Transient headache and neurological deficits with cerebrospinal fluid lymphocytosis (HaNDL) is characterised by migraine-like headache episodes accompanied by neurological deficits consisting of motor, sensory, or aphasic symptoms. Electroencephalogram (EEG) and single photon emission computed tomography (SPECT) may show focal abnormalities that correspond to the neurological deficits. We aim to evaluate the correlation between focal deficit topography and EEG or SPECT abnormalities in 5 new cases.
PatientsWe retrospectively reviewed patients attended in a tertiary hospital (January 2010-May 2014) and identified 5 patients (3 men, 2 women) with a mean age of 30.6±7.7 (21-39) years. They presented 3.4±2.6 episodes of headache (range, 2-8) of moderate to severe intensity and transient neurological deficits over a maximum of 5 weeks. Pleocytosis was detected in CSF in all cases (70 to 312cells/mm3, 96.5-100% lymphocytes) with negative results from aetiological studies.
ResultsAt least one EEG was performed in 4 patients and SPECT in 3 patients. Patient 1: 8 episodes; 4 left hemisphere, 3 right hemisphere, and 1 brainstem; 2 EEGs showing left temporal and bilateral temporal slowing; normal SPECT. Patient 2: 2 episodes, left hemisphere and right hemisphere; SPECT showed decreased left temporal blood flow. Patient 3: 3 left hemisphere deficits; EEG with bilateral frontal and temporal slowing. Patient 4: 2 episodes with right parieto-occipital topography and right frontal slowing in EEG. Patient 5: 2 episodes, right hemisphere and left hemisphere, EEG with right temporal slowing; normal SPECT.
ConclusionThe neurological deficits accompanying headache in HaNDL demonstrate marked clinical heterogeneity. SPECT abnormalities and most of all EEG abnormalities were not uncommon in our series and they did not always correlate to the topography of focal déficits.
El síndrome de cefalea transitoria y déficits neurológicos con linfocitosis de líquido cefalorraquídeo (LCR), conocido por su acrónimo en inglés, HaNDL, se caracteriza por episodios de cefalea de características migrañosas acompañados por síntomas deficitarios motores, sensitivos o de lenguaje. El electroencefalograma (EEG) o la tomografía por emisión de fotón único (SPECT) pueden mostrar anomalías focales consistentes con los déficits neurológicos. Pretendemos evaluar dicha correlación en una serie de 5 nuevos pacientes.
PacientesAnálisis retrospectivo de pacientes atendidos en un hospital terciario (enero del 2010-mayo del 2014, 5 casos [3 varones, 2 mujeres]), de 30,6±7,7 años (21-39). Presentaron 3,4±2,6 (2-8) episodios de cefalea moderada-severa y déficits neurológicos durante un tiempo no superior a 5 semanas. En todos, pleocitosis de LCR (70 a 312 células/mm3, 96,5-100% linfocitos), con estudio etiológico negativo.
ResultadosEEG en 4 pacientes y SPECT en 3. Caso 1: 8 episodios, 4 de hemisferio izquierdo, 3 hemisferio derecho y 1 de tronco, 2 EEG con enlentecimiento temporal izquierdo y bitemporal; SPECT normal. Caso 2: 2 cuadros, hemisférico izquierdo y hemisférico derecho respectivamente y SPECT con flujo disminuido temporal izquierdo. Caso 3: 3 episodios hemisféricos izquierdos; EEG con enlentecimiento temporo-frontal bilateral. Caso 4: 2 cuadros con topografía parieto-occipital derecha y EEG con enlentecimiento frontal derecho. Caso 5: 2 episodios, hemisférico derecho y hemisférico izquierdo, EEG con enlentecimiento temporal derecho; SPECT normal.
ConclusionesExiste gran heterogeneidad clínica en los déficits neurológicos del HaNDL; las alteraciones en SPECT y, sobre todo, en EEG no son infrecuentes y no siempre se relacionan con la topografía clínica.
The syndrome of transient headache and neurological deficits with cerebrospinal fluid (CSF) lymphocytosis (HaNDL) was simultaneously and independently described in 1980 by Swanson et al.,1 who presented 7 cases at the annual meeting of the American Academy of Neurology (which were published a year later),2 and Martí-Massó et al.,3 who presented 3 cases at the extraordinary meeting of the Spanish Society of Neurology, which were subsequently published together with an additional 7 cases in 1984.4–6
HaNDL syndrome is a rare disorder included in the third edition of the International Classification of Headache Disorders (ICHD-3, beta version).7 It is characterised by episodes of migraine-like headache; symptoms of motor, sensory, or language impairment, and lymphocytic pleocytosis, with undetermined aetiology.
Symptoms are benign and self-limiting, and resolve spontaneously in less than 3 months.
Most patients present neurological deficits due to damage to one hemisphere, although several regions may be affected during an episode or successive episodes. Electroencephalography (EEG) and single-photon emission computed tomography (SPECT) have been reported to display focal lesions compatible with neurological deficits.
The purpose of our study is to review the clinical characteristics of HaNDL syndrome and their correlation with EEG and SPECT findings in a series of 5 new patients.
Material and methodsWe retrospectively analysed the clinical histories of the patients diagnosed with HaNDL syndrome in a tertiary care hospital between January 2010 and May 2014. In all cases, we recorded demographic and clinical variables, including a history of headache, any potential trigger factors, number of episodes compatible with HaNDL syndrome, and time elapsed between the first and the last episodes. We analysed the characteristics of the headache and the accompanying clinical symptoms of each episode, CSF parameters, and the results of the complementary tests, with an emphasis on EEG and SPECT findings.
ResultsWe identified a total of 5 patients (3 men and 2 women) with a mean age of 30.6±7.7 years (range, 21-39). Our patients experienced a mean of 3.4±2.62–8 headache episodes of at least moderate intensity and neurological deficits lasting less than 5 weeks in all cases. Two patients had a history of migraine; in these cases, headache episodes compatible with HaNDL syndrome were accompanied by photophobia and/or phonophobia (60%), nausea (20%), or vomiting (20%).
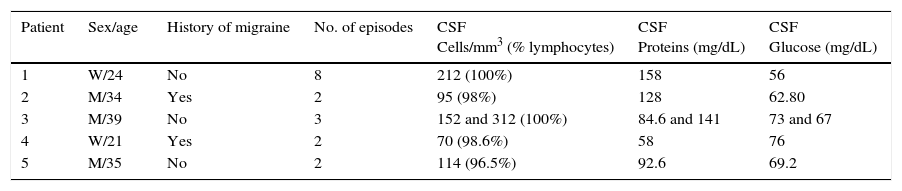
Papilloedema was not observed in any case. In all patients, the CSF analysis revealed pleocytosis (70 to 312cells/mm3), predominantly lymphocytic (96.5%-100%). Table 1 shows the main clinical and CSF characteristics of our 5 patients.
Clinical and demographic characteristics of the 5 patients.
| Patient | Sex/age | History of migraine | No. of episodes | CSF Cells/mm3 (% lymphocytes) | CSF Proteins (mg/dL) | CSF Glucose (mg/dL) |
|---|---|---|---|---|---|---|
| 1 | W/24 | No | 8 | 212 (100%) | 158 | 56 |
| 2 | M/34 | Yes | 2 | 95 (98%) | 128 | 62.80 |
| 3 | M/39 | No | 3 | 152 and 312 (100%) | 84.6 and 141 | 73 and 67 |
| 4 | W/21 | Yes | 2 | 70 (98.6%) | 58 | 76 |
| 5 | M/35 | No | 2 | 114 (96.5%) | 92.6 | 69.2 |
W: woman; M: man.
The results of the bacterial cultures of CSF were negative in all patients. Autoimmune tests and serological tests for neurotropic viruses, Borrelia, Mycoplasma, Brucella, and human immunodeficiency virus yielded negative results in all 5 cases. Brain magnetic resonance imaging (MRI) studies of all patients showed no abnormalities. A computed tomography (CT) perfusion scan was performed in patients 1 and 3 during one of the episodes. In one case the test showed normal results, whereas in the other it revealed a moderate increase in mean transit time in the whole symptomatic hemisphere, with CT angiography results within normal limits.
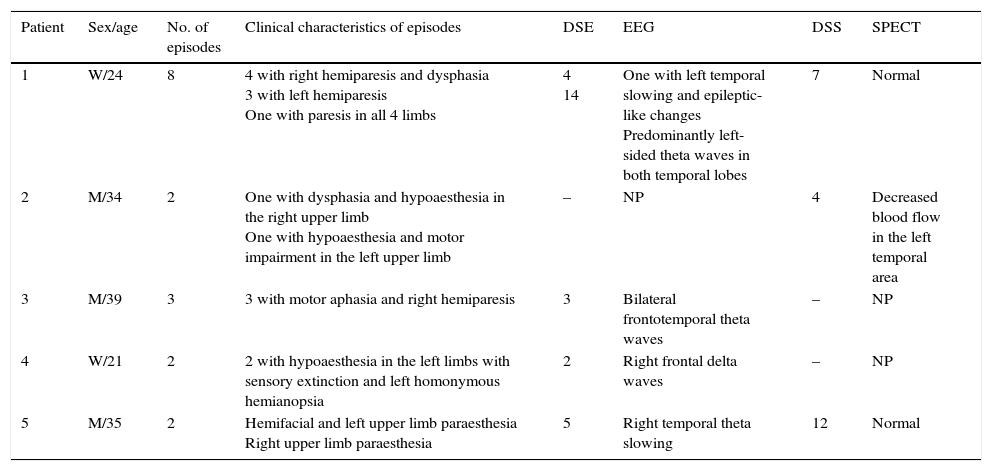
At least one EEG study was available for 4 of the patients, and a SPECT study for 3. Patient 1 had undergone 2 EEG studies. The first of these was conducted during the first episodes and showed focal epileptic-like changes. As symptoms persisted (alternating hemiparesis), an additional study was performed, which showed slower, asymmetrical activity in the cortex of both temporal lobes. As was the case in patient 1, patients 3 and 4 showed EEG changes while they were still symptomatic. Symptoms in patient 5 resolved within 24hours and an EEG study performed 5 days later showed a pathological pattern of cortical activity; solely in this case were EEG changes consistent with the location of the neurological deficit. SPECT findings were normal in patients 1 (performed while the patient was symptomatic) and 5 (after symptoms had resolved). In patient 2, SPECT findings were pathological but did not correspond with the symptoms which had started 4 days previously. Table 2 shows the correlation between the neurological deficit symptoms and the complementary test findings.
Correlation between neurological deficits and EEG and SPECT findings in our series.
| Patient | Sex/age | No. of episodes | Clinical characteristics of episodes | DSE | EEG | DSS | SPECT |
|---|---|---|---|---|---|---|---|
| 1 | W/24 | 8 | 4 with right hemiparesis and dysphasia 3 with left hemiparesis One with paresis in all 4 limbs | 4 14 | One with left temporal slowing and epileptic-like changes Predominantly left-sided theta waves in both temporal lobes | 7 | Normal |
| 2 | M/34 | 2 | One with dysphasia and hypoaesthesia in the right upper limb One with hypoaesthesia and motor impairment in the left upper limb | – | NP | 4 | Decreased blood flow in the left temporal area |
| 3 | M/39 | 3 | 3 with motor aphasia and right hemiparesis | 3 | Bilateral frontotemporal theta waves | – | NP |
| 4 | W/21 | 2 | 2 with hypoaesthesia in the left limbs with sensory extinction and left homonymous hemianopsia | 2 | Right frontal delta waves | – | NP |
| 5 | M/35 | 2 | Hemifacial and left upper limb paraesthesia Right upper limb paraesthesia | 5 | Right temporal theta slowing | 12 | Normal |
DSE: days elapsed from symptom onset to EEG; DSS: days elapsed from symptom onset to SPECT; W: woman; NP: not performed; M: man.
HaNDL syndrome is a disorder included in the differential diagnosis of stroke since it presents with acute neurological deficits.8,9 In emergency care of these patients, a CT perfusion study may reveal significant reductions in cerebral blood flow and, in the case of HaNDL, possibly cerebral blood volume with a prolongation of mean transit time in an arterial territory,8 as observed in one of our cases.
Most of the references to HaNDL syndrome in the literature are isolated cases or small case series, with the exception of the study by Gómez-Aranda et al. which included 50 patients.10 The most frequent neurological deficits in HaNDL syndrome are sensory disorders (70%), frequently manifesting as paraesthesia or decreased sensitivity of the hand which can later affect the arm and the ipsilateral face but rarely involves the lower limbs. Aphasia is also frequent, presenting in 66% of cases, with pure motor aphasia being the most common type (34%). Motor symptoms have been reported in 42% of patients and manifest as a loss of strength on one side of the body. Visual symptoms are less frequent (18%).10–14 In the majority of patients, focal deficits affect one hemisphere only, although several brain regions may be affected during different episodes11–13 in up to 43% of cases.11
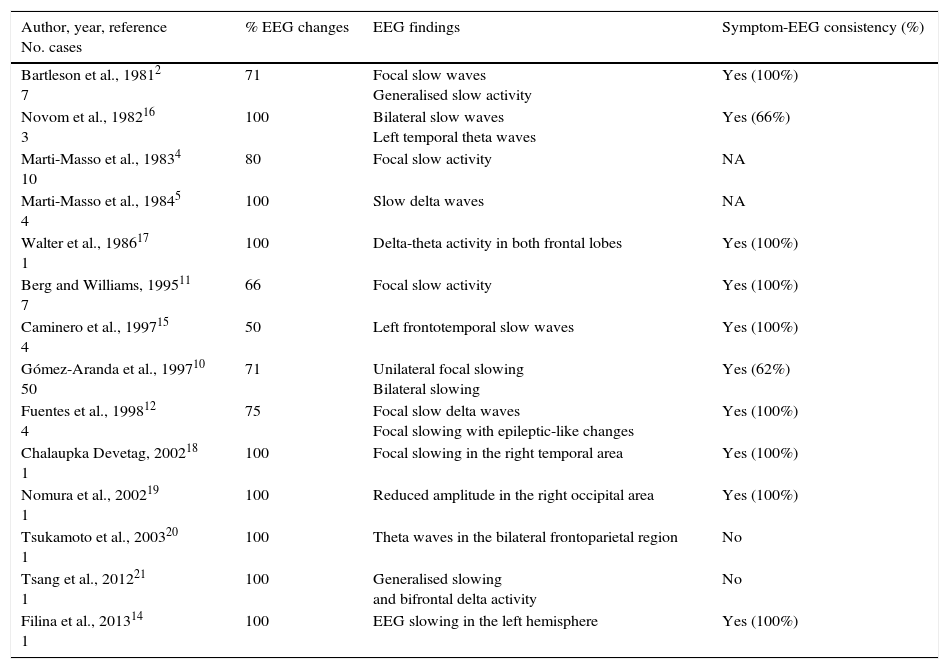
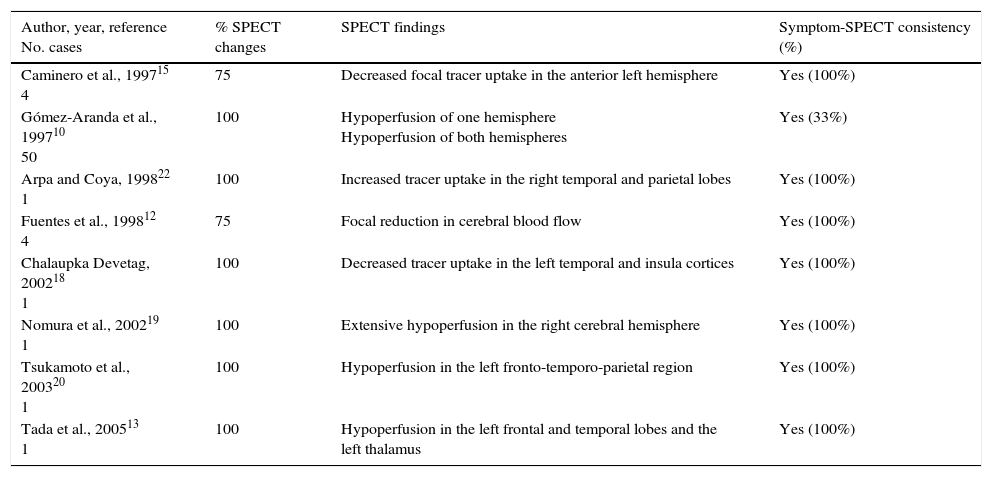
EGG changes and alterations in cerebral blood flow detected by SPECT are traditionally believed to be consistent with focal neurological deficits.12,15 We have found a total of 14 articles describing EEG findings in patients diagnosed with HaNDL syndrome,2,10–12,14–21 and 8 with data from SPECT studies.12,18–20,22 The main findings of these studies are summarised in Tables 3 and 4.
Correlation between EEG findings and neurological deficits in the literature.
| Author, year, reference No. cases | % EEG changes | EEG findings | Symptom-EEG consistency (%) |
|---|---|---|---|
| Bartleson et al., 19812 7 | 71 | Focal slow waves Generalised slow activity | Yes (100%) |
| Novom et al., 198216 3 | 100 | Bilateral slow waves Left temporal theta waves | Yes (66%) |
| Marti-Masso et al., 19834 10 | 80 | Focal slow activity | NA |
| Marti-Masso et al., 19845 4 | 100 | Slow delta waves | NA |
| Walter et al., 198617 1 | 100 | Delta-theta activity in both frontal lobes | Yes (100%) |
| Berg and Williams, 199511 7 | 66 | Focal slow activity | Yes (100%) |
| Caminero et al., 199715 4 | 50 | Left frontotemporal slow waves | Yes (100%) |
| Gómez-Aranda et al., 199710 50 | 71 | Unilateral focal slowing Bilateral slowing | Yes (62%) |
| Fuentes et al., 199812 4 | 75 | Focal slow delta waves Focal slowing with epileptic-like changes | Yes (100%) |
| Chalaupka Devetag, 200218 1 | 100 | Focal slowing in the right temporal area | Yes (100%) |
| Nomura et al., 200219 1 | 100 | Reduced amplitude in the right occipital area | Yes (100%) |
| Tsukamoto et al., 200320 1 | 100 | Theta waves in the bilateral frontoparietal region | No |
| Tsang et al., 201221 1 | 100 | Generalised slowing and bifrontal delta activity | No |
| Filina et al., 201314 1 | 100 | EEG slowing in the left hemisphere | Yes (100%) |
NA: not available.
Correlation between SPECT findings and neurological deficits in the literature.
| Author, year, reference No. cases | % SPECT changes | SPECT findings | Symptom-SPECT consistency (%) |
|---|---|---|---|
| Caminero et al., 199715 4 | 75 | Decreased focal tracer uptake in the anterior left hemisphere | Yes (100%) |
| Gómez-Aranda et al., 199710 50 | 100 | Hypoperfusion of one hemisphere Hypoperfusion of both hemispheres | Yes (33%) |
| Arpa and Coya, 199822 1 | 100 | Increased tracer uptake in the right temporal and parietal lobes | Yes (100%) |
| Fuentes et al., 199812 4 | 75 | Focal reduction in cerebral blood flow | Yes (100%) |
| Chalaupka Devetag, 200218 1 | 100 | Decreased tracer uptake in the left temporal and insula cortices | Yes (100%) |
| Nomura et al., 200219 1 | 100 | Extensive hypoperfusion in the right cerebral hemisphere | Yes (100%) |
| Tsukamoto et al., 200320 1 | 100 | Hypoperfusion in the left fronto-temporo-parietal region | Yes (100%) |
| Tada et al., 200513 1 | 100 | Hypoperfusion in the left frontal and temporal lobes and the left thalamus | Yes (100%) |
EEG changes are present in 86.6% of cases, a percentage comparable with that of our series (100%). The most frequently reported findings consist of focal2,5,10–12,14–16,18 or bilateral10,16,17,20,21 slowing with delta or theta activity, which are again in line with our findings. There is only one previous description of epileptic-like changes similar to those of our patient 1.12 Consistency of EEG changes with neurological deficit topography is far from the traditionally considered 100% (77.3%) but is higher than the 25% found in our series.
SPECT studies show abnormal results in 93.7% of the cases reported in the literature (a percentage above that in our series), and changes are correlated with the topography of the clinical manifestations in 91.6% of the patients. A focal reduction of cerebral blood flow was observed in the vast majority of cases,12,13,15,18,22; furthermore, decreased brain perfusion in a whole hemisphere has also been described.8 We only found one case of focal hyperperfusion in the literature20; this was, however, an atypical case since symptoms recurred after the 3-month time frame defined for HaNDL.
The differential diagnosis between HaNDL and migraine with aura must be established, although CSF changes are specific to this syndrome. Familial hemiplegic migraine is a rare form of migraine with aura that involves motor symptoms: this entity is associated with mutations detected to date in 4 genes (CACNA1A, ATP1A2, SCN1A, and PRRT2).23 Lymphocytosis is characteristic of HaNDL syndrome.11,13 Regarding the complementary test analysed in our study, the SPECT scans performed during migraine auras showed a local reduction of tracer uptake ipsilateral to the origin of the aura symptoms. These abnormalities tend to disappear within 24hours.15 In patients with HaNDL syndrome, in contrast, large hypoperfused brain areas can be found during the acute phase and SPECT tests may display abnormalities for weeks or even months. Regarding EEG findings, 80% of patients with migraine with aura present EEG abnormalities during the ictal phase, corresponding to increased slow-wave baseline cortical EEG activity in 90% of cases; interictal EEG recordings are normal in a higher percentage of cases.24
Lastly, especially during the initial evaluation, HaNDL should be included in the differential diagnosis of stroke, acute meningoencephalitis, and above all meningoencephalitis due to herpes simplex virus 1 (HSV-1). On the one hand, 80% of patients with meningoencephalitis caused by HSV-1 present headaches, 30% to 40% manifest motor deficits, and a third has language disorders.25 EEG abnormalities in these patients can be similar to those described in our series. On the other hand, more than 20% of patients with HaNDL syndrome present fever, and more than half show increased CSF opening pressure.10 In light of the above, the ICHD-3 states that the aetiological study of pleocytosis should be negative.7
In our series, as in previous studies, we observed changes in baseline cortical activity (EEG slowing) and brain hypoperfusion. According to our literature review (Tables 3 and 4), the highest percentages of topographical correlation with clinical deficits correspond to isolated cases or small case series, which possibly overestimate this parameter. The lowest correlations between symptoms and EEG, and symptom and SPECT were observed in a series of 50 patients analysed by Gómez-Aranda et al.10 More than 15 years after that study was published, the aetiology of this entity still remains unknown, as these authors stated. Vascular changes that can be detected during and occasionally weeks after the acute phase, and cortical involvement, as shown by the EEG and maybe related to cortical spreading depression,8 seem diffuse.10 This is why a patient may present different clinical manifestations. Although our study presents some limitations due to its small sample size and retrospective design, the limited correspondence of neurological deficit topography with EEG and SPECT abnormalities in our series support the results obtained by Gómez-Aranda et al. and suggest diffuse involvement in HaNDL syndrome.
Lastly, we recommend performing EEG and SPECT studies in hospitalised patients when there is suspicion of HaNDL syndrome, an infrequent entity whose nature still needs to be understood.
FundingThis study has received no funding of any kind.
Conflicts of interestThere are no conflicts of interest to declare.
Please cite this article as: Barón J, Mulero P, Pedraza MI, Gamazo C, de la Cruz C, Ruiz M, et al. Síndrome HaNDL: correlación entre la topografía del déficit neurológico y las alteraciones en electroencefalograma y SPECT en una serie de 5 nuevos casos. Neurología. 2016;31:305–310.