In most cases, multiple sclerosis (MS) initially presents as clinically isolated syndrome (CIS). Differentiating CIS from other acute or subacute neurological diseases and estimating the risk of progression to clinically definite MS is essential since presenting a second episode in a short time is associated with poorer long-term prognosis.
DevelopmentWe conducted a literature review to evaluate the usefulness of different variables in improving diagnostic accuracy and predicting progression from CIS to MS, including magnetic resonance imaging (MRI) and such biofluid markers as oligoclonal IgG and IgM bands, lipid-specific oligoclonal IgM bands in the CSF, CSF kappa free light-chain (KFLC) index, neurofilament light chain (NfL) in the CSF and serum, and chitinase 3–like protein 1 (CHI3L1) in the CSF and serum.
ConclusionsCodetection of oligoclonal IgG bands and MRI lesions reduces diagnostic delays and suggests a high risk of CIS progression to MS. A KFLC index > 10.6 and CSF NfL concentrations > 1150 ng/L indicate that CIS is more likely to progress to MS within one year (40%–50%); 90% of patients with CIS and serum CHI3L1 levels > 33 ng/mL and 100% of those with lipid-specific oligoclonal IgM bands present MS within one year of CIS onset.
La mayoría de los pacientes con esclerosis múltiple (EM) debutan con un síndrome clínico aislado (SCA). Es importante diferenciar este SCA de otras patologías neurológicas agudas o subagudas y estimar el riesgo de desarrollar una esclerosis múltiple clínicamente definida (EMCD), pues un segundo ataque clínico en un corto periodo de tiempo se asocia con peor pronóstico a largo plazo.
DesarrolloSe realizó una revisión bibliográfica con el objetivo de contrastar diferentes variables, tales como la resonancia magnética (RM) y diferentes marcadores biofluídicos como las bandas oligoclonales IgG (BOC), bandas oligoclonales IgM (BOCM), bandas oligoclonales IgM lípido específicas (BOCM-LE), índice de cadenas ligeras libres Kappa (κ index) mediante la determinación de las cadenas ligera libres kappa en líquido cefalorraquídeo (LCR), neurofilamentos de cadenas ligeras en LCR (NfLL) y suero (NfLS) y la proteína chitinasa 3-like 1 (CHI3L1) en LCR (CHI3L1L) y suero (CHI3L1S), con el objetivo de mejorar la precisión diagnóstica y predecir los riesgos de un segundo ataque clínico tras un SCA.
ConclusiónUnas BOC positivas junto con la identificación de lesiones por RM, reducirán el tiempo de diagnóstico y nos indicaran que la mayoría de los pacientes con SCA evolucionaran a EM. Un κ index >10.6 y una concentración de NfLL > 1150 ng/L, nos indican que los SCA tienen más probabilidades de convertirse en EM durante el primer año (40%/50%). El 90% de los pacientes con SCA y niveles de CHI3L1S >33 ng/mL, y el 100% con presencia BOCM-LE se convierten en EM durante el primer año.
Clinically isolated syndrome (CIS) is the clinical status characterised by central nervous system (CNS) inflammation and demyelination suggestive of multiple sclerosis (MS), in the absence of other alternative diagnoses.1,2 MS initially manifests as CIS in approximately 85% of patients, and is converted to clinically definite multiple sclerosis (CDMS) when patients develop a second relapse. Prospective studies show that between 60% and 70% of patients with CIS develop CDMS within 20 years.3 Currently, no pathognomonic evidence or clinical characteristic exists that diagnoses MS in isolation. Diagnosis is established after exclusion of alternative diagnoses based on medical history, physical examination, MRI findings, and laboratory findings (biofluid markers), together with the clinician’s judgement.4
Identifying risk of conversion from CIS to CDMS is especially relevant, as conversion within a short period of time is associated with poorer prognosis and severe disability. Therefore, patients at greater risk may benefit from highly effective treatments (with greater associated risk), whereas those at lower risk would be prescribed other safer but less effective treatments.5–7 Therefore, it is important to identify cerebrospinal fluid (CSF) and blood biomarkers that may help us predict this conversion.
This review article analyses MRI findings and biofluid markers that may meet this objective (IgG oligoclonal bands [OCB], IgM oligoclonal bands [IgM OCB], lipid-specific oligoclonal IgM OCB, kappa free light chain index [kappa index], neurofilament light chain [NfL] in the CSF and serum, and chitinase 3-like protein 1 [CHI3L1] in the CSF [CSF-CHI3L1] and serum [sCHI3L1]), with the aim of assessing their diagnostic accuracy and identifying which of them may more efficiently predict the risk of a second clinical episode after CIS.
Diagnosis of CISAccording to the McDonald diagnostic criteria, a patient with CIS may be diagnosed with MS if the first MRI study meets criteria for dissemination in space (DIS) and dissemination in time (DIT), or if OCB are detected, which may replace a clinical event or DIT in the MRI study.8
MRI scans are widely used to support this clinical diagnosis,9 as is intrathecal IgG synthesis in patients presenting CIS. MRI is the most widely used tool to assess these patients10,11; it is useful in analysing DIS and DIT, thus contributing to early diagnosis of MS.12 According to several studies, between 50% and 80% of patients with CIS present lesions of demyelinating appearance in the initial brain MRI scan13–15; however, T2-weighted sequences may show at least one white-matter lesion in 91% of these patients.16
When the MRI scan is assessed based on 3 of the 4 Barkhof/Tintoré criteria (MRI-BT), it shows high specificity (70%-88%) and moderate sensitivity (49%–82%),17–20 whereas assessment based on the McDonald criteria for demonstration of DIS9 shows a better sensitivity (72%) without losing specificity, as shown by Swanton et al.21–23
Intrathecal IgG synthesis determined by OCB detection represents an important tool for the diagnosis of MS.24 In CIS, OCB are detected in 61.02%–70% of patients, and MRI findings, when assessed according to the MRI-BT criteria, are positive in 53.5%–64%.25,26 OCB are more accurate overall than MRI-BT (sensitivity 78% vs 70%, specificity 65% vs 48%, positive predictive value [PPV] 62% vs 50%, negative predictive value [NPV] 80% vs 68%, and accuracy 70% vs 58%).26 However, when MRI findings were assessed according to the 2010 McDonald criteria,27 in a study conducted by Teixeira et al.28 in 82 patients with symptoms of CIS, they were classified as positive in 63 (76%), whereas OCB were present in 48 (58%) (it is important to highlight that OCB were not determined in the total sample, only in 69 patients); diagnosis of CIS was confirmed in 10 patients with OCB and MRI findings showing no demyelinating lesions, and another 16 with negative results for OCB and with MRI showing demyelinating lesions.
When patients were diagnosed based on abnormal MRI findings, rather than clinical suspicion of MS, the rate of non-confirmation was much higher (89% vs 54%).29 MRI studies are generally inconclusive, especially in the first stages of the disease30 and in patients with suspected MS and presenting vascular risk factors, migraine, or atypical symptoms; in these cases, OCB are usually negative, but positive findings may play a significant role when ruling out other causes of demyelinating lesions in MRI.31
Furthermore, it should be noted that OCB are not exclusive to MS, as they are also found in other inflammatory and infectious diseases affecting the CNS,32 such as vascular, metabolic, or degenerative diseases.33,34 Also, the methods used to detect OCB require expert laboratories for correct interpretation, which may cause variability in results. However, the McDonald criteria have enhanced the role of OCB results in the CSF, and have increased the value of MRI findings by including cortical and symptomatic lesions. Both biomarkers have potential to decrease the time to diagnosis.8 Thus, presence of OCB together with MRI lesions may improve overall diagnostic accuracy by increasing the specificity and the NPV of MRI findings.12,35
IgM OCB are not a good parameter for diagnosing CIS, as they are only observed in 28%-55% of patients with CDMS,36,37 a much lower rate than OCB and MRI lesions.25,26,28
A κ index > 10.62 is a good parameter to confirm CIS. In the study by Menéndez et al.,38 the κ index did not show statistically significant differences between patients from the 3 control groups: individuals with normal pressure hydrocephalus, patients with other non-inflammatory neurological diseases, and patients with inflammatory neurological diseases other than MS; the value identified in the patients from these 3 groups was 1.96 (1.13–3.56). However, they did observe statistically significant differences between values from individuals in the control groups and those with CIS. The value of the κ index was 35.61 (4.48–132.73) in the latter group. Although κ index > 10.62 presents slightly lower sensitivity and specificity than OCB (93.1 vs 96.5% and 95.7% vs 98.6%), a κ index is the most interesting alternative to OCB determination in the detection of MS.38 A free kappa index ≥ 5.9 is useful as a more sensitive marker of intrathecal IgG synthesis, though it is less specific (sensitivity of 96%; specificity of 86%) than OCB detection. Determination of this parameter may provide valuable diagnostic and prognostic information, especially in patients with CIS or suspected MS with negative results for OCB,39,40 and may reinforce the diagnostic value of negative OCB findings with presence of MRI lesions, or vice-versa.
CSF-NfL and CHI3L1 are not specific to MS. Determining CSF-NfL or sNfL detects axonal lesions, which can result from many neurological diseases, such as white matter lesions and other types of subcortical damage,41–43 vascular disease (small-vessel disease),44,45 frontotemporal dementia,46 MS,47 idiopathic normal pressure hydrocephalus,48 amyotrophic lateral sclerosis,49 and several CNS infections50,51; therefore, these studies may be used mainly as a marker of axonal lesions, and not as a diagnostic marker of any specific neurological disease.52 The CHI3L1 protein is also not specific to MS, and usually increases in disorders potentially involving active inflammation, such as neuromyelitis optica, HIV infection, cerebrovascular disease, and Alzheimer disease. Serum levels of the protein may increase due to several inflammatory diseases, especially those progressing with chronic inflammation.53–56
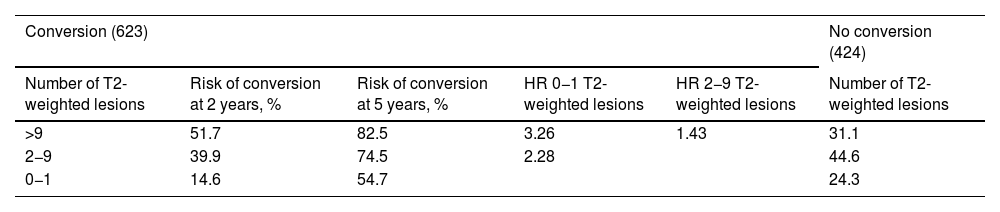
Prediction of conversion of CIS to CDMSSeveral retrospective and prospective hospital studies report a rate of conversion from CIS to CDMS ranging from 16% in the first year to 60% at 2 years of follow-up.57–61 Different variables have shown to be useful in predicting this conversion, although MRI findings are the most reliable marker, presence of OCB and such other markers as NfL and CHI3L1 in both CSF and serum may play an important role.33,62 MRI is the most widely used tool to assess patients with clinical symptoms of CIS suggestive of MS, and its use to predict conversion to CDMS is widely accepted.63 Based on MRI results from 1047 cases of CIS from several studies, Kuhle et al.64 established a scale for predicting the risk of conversion to CDMS (Table 1), taking into account the McDonald criteria for DIS.65
Risk of conversion of CIS to CDMS as a function of the number of T2-weighted lesions on the MRI.
| Conversion (623) | No conversion (424) | ||||
|---|---|---|---|---|---|
| Number of T2-weighted lesions | Risk of conversion at 2 years, % | Risk of conversion at 5 years, % | HR 0−1 T2-weighted lesions | HR 2−9 T2-weighted lesions | Number of T2-weighted lesions |
| >9 | 51.7 | 82.5 | 3.26 | 1.43 | 31.1 |
| 2−9 | 39.9 | 74.5 | 2.28 | 44.6 | |
| 0−1 | 14.6 | 54.7 | 24.3 | ||
CDMS: clinically defined multiple sclerosis; CIS: clinically isolated syndrome; HR: hazard ratio.
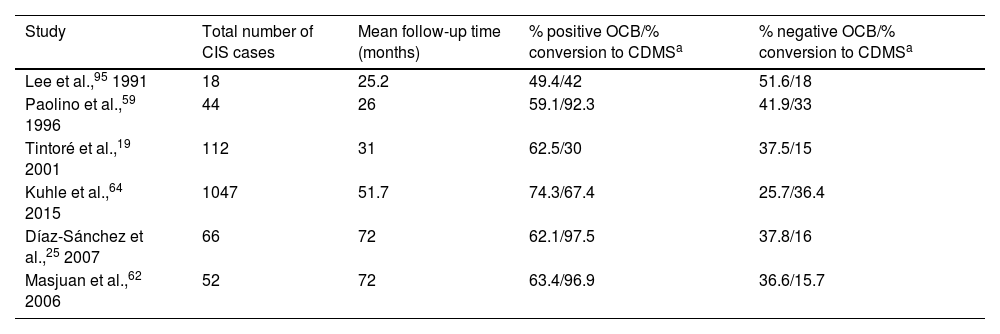
Presence of OCB is suggestive of high risk of conversion to CDMS.25,32 Various studies have shown how longer follow-up times are associated with higher rates of conversion in patients with OCB (Table 2).
Rate of conversion to CDMS as a function of the percentage of patients with positive and negative OCB.
| Study | Total number of CIS cases | Mean follow-up time (months) | % positive OCB/% conversion to CDMSa | % negative OCB/% conversion to CDMSa |
|---|---|---|---|---|
| Lee et al.,95 1991 | 18 | 25.2 | 49.4/42 | 51.6/18 |
| Paolino et al.,59 1996 | 44 | 26 | 59.1/92.3 | 41.9/33 |
| Tintoré et al.,19 2001 | 112 | 31 | 62.5/30 | 37.5/15 |
| Kuhle et al.,64 2015 | 1047 | 51.7 | 74.3/67.4 | 25.7/36.4 |
| Díaz-Sánchez et al.,25 2007 | 66 | 72 | 62.1/97.5 | 37.8/16 |
| Masjuan et al.,62 2006 | 52 | 72 | 63.4/96.9 | 36.6/15.7 |
CDMS: clinically definite multiple sclerosis; CIS: clinically isolated syndrome; OCB: oligoclonal bands.
Presence of OCB is associated with a two-fold increase in the risk of conversion to MS, compared to patients with negative OCB,66 with a hazard ratio (HR) of 2.49.64
The presence and number of CNS lesions in MRI and positive CSF OCB findings have been independently associated with a higher risk of conversion to MS.33,62,63,67 Patients with CIS who present OCB and more than 2 lesions on T2-weighted MRI sequences are at 21.69 times greater risk of conversion to CDMS.68
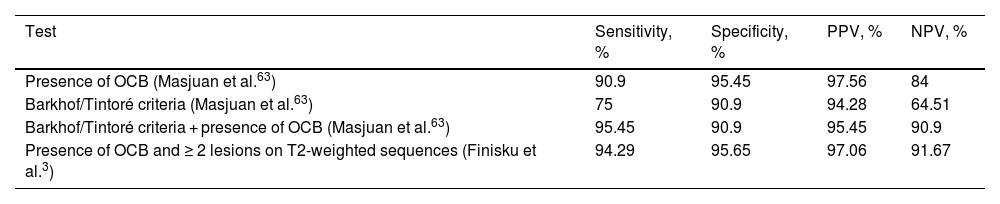
When both examinations are analysed together, results substantially improve (Table 3).
Comparison of lesions on MRI according to the Barkhof/Tintoré criteria and the 2010 McDonald criteria, and presence of OCB in predicting the conversion of CIS to CDMS.
| Test | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|
| Presence of OCB (Masjuan et al.63) | 90.9 | 95.45 | 97.56 | 84 |
| Barkhof/Tintoré criteria (Masjuan et al.63) | 75 | 90.9 | 94.28 | 64.51 |
| Barkhof/Tintoré criteria + presence of OCB (Masjuan et al.63) | 95.45 | 90.9 | 95.45 | 90.9 |
| Presence of OCB and ≥ 2 lesions on T2-weighted sequences (Finisku et al.3) | 94.29 | 95.65 | 97.06 | 91.67 |
CDMS: clinically defined multiple sclerosis; CIS: clinically isolated syndrome; MRI: magnetic resonance imaging; NPV: negative predictive value; OCB: oligoclonal bands; PPV: positive predictive value.
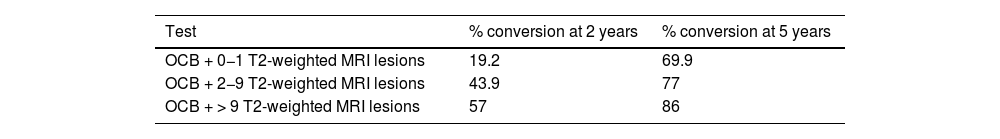
Thus, presence of OCB and MRI lesions suggest that the great majority of patients with CIS would progress to CDMS. However, these parameters are less useful in predicting progression at very early stages of the disease.16,69 Subjects at higher risk, those presenting OCB and more than 9 lesions on T2-weighted MRI sequences, are more likely to progress early (Table 4).
Risk of conversion from CIS to CDMS according to presence of OCB and number of T2-weighted lesions (MRI).
| Test | % conversion at 2 years | % conversion at 5 years |
|---|---|---|
| OCB + 0−1 T2-weighted MRI lesions | 19.2 | 69.9 |
| OCB + 2−9 T2-weighted MRI lesions | 43.9 | 77 |
| OCB + > 9 T2-weighted MRI lesions | 57 | 86 |
CDMS: clinically definite multiple sclerosis; CIS: clinically isolated syndrome; MRI: magnetic resonance imaging; OCB: positive findings for oligoclonal bands.
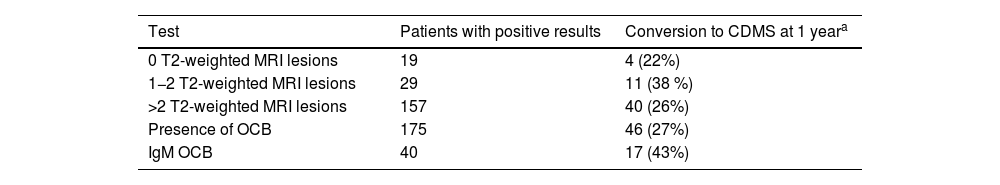
Between 28% and 55% of patients with CDMS present positive IgM OCB findings.36,37 In a study by Ferraro et al.,16 including 55 patients with CIS who progressed to CDMS, 40 presented IgM OCB. Median time until relapse was 35 months for patients with negative IgM OCB. In patients with presence of OCB, the median time was 20 months, decreasing to 12 months if the CIS manifested before the age of 30. These results are similar to those reported by Villar et al.69 and Boscá et al.66 The risk of relapse increased with presence of IgM OCB in the CSF only, with 17 of these patients (43%) progressing to CDMS during the first year of follow-up (Table 5).16
Rate of conversion of CIS to CDMS according to the number of T2-weighted lesions (MRI), presence of OCB and IgM OCB. Of 205 patients with CIS, 150 progressed to CDMS (55 [36%] within a year).
| Test | Patients with positive results | Conversion to CDMS at 1 yeara |
|---|---|---|
| 0 T2-weighted MRI lesions | 19 | 4 (22%) |
| 1−2 T2-weighted MRI lesions | 29 | 11 (38 %) |
| >2 T2-weighted MRI lesions | 157 | 40 (26%) |
| Presence of OCB | 175 | 46 (27%) |
| IgM OCB | 40 | 17 (43%) |
CDMS: clinically definite multiple sclerosis; CIS: clinically isolated syndrome; IgM OCB: IgM oligoclonal bands; MRI: magnetic resonance imaging; OCB: IgG oligoclonal bands.
Presence of IgM OCB targeting myelin lipids leads to greater progression of disability.69–76 All patients with IgM OCB targeting myelin lipids (68% of IgM OCB) experienced a second relapse at 11 months of disease onset. Presence of IgM OCB was associated with higher risk of a second relapse (HR: 3.54; confidence interval: 1.65–7.55); however, the risk was considerably higher in patients with IgM OCB targeting myelin lipids (HR: 11.41).77
High levels of free light chains in the CSF also predict conversion of CIS to CDMS78–81; furthermore, previous studies correlate kappa free light chain levels in the CSF with MRI lesion load, and progression to MS in patients with isolated neuritis optica.81 Patients with CIS with a κ index > 10.62 present 7.34 times higher risk of conversion to MS. This index was also correlated with the presence of OCB and criteria for lesions in the MRI. With a κ index > 10.62, 71.4% of patients with CIS (35/49) progressed to MS, with a mean conversion time of 19 months. Approximately 40% progressed during the first year. Of the 28 patients with CIS and a κ index > 10.62, 89.3% of them did not progress to MS.38
CSF-NfL level is well correlated with lesions in the MRI.82–88 With a cut-off point > 500 ng/L, the great majority progressed to MS within 4–6 years.82 However, CSF-NfL level is a weak risk factor for predicting conversion to MS, and its value should be considered in the context of presence of OCB and radiological MRI findings (number of lesions in T2-weighted sequences).84,89,90 Despite this, high levels of CSF-NfL (>1208 ng/L) indicate higher risk of progression to MS, and have been associated with high numbers of lesions in T2-weighted sequences at baseline and during the first year of follow-up.84 Håkansson et al.82 observed that with values >1348 ng/L, 7 out of 9 (78%) cases of CIS progress within 2 years, whereas only one in 10 patients with lower values present conversion. Similar findings are reported by Mañé-Martínez et al.91 in 109 patients with CIS, with 53 showing values above 1150 ng/L. Of these, 50% presented conversion within one year (90% at 4 years). Lower values are associated with a one-year conversion rate of 18% (70% at 4 years).91 In a study of the serum of 222 patients, Dalla et al.92 observed high sNfL levels, above 40.4 pg/mL (75th percentile), in 78. These high values were correlated with presence in the baseline MRI of a high number of T2 and gadolinium-enhancing lesions, in addition to high risk of developing CDMS in 2 years.92 However, in a study comparing sNfL levels in patients with CIS who rapidly progressed to CDMS (79–139 days) and in those who did not progress (follow-up of 5.3−.9 years), Disanto et al.83 found that sNfL levels were not associated with faster progression to CDMS. The difference in sNfL levels between patients presenting rapid conversion and those without conversion was not statistically significant.84
CSF-CHI3L1 concentrations were significantly higher in patients with CIS who progressed to CDMS than in those who did not.54,91,93,94 However, the role of this protein as a biomarker associated with progression to MS is probably modest, although its function as prognostic biomarker is particularly relevant as predictor of disability. Nevertheless, patients presenting high sCHI3L1 levels (> 33 ng/mL) more rapidly progress to CDMS, with a likelihood of no conversion at 10 months of follow-up of 10%.94
DiscussionCurrently, there is no single test that confirms a first clinical episode of MS. MRI studies are widely used to support this clinical diagnosis.9 When MRI is assessed according to the McDonald criteria for DIS,95 it shows good sensitivity (72%)21 and specificity (70%–88%),23 but is sometimes inconclusive.30 Detection of OCB presents higher sensitivity (78%) but poorer specificity (65%).26 However, the joint performance of MRI studies and OCB determination for the diagnosis of CIS presents greater overall diagnostic accuracy than either study in isolation, increasing the specificity and the NPV.12,35 A κ index > 10.62 is not present in other neurological diseases, whether inflammatory or non-inflammatory,78 and may help to confirm a clinical diagnosis in patients with negative results for OCB or inconclusive MRI findings.
MS begins as CIS in approximately 85% of patients,96,97 and 60%–70% of them will develop CDMS within a period of 20 years.3 It is very important to predict conversion to MS in early stages of CIS, as MS in these patients usually progresses more aggressively, and effective treatments may be prescribed to reduce disability progression. Both MRI and OCB determination are weak predictors of conversion from CIS to CDMS in the short term.70,79 Although this is also the case with the κ index and CSF-NfL concentration, patients with a κ index above 10.62 and CSF-NfL level >1150 ng/L present a higher risk of conversion (40% and 50%, respectively).38,92 In the study published by Hinsinger et al.,94 patients with sCHI3L1 levels >33 mg/mL more rapidly progressed to CDMS (90% in less than one year). The probability of avoiding a second episode at one year is very low (<10%).95
The presence of IgM OCB and lipid-specific IgM OCB limited to the CSF enables us to identify a group of patients with an immunopathogenic mechanism mediated by IgM antibodies and high likelihood of conversion to CDMS in a short period of time. Approximately 43% of patients with CIS and presenting a pattern of IgM OCB and age below 30 years progress to CDMS before one year; however, this risk increases to 100% when these patients present lipid-specific IgM OCB. All patients with lipid-specific IgM OCB (68% of all patients with IgM OCB) presented a second relapse before one year after disease onset.78
ConclusionThe identification of lesions in MRI studies according to McDonald criteria for DIS,65 together with presence of OCB, are indicators that the majority of patients with CIS progress to CDMS.
A κ index > 10.6, used as a marker of the immune system, and CSF-NfL > 1150 ng/L (marker of axonal damage) alert us that patients with CIS are more likely to progress to CDMS within one year (40%–50%).
Markers associated with the immune system (IgM OCB, and lipid-specific IGM OCB) identify a group of patients with CIS with a high probability of conversion to CDMS, especially those presenting lipid-specific IgM OCB (100%).
Serum CHI3L1 (>33 mg/mL) may enable us to detect a majority of patients at a higher risk of conversion to CDMS during the first year and with a more aggressive disease course.
FundingThis study received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.