We conducted an extensive bibliometric study into cohorts of patients with Guillain-Barré syndrome (GBS) associated with Zika virus infection. We searched numerous databases including Scopus, Medline/PubMed, Web of Science, Science Direct, Scielo, and Imbiomed.
We selected studies conducted in the Americas and grouped them by country and cohort size; evaluated the methodology used to identify infectious agents (selecting those that screened for Zika virus as a primary objective and other agents [viruses and bacteria] as a secondary objective); and analysed the percentage of patients testing positive for Zika virus, global or average incidence, and the nerve conduction findings reported. We excluded case reports, series of fewer than 5 cases, and cohort studies not reporting serology findings for Zika virus.
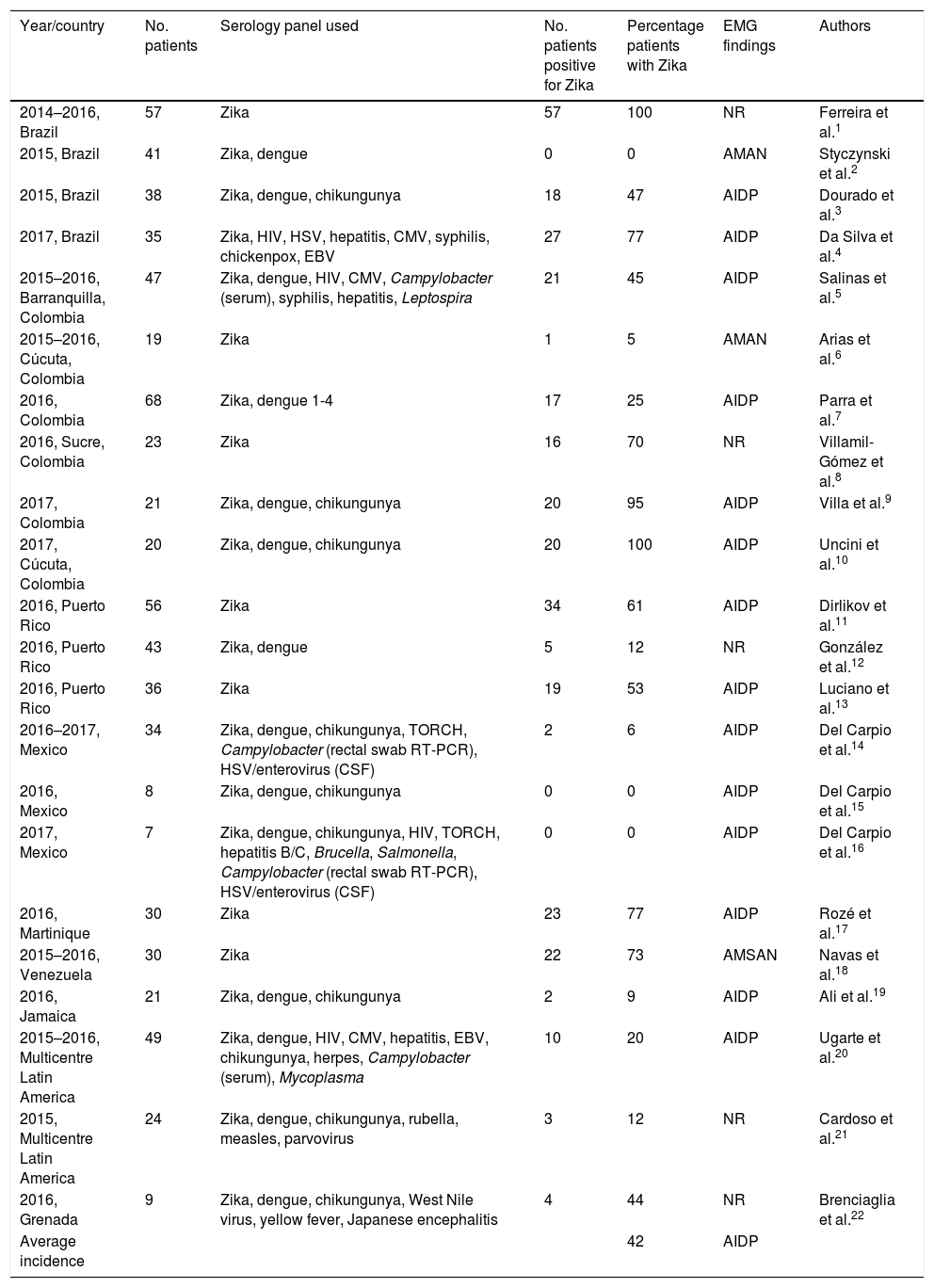
We identified 22 studies analysing cohorts of patients with GBS associated with Zika virus infection, published between 2014 and 2017; cohort size varied between studies. Brazil and Colombia had the most studies published (4 and 6, respectively), followed by Puerto Rico and Mexico (3 each), and Venezuela, Jamaica, Grenada, and Martinique (one study each). We found 2 multicentre studies, with participants from different Central and South American countries (Table 1).
Main cohort studies conducted in the Americas.
| Year/country | No. patients | Serology panel used | No. patients positive for Zika | Percentage patients with Zika | EMG findings | Authors |
|---|---|---|---|---|---|---|
| 2014–2016, Brazil | 57 | Zika | 57 | 100 | NR | Ferreira et al.1 |
| 2015, Brazil | 41 | Zika, dengue | 0 | 0 | AMAN | Styczynski et al.2 |
| 2015, Brazil | 38 | Zika, dengue, chikungunya | 18 | 47 | AIDP | Dourado et al.3 |
| 2017, Brazil | 35 | Zika, HIV, HSV, hepatitis, CMV, syphilis, chickenpox, EBV | 27 | 77 | AIDP | Da Silva et al.4 |
| 2015–2016, Barranquilla, Colombia | 47 | Zika, dengue, HIV, CMV, Campylobacter (serum), syphilis, hepatitis, Leptospira | 21 | 45 | AIDP | Salinas et al.5 |
| 2015–2016, Cúcuta, Colombia | 19 | Zika | 1 | 5 | AMAN | Arias et al.6 |
| 2016, Colombia | 68 | Zika, dengue 1-4 | 17 | 25 | AIDP | Parra et al.7 |
| 2016, Sucre, Colombia | 23 | Zika | 16 | 70 | NR | Villamil-Gómez et al.8 |
| 2017, Colombia | 21 | Zika, dengue, chikungunya | 20 | 95 | AIDP | Villa et al.9 |
| 2017, Cúcuta, Colombia | 20 | Zika, dengue, chikungunya | 20 | 100 | AIDP | Uncini et al.10 |
| 2016, Puerto Rico | 56 | Zika | 34 | 61 | AIDP | Dirlikov et al.11 |
| 2016, Puerto Rico | 43 | Zika, dengue | 5 | 12 | NR | González et al.12 |
| 2016, Puerto Rico | 36 | Zika | 19 | 53 | AIDP | Luciano et al.13 |
| 2016–2017, Mexico | 34 | Zika, dengue, chikungunya, TORCH, Campylobacter (rectal swab RT-PCR), HSV/enterovirus (CSF) | 2 | 6 | AIDP | Del Carpio et al.14 |
| 2016, Mexico | 8 | Zika, dengue, chikungunya | 0 | 0 | AIDP | Del Carpio et al.15 |
| 2017, Mexico | 7 | Zika, dengue, chikungunya, HIV, TORCH, hepatitis B/C, Brucella, Salmonella, Campylobacter (rectal swab RT-PCR), HSV/enterovirus (CSF) | 0 | 0 | AIDP | Del Carpio et al.16 |
| 2016, Martinique | 30 | Zika | 23 | 77 | AIDP | Rozé et al.17 |
| 2015–2016, Venezuela | 30 | Zika | 22 | 73 | AMSAN | Navas et al.18 |
| 2016, Jamaica | 21 | Zika, dengue, chikungunya | 2 | 9 | AIDP | Ali et al.19 |
| 2015–2016, Multicentre Latin America | 49 | Zika, dengue, HIV, CMV, hepatitis, EBV, chikungunya, herpes, Campylobacter (serum), Mycoplasma | 10 | 20 | AIDP | Ugarte et al.20 |
| 2015, Multicentre Latin America | 24 | Zika, dengue, chikungunya, rubella, measles, parvovirus | 3 | 12 | NR | Cardoso et al.21 |
| 2016, Grenada | 9 | Zika, dengue, chikungunya, West Nile virus, yellow fever, Japanese encephalitis | 4 | 44 | NR | Brenciaglia et al.22 |
| Average incidence | 42 | AIDP |
AMAN: acute motor axonal neuropathy; AMSAN: acute motor-sensory axonal neuropathy; AIDP: acute inflammatory demyelinating polyneuropathy; CMV: cytomegalovirus; CSF: cerebrospinal fluid; EBV: Epstein-Bar virus; EMG: electromyography; HIV: human immunodeficiency virus; HSV: herpes simplex virus; NR: not reported; RT-PCR: reverse transcription polymerase chain reaction; TORCH: panel of toxoplasmosis, other, rubella, cytomegalovirus, herpes simplex virus.
Two studies, conducted in Brazil and Colombia, reported Zika virus positivity in every patient with GBS; another found no case of Zika virus infection among the 41 patients studied. Incidence varied in the remaining studies.
Incidence rates were lower in Central America and the Caribbean, but the majority of studies reported rates above 50%; in North America and specifically Mexico, the incidence of positive results dropped considerably, with no association found between GBS and Zika virus infection.
The average rate of Zika virus infection in the cohorts of patients with GBS was 42%.
A meta-analysis including 3 large South American studies estimated the prevalence of GBS associated with Zika virus at 1.23%.23
In terms of the neurophysiological patterns reported, 12 studies reported acute inflammatory demyelinating polyneuropathy (AIDP), 2 (from Brazil and Colombia) reported acute motor axonal neuropathy (AMAN), and one (Venezuela) reported acute motor-sensory axonal neuropathy (AMSAN).
The serology tests performed vary between studies: 7 only tested for Zika virus, 5 studied the 3 main arboviruses (Zika, dengue, and chikungunya), and 3 studied Zika and dengue viruses only; others additionally searched for other viruses and bacteria, with Campylobacter and the TORCH panel (toxoplasma, other, rubella, cytomegalovirus, and herpes simplex) being the most frequent.
The association between Zika virus infection and GBS is variable, even between studies conducted within the same geographical region; we are therefore unable to reliably establish a causal relationship. As the Zika outbreak spread towards Central America and the Caribbean, incidence of Zika virus infection among patients with GBS decreased, approaching zero in North America.
The predominant neurophysiological pattern was AIDP. AIDP is the most frequent pattern in infectious processes and the predominant type observed in Europe and North America, whereas AMAN is usually more frequent in Mexico and Central and South America; this was not the case in these cohorts. AMSAN was predominant in a study conducted in Venezuela; this pattern has not previously been reported in the region.24
In conclusion, there is a need for additional clinico-epidemiological studies following a uniform protocol that may be applied and reproduced in any population; this would include testing for known pathogens of significant incidence (e.g., Campylobacter); major neurotropic arboviruses such as dengue, Zika, and chikungunya viruses; the TORCH panel; enteroviruses; and West Nile virus, among others.
Please cite this article as: del Carpio Orantes L. Síndrome de Guillain-Barré asociado a zika, experiencia americana. Estudio bibliométrico. Neurología. 2020;35:426–429.