phi coefficient, and accuracy; these parameters are frequently used in the validation of prediction models. These data enabled the calculation of classical measures of the diagnostic accuracy of a test (specificity, predictive values, likelihood ratios), and the creation of the corresponding contingency table.
Statistical analysis was performed with SPSS, version 19.0.0,43 and MedCalc, version 18.9.1.44
Formal considerationsStudy design and manuscript drafting followed the recommendations of the STARD 2015 guidelines45 for reporting diagnostic accuracy studies, the STARDdem statement46 for reporting the diagnostic accuracy of studies into cognitive disorders, and the guidelines for the development of predictive models in biomedicine.47
The study complies with the ethical principles for medical research established in the latest revision of the World Medical Association’s Declaration of Helsinki (Edinburgh, 2000).
ResultsThe study sample included 448 individuals with a mean age (SD) of 60.5 (17.7) years, with a slight predominance of women (53.8%) and individuals with more than primary education (52.2%); the prevalence of CI in this sample was 46.4%. The 2 randomly generated datasets (base and test, including 358 and 90 individuals, respectively) showed no significant differences in any of the sociodemographic variables studied, in the prevalence of CI, or in Fototest and Mini-Cog results.
The external sample, in contrast, presented a much higher prevalence of CI (77.0%), an older mean age (71.7 [10.0] years), a higher education level (60.7% of individuals had at least secondary education), and a lower prevalence of women (47.5%), although differences were not significant in the latter 2 variables. Fototest and Mini-Cog results were significantly lower in the external sample than in the study sample.
Table 1 summarises the sociodemographic characteristics, prevalence of CI, and Fototest and Mini-Cog results of the 2 samples and the 2 datasets.
Sociodemographic characteristics and cognitive test results, by sample and dataset.
| Base dataset (80%) | Test dataset (20%) | Study sample | External sample | a | b | |
|---|---|---|---|---|---|---|
| No. subjects | 358 | 90 | 448 | 61 | ||
| CI | 166 (46.4%) | 42 (46.7%) | 208 (46.4%) | 47 (77.0%) | 0.03 (0.96) | 20.14 (0.0001) |
| Age (years) | 60.7 (17.4) | 60.1 (18.7) | 60.7 (17.7) | 71.7 (10.0) | 0.08 (0.78) | 23.28 (0.0001) |
| Sex (women) | 193 (53.9%) | 48 (53.3%) | 241 (53.8%) | 29 (47.5%) | 0.01 (0.92) | 0.84 (0.36) |
| Education level | ||||||
| Primary education | 171 (47.8%) | 46 (51.1%) | 217 (48.4%) | 24 (39.3%) | 0.31 (0.57) | 1.78 (0.18) |
| > Primary education | 187 (52.2%) | 44 (48.9%) | 231 (51.6%) | 37 (60.7%) | ||
| GDS | ||||||
| 1 | 130 (36.3%) | 32 (35.6%) | 162 (36.2%) | – | 4.30 (0.51) | 37.78 (0.0001) |
| 2 | 62 (17.3%) | 16 (17.8%) | 78 (17.4%) | 14 (23.0%) | ||
| 3 | 75 (20.9%) | 19 (21.1%) | 94 (21.0%) | 28 (45.9%) | ||
| 4 | 71 (19.8%) | 16 (17.8%) | 87 (19.4%) | 15 (24.6%) | ||
| 5−6 | 20 (5.6%) | 7 (7.8%) | 27 (6.0%) | 4 (6.6%) | ||
| FCA | 228 (63.7%) | 58 (64.4%) | 286 (63.8%) | 61 (100%) | 0.01 (0.98) | 32.34 (0.0001) |
| Fototest | 32.6 (7.8) | 33.0 (7.8) | 32.7 (7.8) | 29.5 (6.9) | 0.22 (0.64) | 9.28 (0.002) |
| Time (s) | 130.2 (20.2) (n = 317) | 128.7 (19.3) (n = 83) | 129.9 (20.0) (n = 400) | 136.7 (14.8) (n = 49) | 0.38 (0.054) | 5.36 (0.02) |
| Mini-Cog | 2.3 (1.8) | 2.4 (1.8) | 2.4 (1.8) | 1.3 (1.4) | 0.10 (0.75) | 17.83 (0.0001) |
| Time (s) | 103.3 (33.7) (n = 310) | 100.2 (34.9) (n = 81) | 102.7 (33.9) (n = 391) | 109.7 (34.8) (n = 48) | 0.42 (0.52) | 1.84 (0.17) |
Data are presented as either number (%) or mean (standard deviation). CI: cognitive impairment; FCA: formal cognitive assessment; GDS: Global Deterioration Scale. Data are presented as either number (%) or mean (standard deviation).
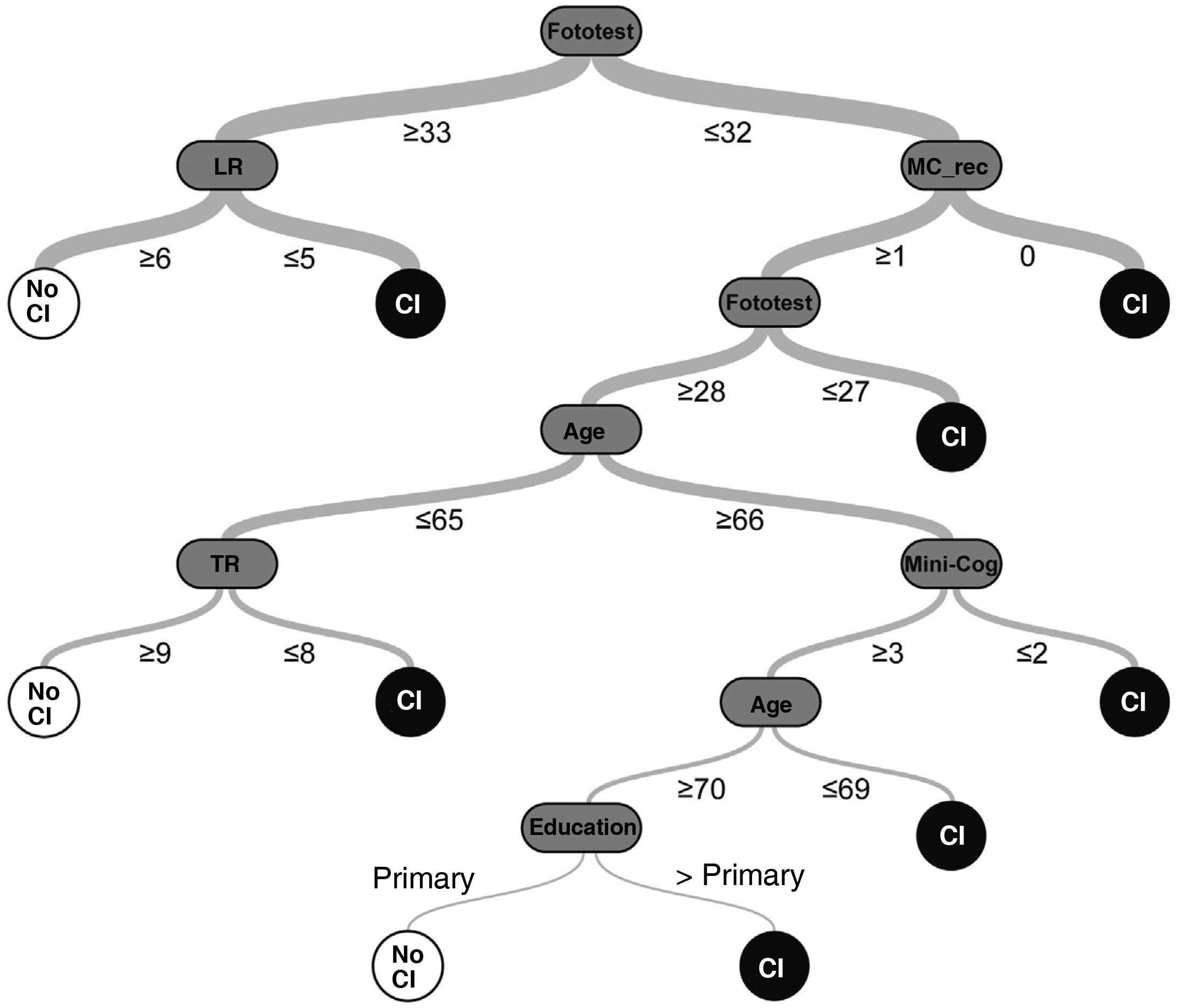
The random decision tree model selected (among the 98 models generated by the platform) had an accuracy of 92.2% and a phi coefficient of 0.84 in the test sample, with a total of 7 levels (Fig. 1) (Supplementary Material, Table 3).
Table 2 summarises the diagnostic parameters of the different models in the different samples and datasets. The diagnostic accuracy of the Fototest was moderate, but significantly higher than that of the Mini-Cog (0.93 ± 0.01 [AUC ± SE] vs 0.89 ± 0.02; t = 2.06; P = .04), while the diagnostic accuracy of the Comb-simple (0.95 ± 0.01) and Comb-LR models (0.98 ± 0.01) was significantly higher than that of either test alone (t > 4.00 and P < .0001 for all comparisons) (Fig. 2, Table 3). The Comb-DT model does not have an AUC, since it only includes one alternative for classification; however, its accuracy (95.2%) was significantly higher than that of the Mini-Cog (81.6%; χ2 = 32.24; P < .0001), the Fototest (84.9%; χ2 = 21.16; P < .001), and the Comb-simple (88.3%; χ2 = 11.2; P = .001) and Comb-LR models (91.6%; χ2 = 3.76; P = .05). Stepped models present the exact same diagnostic accuracy as the combined models they include, with the added benefit of significantly shorter administration times (197.3 [56.7] vs 233.9 [45.2]; t = 8.8; P < .0001).
Diagnostic accuracy in the base dataset and assessment in the test dataset and external sample.
| Test sample | Base dataset (n = 358; 166 CI) | Test dataset (n = 90; 42 CI) | External sample (n = 61; 47 CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Cut-off | Accuracy | Time | Sens. | Precision | Phi | Accuracy | Sens. | Precision | Phi | Accuracy | |
| Fototest | 0.93 (0.01) | ≤ 32 | 84.9 (80.8−88.5) | 130.2 (20.2) | 0.93 | 0.78 | 0.70 | 84.4 | 0.91 | 0.98 | 0.79 | 91.8 |
| Mini-Cog | 0.89 (0.02) | ≤ 2 | 81.6 (77.1−85.4) | 103.3 (33.7) | 0.86 | 0.86 | 0.73 | 86.7 | 0.85 | 0.87 | 0.41 | 78.7 |
| Comb-simple | 0.95 (0.01) | ≤ 34 | 88.3 (88.5−91.4) | 233.9 (45.2) | 0.93 | 0.85 | 0.78 | 88.9 | 0.91 | 0.98 | 0.79 | 91.8 |
| Comb-LRa | 0.98 (0.01) | ≥ 0.50 | 91.6 (88.2−94.3) | 0.95 | 0.95 | 0.91 | 95.6 | 0.91 | 0.96 | 0.74 | 90.2 | |
| Comb-DT | – | b | 95.2 (92.5−97.2) | 0.90 | 0.93 | 0.87 | 92.2 | 0.91 | 0.93 | 0.68 | 88.5 | |
| Step-simple | – | c | 88.3 (88.5−91.4) | 197.3 (56.7) | 0.93 | 0.85 | 0.78 | 88.9 | 0.91 | 0.98 | 0.79 | 91.8 |
| Step-LR | – | d | 91.6 (88.2−94.3) | 0.95 | 0.94 | 0.89 | 94.4 | 0.94 | 0.94 | 0.72 | 90.2 | |
| Step-DT | – | e | 95.2 (92.5−97.2) | 0.90 | 0.93 | 0.87 | 92.2 | 0.91 | 0.91 | 0.63 | 86.9 | |
Results for the base dataset are expressed as mean (standard deviation) or percentage (exact confidence interval).
AUC: area under the curve; CI: cognitive impairment; Comb: combined model; DT: decision tree; LR: logistic regression; Phi: Matthew’s correlation coefficient; Sens.: sensitivity; Step: stepped model.
Diagnostic accuracy of the Fototest and Mini-Cog, used alone or in combination.
| Fototest | Mini-Cog | Comb-simple | Comb-LR | |
|---|---|---|---|---|
| Fototest | – | 0.036 (0.02) (2.06; 0.04) | 0.024 (0.004) (5.59; < 0.0001) | 0.017 (0.018) (5.04; <0.0001) |
| Mini-Cog | – | 0.060 (0.015) (4.04; < 0.0001) | 0.080 (0.014) (5.73; <0.0001) | |
| Comb-simple | – | 0.020 (0.006) (3.56; 0.0004) | ||
| Comb-LR | – |
Results are expressed as the difference between AUCs (SE) (z; P).
AUC: area under the curve; LR: logistic regression; SE: standard error.
The validation of the models in the test dataset largely replicates the predictions of the models generated in the base dataset, except for the models using random decision trees, which present a slightly lower diagnostic accuracy, as may be expected due to the overadjustment effect. The models also showed a very high diagnostic accuracy in the external sample, with accuracy values ranging from 86.9% to 91.8% (except for the Mini-Cog, at 78.7%), although the difference between the external sample and the base dataset was not statistically significant (81.6%; χ2 = 0.29; P = .59).
The Supplementary Material (Tables 4–11) presents the contingency tables and classical measures of diagnostic accuracy (sensitivity, specificity, predictive values, likelihood ratios) for each model and in each sample for the selected cut-off points and criteria.
DiscussionThe results of this prospective study, which was conducted under conditions of routine clinical practice, clearly show that the combined use of the Fototest and Mini-Cog surpasses the diagnostic accuracy of either instrument alone. These results are consistent, as they were replicated in 2 independent samples: one with the same sociodemographic and clinical characteristics as the sample used to develop the models, and another sample that was completely different in terms of sociodemographic and clinical characteristics (drawn from a cognitive-behavioural neurology consultation, older age, higher prevalence of CI, and different geographical location). The greater diagnostic accuracy of the combined models is probably due to greater comprehensiveness of the cognitive assessment, as these models evaluate more cognitive domains. This, in turn, facilitates the identification of different profiles of CI, which could enhance diagnostic studies. However, our study did not address this issue, which should be explored in future research.
Our results are also consistent with and support the available data from Spain regarding the diagnostic accuracy of the instruments used. The only previous study of Mini-Cog in Spain reported an AUC of 0.88 (SE: 0.01),29 which is practically identical to that found in our study (0.89 [0.02]); one study reconstructed the Mini-Cog score from the Clock-Drawing Test and the Mini–Mental State Examination, so we do not consider it comparable.28 Similarly, the diagnostic accuracy of the Fototest in the present study (0.93 [0.01]) is very similar to that reported in previous studies in Spain (0.86 [0.02],48 0.93 [0.02],49 0.95 [0.01]20) and even in Argentina (0.93 [0.03]50).
The complex models (Comb-LR and Comb-DT) provide higher diagnostic accuracy than the Comb-simple model, probably due to the weighting of diagnostic contributions from each test and the inclusion of sociodemographic variables (sex, age, education level) in these models. These variables can be especially relevant, particularly in the case of the Mini-Cog.29 These complex models have the disadvantage that they involve a computational process that may be difficult to apply during consultations. The simple model, in contrast, is more practical for use in the clinical setting, as it simply requires adding the scores from both instruments. However, one of the authors (RVC) developed a program to facilitate the application of these instruments, incorporating a calculator that performs these computations automatically, providing a report that includes all results (Supplementary Material, Fig. 1). This application, Predi-Cog51 (https://www.hipocampo.org/Predi-Cog.asp), is available online, free of cost, at www.hipocampo.org.52
Based on our results, we recommend applying the tests in a stepped manner (Fig. 3), which allows direct classification of a substantial percentage of individuals, without compromising diagnostic accuracy, using only the Fototest (base dataset, 36.6%; test dataset, 37.8%; external sample, 42.6%), saving time (197 [56.7] vs 233.9 [45.2] seconds).
Our study has some limitations, such as the exclusion of individuals without at least primary education, which limits the possibility of extrapolating our results to the population with lower education levels. In any case, our previous data already recommended avoiding the use of Mini-Cog in this population.28,29 Another Fototest weakness is that only 63.9% of the individuals in the study sample underwent formal cognitive assessment. However, we only excluded individuals without cognitive complaints and with Fototest scores above the 10th percentile, which makes it unlikely that these individuals would have CI, thus minimising the risk of partial verification bias.53 Lastly, while the index and reference assessments were performed by different professionals who were blinded to other results, and diagnosis was based on reference test results, the professional who issued the diagnosis was not blinded to the results of the BCTs. Our study also has several strengths, such as its naturalistic, pragmatic nature, the large sample size, and, most importantly, the double validation of the results in 2 independent samples with very different characteristics.
In conclusion, the combined use of the Fototest and Mini-Cog, 2 very brief tests that are simple to administer and that evaluate all the cognitive functions that should be addressed in a brief cognitive assessment, enhances the already high diagnostic accuracy of both instruments. Stepped use of these tests is more efficient than their combined use, since it achieves a significant decrease in mean administration time (< 200 seconds) while maintaining accuracy.
Data availability statementThe data that support the findings of this study are available upon reasonable request.
Conflicts of interestC. Carnero Pardo is the creator of the Fototest. Under the terms of its Creative Commons licence, the Fototest may be used and distributed for non-commercial purposes provided that it is not modified and its authorship is explicitly acknowledged.
All figures were created by Marc Torres Ciuró.
An interim analysis of this study was presented at the 71st Annual Meeting of the Spanish Society of Neurology.