We performed an observational, descriptive, retrospective study analysing cases of acute flaccid paralysis in the North Veracruz district of the Mexican Institute of Social Security in 2016 and 2017. In order to be included in the study, patients had to meet the Asbury and Cornblath criteria and Brighton criteria 1-3, and data had to be available from serological tests for arboviruses (dengue virus, Zika virus, and chikungunya virus), in accordance with the recommendations of the World Health Organization/Pan American Health Organization recommendations for the study of cases of Guillain-Barré syndrome during the arbovirus season. We also analysed the neurophysiological pattern of the disease, treatment outcomes, and mortality. Patients not meeting the inclusion criteria or not wishing to participate in the study were excluded.1
Patients attended in 2016 underwent testing for dengue virus (reverse-transcriptase polymerase chain reaction [RT-PCR] within 7 days of onset and IgG/IgM within 30 days), chikungunya virus (RT-PCR and IgM with a similar timeframe to testing for dengue virus), and Zika virus (serum RT-PCR within 7 days and urine RT-PCR within 14; IgG and IgM within 30 days); to identify other aetiologies, patients attended in 2017 additionally underwent an extended protocol testing for other viral and bacterial pathogens, including RT-PCR for enterovirus and herpes virus in the cerebrospinal fluid (CSF), serum IgG and IgM TORCH screen (toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex), and RT-PCR to detect Campylobacter in the stool.
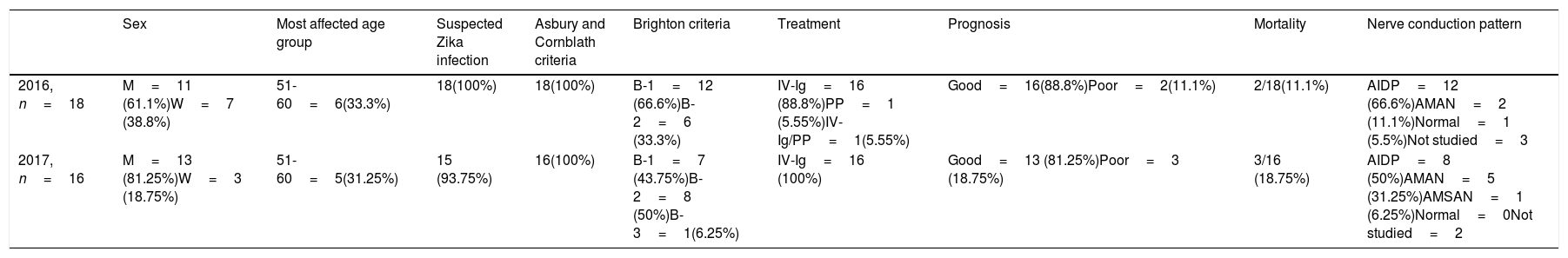
The sample included 34 patients, of whom 24 were men (70.6%) and 10 were women (29.4%); 33 patients (97.1%) met the working definition for suspected Zika virus infection. CSF findings were normal in 19 patients (55.9%), with albuminocytological dissociation in 10 (29.4%) and low glucose levels in 3 (8.8%); no CSF findings were available in 2 patients (5.9%). Head CT scans were performed in 28 patients, with normal results in all cases. Neurophysiological studies identified acute inflammatory demyelinating polyneuropathy (AIDP) in 20 patients (58.8%), acute motor axonal neuropathy (AMAN) in 7 (20.6%), and acute motor-sensory axonal neuropathy (AMSAN) in one (2.9%); the study yielded normal findings in one patient (2.9%) and was not performed in 5 (14.7%). According to the Brighton criteria, the level of diagnostic certainty was level 1 in 19 patients (55.9%), level 2 in 14 (41.2%), and level 3 in one patient (2.9%). Thirty-two patients (94%) were treated with intravenous immunoglobulins (IV-Ig), one was treated with plasmapheresis (2.9%), and one received both treatments in combination (Table 1).
Characteristics of the groups studied.
| Sex | Most affected age group | Suspected Zika infection | Asbury and Cornblath criteria | Brighton criteria | Treatment | Prognosis | Mortality | Nerve conduction pattern | |
|---|---|---|---|---|---|---|---|---|---|
| 2016, n=18 | M=11 (61.1%)W=7 (38.8%) | 51-60=6(33.3%) | 18(100%) | 18(100%) | B-1=12 (66.6%)B-2=6 (33.3%) | IV-Ig=16 (88.8%)PP=1 (5.55%)IV-Ig/PP=1(5.55%) | Good=16(88.8%)Poor=2(11.1%) | 2/18(11.1%) | AIDP=12 (66.6%)AMAN=2 (11.1%)Normal=1 (5.5%)Not studied=3 |
| 2017, n=16 | M=13 (81.25%)W=3 (18.75%) | 51-60=5(31.25%) | 15 (93.75%) | 16(100%) | B-1=7 (43.75%)B-2=8 (50%)B-3=1(6.25%) | IV-Ig=16 (100%) | Good=13 (81.25%)Poor=3 (18.75%) | 3/16 (18.75%) | AIDP=8 (50%)AMAN=5 (31.25%)AMSAN=1 (6.25%)Normal=0Not studied=2 |
AIDP: acute inflammatory demyelinating polyneuropathy; AMAN: acute motor axonal neuropathy; AMSAN: acute motor-sensory axonal neuropathy; IV-Ig: intravenous immunoglobulins; M: men; n: number of patients; PP: plasmapheresis; W: women.
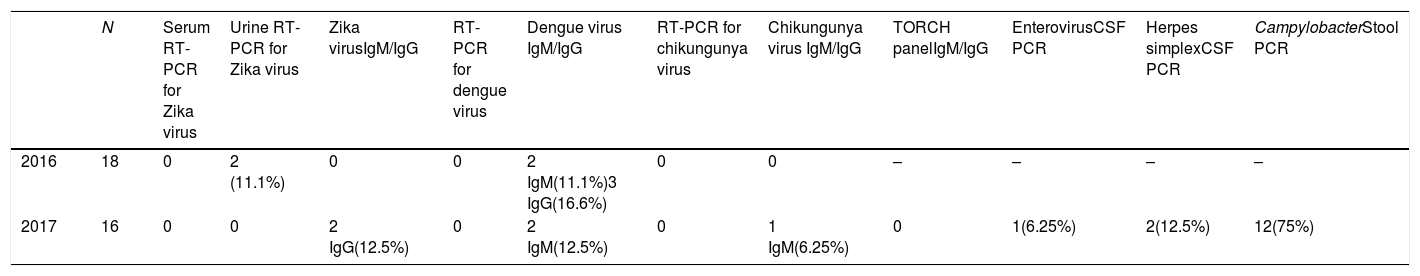
Results of the laboratory analysis of virus infection were as follows: Zika virus serology was performed in 28 patients, of whom 2 (7.1%) presented positive IgG antibodies and none tested positive in the serum RT-PCR or IgM studies; an RT-PCR study for Zika virus was also performed in the urine of 33 patients, with 2 (6.0%) testing positive. Serology testing for chikungunya virus was performed in 32 patients; only one (3.1%) tested positive for IgM antibodies. Serology testing for dengue virus was performed in 32 patients, revealing positive IgM antibodies in 4 (12.5%) and positive IgG antibodies in 3 (9.3%). Testing for the presence of less common viruses detected enterovirus in one (6.25%) and herpes simplex in 2 (12.5%) of the 16 patients tested. Stool PCR studies for Campylobacter were also performed in the 16 patients attended in 2017. PCR findings were positive in 12 patients (75%) and negative in one (6.3%); samples were contaminated in 3 cases (18.8%). Functional and vital prognosis was good in 29 patients (85% of the total sample), with an initial Hughes grade of 4 and a final grade of 2. At the end of the period analysed, 5 patients had died, despite treatment with IV-Ig (Table 2); this represents a mortality rate of 14.7%.
Serological panel studied.
| N | Serum RT-PCR for Zika virus | Urine RT-PCR for Zika virus | Zika virusIgM/IgG | RT-PCR for dengue virus | Dengue virus IgM/IgG | RT-PCR for chikungunya virus | Chikungunya virus IgM/IgG | TORCH panelIgM/IgG | EnterovirusCSF PCR | Herpes simplexCSF PCR | CampylobacterStool PCR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 18 | 0 | 2 (11.1%) | 0 | 0 | 2 IgM(11.1%)3 IgG(16.6%) | 0 | 0 | – | – | – | – |
| 2017 | 16 | 0 | 0 | 2 IgG(12.5%) | 0 | 2 IgM(12.5%) | 0 | 1 IgM(6.25%) | 0 | 1(6.25%) | 2(12.5%) | 12(75%) |
CSF: cerebrospinal fluid; IgG: immunoglobulin G; IgM: immunoglobulin M; RT-PCR: reverse-transcriptase polymerase chain reaction.
The analysis of correlations between variables found that age and Brighton score showed significant positive correlations with patient death (r=0.360, P=.036; and r=0.350, P=.042, respectively). Normal CSF findings showed a significant negative correlation with death (r=−0.360, P=.037).
Despite increases in the incidence of Guillain-Barré syndrome, the direct causal association with Zika virus was weak in this cohort (Zika virus infection was confirmed by urine RT-PCR in only 2 patients) and we were unable to demonstrate cases in which Zika virus was the probable cause; similar results were reported in a cohort in 2016. The search for other pathogens identified Campylobacter as the main aetiological factor in this cohort; this is consistent with the findings of other authors. Testing for agents other than Zika virus confirmed that other neurotropic viruses may also be involved, and should not be disregarded.2
AIDP was the most frequent neurophysiological pattern, unlike in previous studies, in which AMAN was the most significant. This is a relevant finding and is of particular importance in the region of North Veracruz due to its implications on prognosis, which is almost always better for AIDP than for AMAN.3–5
The main limitation of the study is that, despite our planning, it was not always possible to perform all serology studies due to issues with packaging, sample collection, urine or serum alterations, and other problems. Similarly, it was not possible to perform the plaque reduction neutralisation test to distinguish between arboviral infections as the equipment was unavailable; however, positive Zika virus IgG was considered to signal cross-reactivity with other arboviruses rather than an active infection.
Studies with cohorts of patients with Guillain-Barré syndrome should include a complete protocol of infectious diseases, including neurotropic viruses and bacteria known to be potential aetiological agents, enabling better analysis of each case. In addition to RT-PCR of serum and other fluids and serum IgG and IgM studies, studies should ideally include the plaque reduction neutralisation test, in order to distinguish between emerging arboviruses. It should be noted that Zika virus may be found in several bodily fluids; therefore, saliva, semen, and vaginal discharge should also be considered for study.6
Please cite this article as: del Carpio-Orantes L, Peniche Moguel KG, Sánchez Díaz JS, Pola-Ramirez MdR, Mata Miranda MdP, García-Méndez S, et al. Síndrome de Guillain-Barré asociado a zika; análisis de la cohorte delegacional en la región Veracruz norte durante 2016-2017. Neurología. 2020;35:429–431.