Patients managed in the Pediatric Palliative Care Integral Unit (PPCIU) have serious neurological conditions that involve significant damage at central nervous system level. The movement disorder is a very common clinical problem and for the patients where an adequate control of muscle tone is not achieved with usual techniques or drugs, intrathecal baclofen therapy (IBT) should be considered.
Materials and methodsDescriptive retrospective study based on the review of clinical records of patients who received IBT being followed by the PPCIU of Madrid Autonomous Region in the timeframe between September 2012 and February 2021.
ResultsIBT was implanted in 8 patients affected by infantile cerebral palsy (ICP) with a Gross Motor Function Scale (GMFCS) IV–V, 3 patients was a Pantothenate kinase deficit-associated neurodegeneration (PKAN), 2 had Acquired Brain Damage, and the remaining 3 had, respectively, 2 glutaric aciduria type I (GA-1), and poly-malformative syndrome. In all patients we observed a period of clinical stability after IBT, we call this period “honeymoon”. Two patients died while in the honeymoon period, at 24.9 and 19.6 months from implantation of the pump; the median of duration of the honeymoon period in the remaining 14 was 14.4 months (IQ: 8.3–25.8).
ConclusionsIBT was not only used in patients with non-progressive diseases, but also in the group of patients with neurodegenerative or progressive diseases. In all of them, after implantation of the device, we have objectified a period of clinical stability and a better control of muscle tone disorders.
Los pacientes que atiende la unidad de atención integral paliativa pediátrica (UAIPP) presentan condiciones neurológicas graves que implican daños importantes del sistema nervioso central. Los trastornos del movimiento y tono muscular son un problema clínico frecuente en este grupo de pacientes, sin conseguirse en muchas ocasiones un adecuado control de los síntomas con las técnicas o fármacos habituales, siendo necesario considerar en estos casos el empleo de la bomba intratecal de baclofeno (ITB).
Material y métodosEstudio descriptivo retrospectivo basado en la revisión de la historia clínica de pacientes que recibieron la ITB en seguimiento por la UAIPP de la Comunidad de Madrid, entre el periodo comprendido de septiembre de 2012 a febrero de 2021.
ResultadosLa ITB fue implantada a 8 pacientes diagnosticados de parálisis cerebral infantil con un Gross Motor Function Scale IV-V o equivalente, 3 pacientes tenían enfermedad neurodegenerativa asociada a déficit de pantotenato quinasa, 2 daño cerebral adquirido y los últimos 3 pacientes padecían respectivamente 2 de aciduria glutárica tipo i y uno de síndrome polimalformativo. En todos los pacientes estudiados se identificó un periodo de estabilidad clínico de los trastornos motores tras la implantación de la bomba que denominamos «luna de miel». Dos pacientes fallecieron encontrándose en periodo de luna de miel con 24,9 y 19,6 meses desde la implantación de la bomba; en los 14 restantes la mediana de duración de luna de miel fue de 14,4 meses (IQ: 8,3-25,8).
ConclusionesEn nuestro estudio la ITB se empleó en pacientes no solo con enfermedades estáticas, sino también en el grupo de pacientes con enfermedades neurodegenerativas o progresivas. En todos ellos, tras la implantación del dispositivo, hemos objetivado un periodo de estabilidad clínica con un mejor control de los trastornos motores.
Patients managed in the Pediatric Palliative Care Integral Unit (PPCIU) have serious neurological conditions that involve significant damage at central nervous system level. For the most part, they are patients with grave infantile cerebral palsy (ICP), since in addition to their baseline disease and associated comorbidity, it is them who will benefit most from the unit's services.1
Owing to the aforementioned central injury, most of the patients will present motor-type disorders 2 Although spasticity is the main clinical picture in 80% of the patients, it is not uncommon to find a combination of spasticity, weakness and/or dystonia all in one patient, which not only hampers movement and is a cause of limited mobility, but also contributes to secondary pain, discomfort, and the appearance of contractures and deformities over time. Finally, if all this is not properly treated in time, it will result in a significant limitation in their activities of daily living, decreasing their quality of life .2,3
Movement disorder management is currently changing. Nowadays, we know that a multimodal, multidisciplinary management is necessary to achieve their optimal, adequate treatment (rehabilitation therapy, physical therapy, muscle relaxant drugs, and botulinum toxin among others) .4,5 For those patients where an adequate control of muscle tone is not achieved through the previously mentioned techniques, or who present a limitation of drug dosage due to the occurrence of secondary effects, other existing therapeutic options such as intrathecal baclofen therapy (IBT) or dorsal rhizotomy and deep brain stimulation should be considered.5
While IBT use is restricted to reducing generalized spasticity, in situations of grave spasticity in which an acceptable control of muscle tone is not achieved despite treatment with botulinum toxin or oral antispastics, its use has been later extended to treating dystonia .6,7 Although studies have mainly focused in spastic ICP, in clinical practice IBT is also used in dystonic ICP and in other progressive and/or non-progressive neurological conditions .8,9 Many of these diseases fall into the scope of those that are usually managed in pediatric palliative care (PPC) .10,11
The aim of this study is to analyze the characteristics of patients managed in a PPC unit who have received IBT (age, sex, diagnosis of disease, motor disability and treatment of baseline), describe the IBT implantation procedure, as well as its impact on treated patients and global management of spasticity and dystonia symptoms.
Materials and methodsDescriptive retrospective study based on the review of clinical records of patients who received IBT being followed by the PPCIU of Madrid Autonomous Region in the timeframe between September 2012 and February 2021.
The UAIPP operating since 2008 provides care for all children requiring pediatric palliative care in Madrid Autonomous Region. Its main characteristic is that it consists of an interdisciplinary team (physicians, nurses, social worker, psychologists), and has the sufficient capacity and infrastructure to provide pediatric care both in the hospital environment or at the patient's home, 24h a day, 365 days a year. It should be noted that 70% of the patients in our unit have serious neurological diseases.
The variables collected in this study were: age and sex of the patients; diagnosis of baseline disease; main motor disability (spasticity, dystonia, or mixed); dosage and drugs used before and after baclofen pump implantation; reason for pump implantation; pump's infusion mode and size of the reservoir; complications due to the device; and time of clinical stability.
Data analysis was carried out using software Stata® v16.0. Quantitative variables are summarized by their median, standard deviation (SD), and interquartile range (IQ); categorical variables are described using percentages.
ResultsIn the period under study, a baclofen pump was implanted in 16 patients, 10 of whom were male and 6 female. The median age at surgery was 13.0 years (IQ: 7.9–15.4), the median time of prior time of evolution of the disease was 8.1 years (IQ: 3.5–12.8). Regarding the diagnosis of the patients treated, the most frequent one was infantile cerebral palsy (ICP) with a Gross Motor Function Scale (GMFCS) 12 IV–V or equivalent, 3 patients had a Pantothenate kinase deficit -associated neurodegeneration (PKAN), 2 had Acquired Brain Damage, and the remaining 3 had, respectively, 2 glutaric aciduria type I (GA-1), and poly-malformative syndrome. In 9 of the patients there was a prevalence of the spastic component (all of them with ICP diagnosis and 1 PKAN), and there was dystonic prevalence in 7 patients (2 patients with ICP, 2 patients with PKAN, 2 with GA 1, and the patient who had the poly-malformative syndrome).
With regards to the drug treatments the patients received in a phase prior to the pump implantation, there were available data for 15 out of the 16 patients (one of them was transferred from another hospital facility, and it was not possible to collect previous reports and documents). Out of the 15 patients we had information of, 15 received treatment with oral baclofen (mean dosage=1.7mg/kg/day; SD: 1.07), 15 with benzodiazepines (10 diazepam, 4 clonazepam, and 1 clobazam) (mean dosage equivalent to diazepam 0.8mg/kg; SD: 0.6), 13 with tizanidine (mean dosage=0.8mg/kg/day; SD: 0.6), and 5 with trihexyphenidyl (mean dosage 1.0mg/kg; SD: 0.9). All 16 patients received physical therapy, and 15 received infiltrations of botulinum toxin.
In 12 of the 16 patients, surgery was indicated due to progressive worsening of their motor impairment despite using conventional muscle relaxants, and in the remaining 4 for being refractory to the base treatment and the appearance of side effects with the medication. All 16 patients were performed a baclofen test prior to the surgical intervention with good response, therefore surgery was indicated in all of them.
All patients were implanted a SynchroMed type II pump, which are placed in the subcutaneous abdominal tissue, all of them are Medtronic products. For 13 patients, the pump had a 20ml reservoir, and a 40ml reservoir pump was used for the other 3. The median of days of post intervention hospitalization was 7 days (IQ: 2.5–13.0). With respect to complications in the period immediately after the surgery, 2 patients had subcutaneous seromas in the abdominal region corresponding to the placement of the pump which required drainage, and 1 patient suffered from meningitis, and required intravenous antibiotherapy and surgical replacement of the device.
The pump's programming mode was simple continuous infusion for 15 patients, and flexible infusion (continuous with programmed boluses) for 1. Over time, later in the evolution, the continuous programming was changed to a flexible one in 12 of the 15 patients receiving it. The median of maximum daily dosage of intrathecal baclofen was 1375 ug/day (IQ: 1078–1908). The median of programmed daily boluses in the 13 patients who received them was 5 (IQ: 5–6).
Oral drug treatment prior to the intervention could be reduced in all patients. Oral baclofen was stopped immediately following the move to intrathecal medication thereof. Concerning the 13 patients receiving treatment with tizanidine, dosage was modified in 1 patient, whereas in the remaining 12 there was a mean daily-dosage reduction of 74.4% (SD: 32.3), being able to suspend the drug after pump implantation in 6 patients. Of the 15 patients receiving benzodiazepines, one needed to temporarily increase dosage by 79% due to clinical decompensation, while in the remaining 14 patients mean dosage could be reduced by 72% (SD: 27.4), being able to suspend it in 8 patients. Of the 5 patients being treated with trihexyphenidyl, medication could be totally suspended in 3 of them.
With reference to the subsequent follow-up of the patient's progress, only 2 of them had complications related to device malfunction over time. One single patient experienced one episode of catheter blockage, which required its realigning, and a malfunction of the pump that indicated its replacement; and the second one also experienced a malfunction of the device, which was also solved by scheduling a new surgery and placing of a new device.
In all patients under study, we identified a period of clinical stability of motor disorders rendered by the reduction and withdrawal of drugs used before IBT and up to a new decompensation. We call this period “honeymoon”. Two patients died while in the honeymoon period, at 24.9 and 19.6 months from implantation of the pump; the median of duration of the honeymoon period in the remaining 14 was 14.4 months (IQ: 8.3–25.8). Upon completion of the study, 5 patients stayed in clinical stability period, and the other 11 died. The median of months from pump implantation until their death was 25.9 months (IQ: 13–30; range: 7.2–68.3) for the deceased patients.
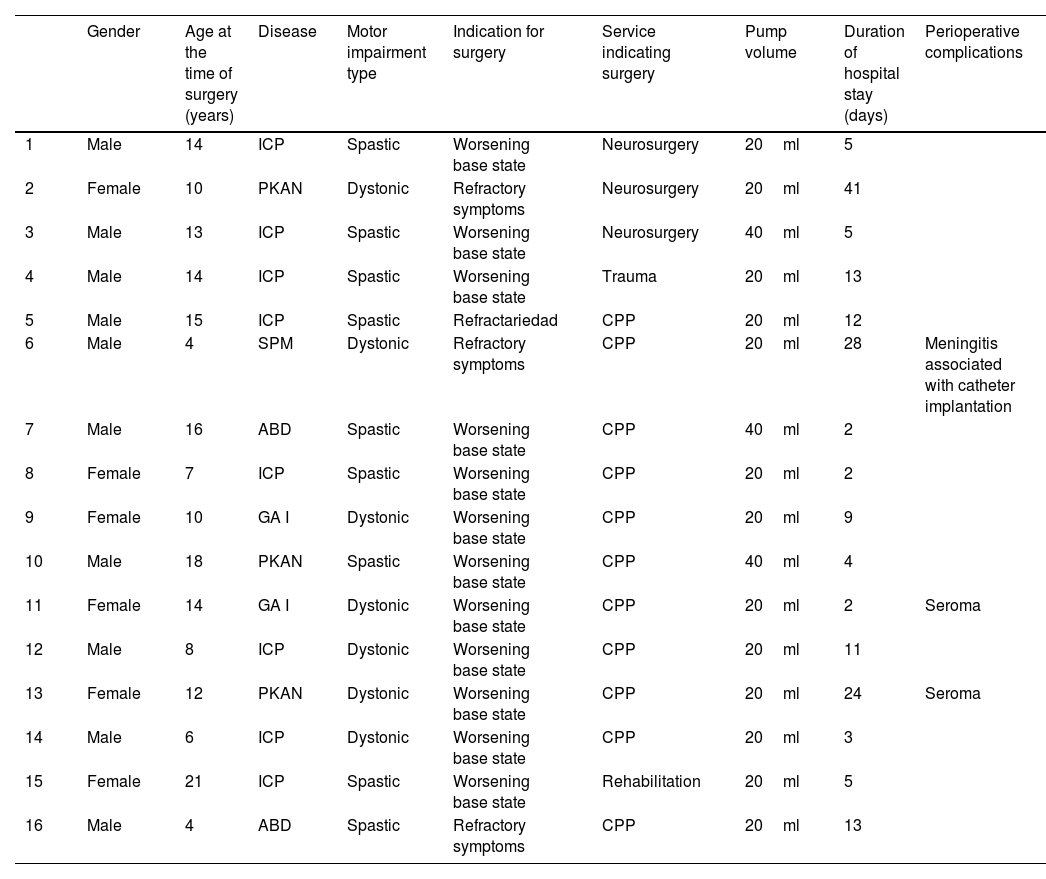
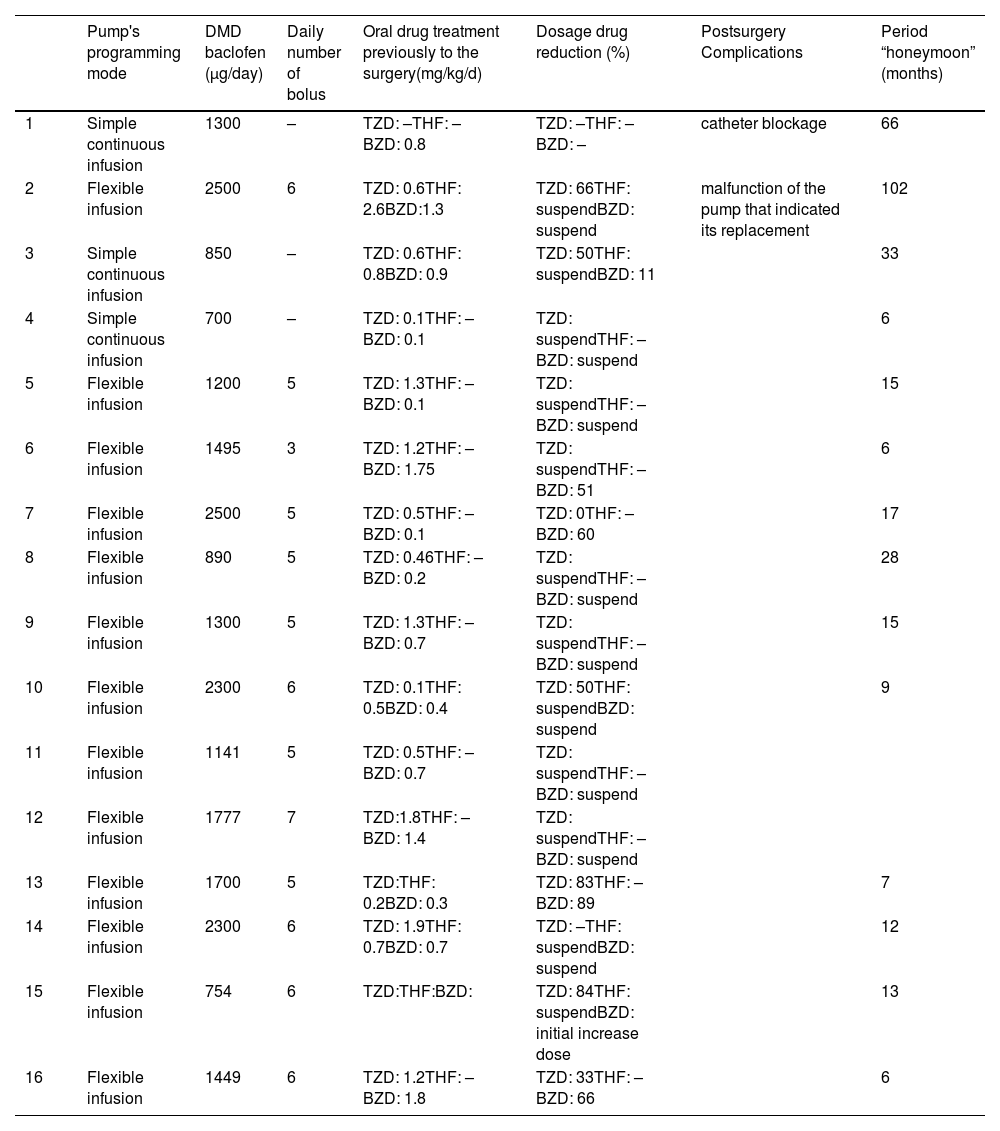
The main clinical characteristics of the patients are represented in Tables 1 and 2.
Clinical characteristics of patients who received intrathecal baclofen pump.
| Gender | Age at the time of surgery (years) | Disease | Motor impairment type | Indication for surgery | Service indicating surgery | Pump volume | Duration of hospital stay (days) | Perioperative complications | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 14 | ICP | Spastic | Worsening base state | Neurosurgery | 20ml | 5 | |
| 2 | Female | 10 | PKAN | Dystonic | Refractory symptoms | Neurosurgery | 20ml | 41 | |
| 3 | Male | 13 | ICP | Spastic | Worsening base state | Neurosurgery | 40ml | 5 | |
| 4 | Male | 14 | ICP | Spastic | Worsening base state | Trauma | 20ml | 13 | |
| 5 | Male | 15 | ICP | Spastic | Refractariedad | CPP | 20ml | 12 | |
| 6 | Male | 4 | SPM | Dystonic | Refractory symptoms | CPP | 20ml | 28 | Meningitis associated with catheter implantation |
| 7 | Male | 16 | ABD | Spastic | Worsening base state | CPP | 40ml | 2 | |
| 8 | Female | 7 | ICP | Spastic | Worsening base state | CPP | 20ml | 2 | |
| 9 | Female | 10 | GA I | Dystonic | Worsening base state | CPP | 20ml | 9 | |
| 10 | Male | 18 | PKAN | Spastic | Worsening base state | CPP | 40ml | 4 | |
| 11 | Female | 14 | GA I | Dystonic | Worsening base state | CPP | 20ml | 2 | Seroma |
| 12 | Male | 8 | ICP | Dystonic | Worsening base state | CPP | 20ml | 11 | |
| 13 | Female | 12 | PKAN | Dystonic | Worsening base state | CPP | 20ml | 24 | Seroma |
| 14 | Male | 6 | ICP | Dystonic | Worsening base state | CPP | 20ml | 3 | |
| 15 | Female | 21 | ICP | Spastic | Worsening base state | Rehabilitation | 20ml | 5 | |
| 16 | Male | 4 | ABD | Spastic | Refractory symptoms | CPP | 20ml | 13 |
ICP: infantile cerebral palsy; (PKAN): Pantothenate kinase deficit-associated neurodegeneration; (ABD): acquired brain damage; (GA-1): glutaric aciduria type I; (SPM): poly-malformative syndrome; CPP: pediatric palliative care.
Clinical characteristics of patients who received intrathecal baclofen pump.
| Pump's programming mode | DMD baclofen (μg/day) | Daily number of bolus | Oral drug treatment previously to the surgery(mg/kg/d) | Dosage drug reduction (%) | Postsurgery Complications | Period “honeymoon” (months) | |
|---|---|---|---|---|---|---|---|
| 1 | Simple continuous infusion | 1300 | – | TZD: –THF: –BZD: 0.8 | TZD: –THF: –BZD: – | catheter blockage | 66 |
| 2 | Flexible infusion | 2500 | 6 | TZD: 0.6THF: 2.6BZD:1.3 | TZD: 66THF: suspendBZD: suspend | malfunction of the pump that indicated its replacement | 102 |
| 3 | Simple continuous infusion | 850 | – | TZD: 0.6THF: 0.8BZD: 0.9 | TZD: 50THF: suspendBZD: 11 | 33 | |
| 4 | Simple continuous infusion | 700 | – | TZD: 0.1THF: –BZD: 0.1 | TZD: suspendTHF: –BZD: suspend | 6 | |
| 5 | Flexible infusion | 1200 | 5 | TZD: 1.3THF: –BZD: 0.1 | TZD: suspendTHF: –BZD: suspend | 15 | |
| 6 | Flexible infusion | 1495 | 3 | TZD: 1.2THF: –BZD: 1.75 | TZD: suspendTHF: –BZD: 51 | 6 | |
| 7 | Flexible infusion | 2500 | 5 | TZD: 0.5THF: –BZD: 0.1 | TZD: 0THF: –BZD: 60 | 17 | |
| 8 | Flexible infusion | 890 | 5 | TZD: 0.46THF: –BZD: 0.2 | TZD: suspendTHF: –BZD: suspend | 28 | |
| 9 | Flexible infusion | 1300 | 5 | TZD: 1.3THF: –BZD: 0.7 | TZD: suspendTHF: –BZD: suspend | 15 | |
| 10 | Flexible infusion | 2300 | 6 | TZD: 0.1THF: 0.5BZD: 0.4 | TZD: 50THF: suspendBZD: suspend | 9 | |
| 11 | Flexible infusion | 1141 | 5 | TZD: 0.5THF: –BZD: 0.7 | TZD: suspendTHF: –BZD: suspend | ||
| 12 | Flexible infusion | 1777 | 7 | TZD:1.8THF: –BZD: 1.4 | TZD: suspendTHF: –BZD: suspend | ||
| 13 | Flexible infusion | 1700 | 5 | TZD:THF: 0.2BZD: 0.3 | TZD: 83THF: –BZD: 89 | 7 | |
| 14 | Flexible infusion | 2300 | 6 | TZD: 1.9THF: 0.7BZD: 0.7 | TZD: –THF: suspendBZD: suspend | 12 | |
| 15 | Flexible infusion | 754 | 6 | TZD:THF:BZD: | TZD: 84THF: suspendBZD: initial increase dose | 13 | |
| 16 | Flexible infusion | 1449 | 6 | TZD: 1.2THF: –BZD: 1.8 | TZD: 33THF: –BZD: 66 | 6 |
DMD baclofen: maximum daily dosage of intrathecal baclofen; TZD: tizanidine; THF: trihexyphenidyl; BZD: benzodiazepines.
The importance of this study lies in considering IBT an efficient treatment for patients where an adequate muscle tone control cannot be achieved through conventional drugs, or where there is a limitation of drug dosage increase due to side effects.
There is clear evidence regarding IBT being an effective therapy for short-term reduction of spasticity in children with ICP. This facilitates patient care, comfort, sleep, and a decrease in skeletal muscle deformity in spastic ICP .13 Evidence is much lower in the case of dystonic ICP, given the small number of studies and of the sampling size studied hitherto .13–15 This is likewise related in our study, since as we mentioned above, 8 of the patients treated were diagnosed ICP and 1 PKAN with prevalence of the spastic component, and 7 had movement disorders with dystonic prevalence: 2 patients with ICP, 2 patients with PKAN, 2 patient with GA-1, and 1 patient with poly-malformative syndrome. All of these patients had serious neurological conditions, both due to their baseline disease or to associated comorbidity, with GMFCS IV–V or equivalent.
With regards to age and sex data, ours were similar to those described in literature,16 only we would like to point out that the mean age at the time of surgery in our sample was 12.1 years. Our unit, despite being a pediatric-focused one, also serves patients over 18, on account of them having diseases related with pediatrics, since they are more easily managed by experienced pediatric teams. The youngest patient described in literature at the moment of surgery was 11 months and weighed 6.4Kg .16 In our sample, the patient of younger age at the time of surgery was 4 years.
Regarding the oral drugs used prior to the moment of deciding on the surgery, out of the 15 patients we had information on, 15 were receiving oral baclofen with a mean dosage of 1.7mg/kg/day (literature recommends 0.75–2mg/kg/day) .17,18 Oral baclofen is very hydrophilic, and its low lipid solubility causes it to cross the hematoencephalic barrier poorly. However, that does not happen with its direct intrathecal administration .18 Directly infusing baclofen into the subarachnoid space of the spinal cord produces a lower incidence of side effects at the brain level, thus achieving a greater reduction of muscle tone compared to orally administered baclofen .19–21 Concerning the rest of the drugs used prior to IBT, 15 patients received treatment with benzodiazepines with a mean dosage in diazepam equivalence of 0.75mg/kg/day (recommended dosage in literature is 0.12–0.8mg/kg/day) .17,18 High doses of the medication might be due to the appearance of tolerance by maintained use of the drug. Thirteen of the patients were also receiving treatment with tizanidine with a mean dosage of 0.8mg/kg/day (much higher doses that those reflected in literature, which recommends 0.2–0.3mg/kg/day),18,19 and with trihexyphenidyl with a mean dose of 0.96mg/kg/day.18,19 All 16 patients received motor physical therapy, and 15 received infiltrations of botulinum toxin periodically. The fact that maximum recommended mean dosage was reached and even exceeded in most drugs may be related with all the patients having muscle tone disturbances with serious or severe hypertonia. Additionally, we count with the limitation that the use and safety of drugs such as trihexyphenidyl and tizanidine has not been established in pediatric population.
In our series, in 12 of the 16 patients, surgery was indicated for showing a progressive worsening of the disease and their motor impairment despite using muscle relaxants, and in the other 4 it was indicated for being refractory to the treatment and the appearance of side effects with the medication.
It is necessary for patients to be of sufficient size for pump placement in the subcutaneous tissue at abdominal level. Patients need at least a minimum weight of 15kg for surgery to be possible .16 There is, however, registration in literature of the aforementioned case of placement of a device in a patient with 11 months and a weight of 6.4kg .16
All 16 patients were implanted pumps in the subcutaneous abdominal tissue. A 20ml reservoir pump was placed in 13 patients, and a 40ml reservoir pump was placed in the other 3. In our hospital, the size of the reservoir used is selected by the Neurosurgery Service, according to the patient's size and weight, thus, most of the reservoirs placed are the 20ml ones, since patients are pediatric population and not adult one. The median in-hospital admission time was 7 days, similar to those in literature relaying stays around 7–10 days .16 Only one patient needed to extend the days for having meningitis and required admission in the intensive care unit and a reintervention after resolution of the infection.
Slow baclofen dose titration to prevent bradycardia and apnea in pediatric population was carried out during hospital admission .22
Regarding the pump's infusion modes programmed, there were two of them: a simple continuous mode, where baclofen infusion is programmed at a specific maintained pace; and a flexible mode, in which there is the option to program boluses (with a determined dose and duration) over a continuous basal dosage. No information regarding IBT infusion modes was found in literature with which to compare our sample. Initial baclofen pump programming mode used in our patients was the simple continuous one mode in 15 patients, and the flexible infusion mode in 1 patient (continuous infusion with programmed boluses). In 12 of the 15 patients who had continuous infusion programmed, it was later changed to flexible mode. The programming mode will depend on the characteristics and needs of each particular patient. However, we found very useful the possibility of programming boluses at predictable times of the day such as washing, physical therapy, night rest, etc. over the continuous basal dosage. The median of maximum dosage of intrathecal baclofen administered was 1375μg/day; the highest registered dosage administered was of 2500μg/day, received by one of the patients with PKAN. In the 13 patients receiving boluses, the median of programmed boluses per day was 5.
As with the implantation of any other device, we may find complications arise from surgery. Cases of infection are rare, those registered in literature vary, but they may reach 9% .16,23 There are registered cases of overdosage by programming error or drug-refilling error in the pump, technical errors related to the functioning of the device, either in the form of malfunction or breakage of the pump, or of disconnected catheter.24 Cases of surgical wound infection and meningitis have also been described. Complications in the long-term may involve increased scoliosis and deformities of the patient, in the form of migration of the pump, breakage of the catheter and cerebrospinal fluid leakage. However, there has been no correlate or comparison with the natural development of the scoliosis deformity in literature .25 With regards to complications in the follow-up period immediately after the surgery, 2 patients had subcutaneous seromas in the abdominal region requiring drainage, and one patient suffered meningitis, requiring intravenous antibiotherapy and surgical replacement of the device after resolution of the infectious process. In clinical follow-up of all the patients, only 2 of them developed complications: one experienced both an episode of catheter blockage which required replacement, and a malfunction of the pump that required replacement six years after its initial placement; and the second patient also experienced a device malfunction four years after its initial placement, which was solved by a new surgery scheduling and placement of a new device. Both patients needed in-hospital admission, and their withdrawal syndrome was treated as an emergency, requiring the administration of benzodiazepines in continuous infusion until achieving the patient's stabilization and solving the problem with a new surgery scheduling.
In the follow-up of all patients, a decrease and significant withdrawal of all drugs used prior to IBT has been registered. In the case of oral baclofen, it was suspended immediately following the move to intrathecal medication. Regarding the 13 patients receiving treatment with tizanidine, dosage could not be modified in 1 patient, whereas in the remaining 12 there was a mean daily dosage reduction by 74.4%, being able to suspend the drug totally in 6 patients after pump implantation. Of the 15 patients being treated with benzodiazepines, dosage had to be temporarily increased by 79% in 1 patient due to clinical decompensation of the motor disorder (patient with ICP with prevalence of dystonic component) who had a decompensation of the baseline status during the first days after surgical intervention, while in the other 14 patients, dosage could be reduced by a median 72%, being able to suspend it in 8 patients. In 3 of the 5 patients being treated with trihexyphenidyl, this could be suspended.
In all patients studied, we identified a period of clinical stability of the motor disorders following pump implantation. This was rendered by a better control of the muscle tone conterminous with IBT, and a decrease and significant withdrawal of the drugs used previously and until the time of a new decompensation or the need to reintroduce or increase oral dosage of drugs.
Inevitably, due to the natural history of the disease, at some point these patients suffer a decompensation, and it is necessary to reintroduce said medication again.26 We call this period of transient clinical stability “honeymoon”. The median duration of this period in 14 patients was 14.4 months (IQ: 8.3–25.8), 2 patients died while in the honeymoon period at 24.9 and 19.6 months since pump implantation. Upon completion of the study, 5 patients remained in clinical stability period without experiencing significant decompensations in their basal status that required reintroduction or dosage increase of muscle relaxant drugs. The remaining 11 patients died on account of their basal disease, or of complications derived from it. In the deceased patients, the median of months from pump implantation until their death was 25.9 months.
We could consider as a limitation of our study its retrospective nature, and the fact that the data collection was based on information from the patients’ clinical records, with its possible and subsequent loss of information. Furthermore, we have not used Muscle tone assessment scales (Ashwort scale),27,28 nor Quality of life assessment scale, or comparison group. An assessment measure used in several studies reported in literature was the COPM (Canadian Occupational Performance Measure) 29 in Occupational Therapy. This assessment measure is used to identify, designate, assess, and validate areas of concern in the patient and their families, focusing on their needs, which also favors treatment adherence. The most frequently identified items in these studies were facilitating getting dressed, improvement in body position and/or transferring, improvement in pain, hygiene/washing among others. In overall, these studies conclude that a greater comfort of the patients has been achieved with IBT, by favoring global care of the patient and increased satisfaction of parents and/or caregivers.4 It is true that the limitation of these studies lies in the subjectivity of the criteria and opinions of the parents who have completed the questionnaire. It is therefore necessary to establish more objective measurement criteria in order to be able to establish a better correlation, taking also into account the heterogeneity of the group of patients, environmental and individual factors, and health factors related to quality of life. The purpose of this studies is to show evidence of the benefit of IBT in pediatric population toward improving their motor function, reducing pain secondary to spastic muscle, and preventing possible future complications and orthopedic deformities that may arise.
ConclusionsIn our study, IBT was not only used in patients with non-progressive diseases, but also in the group of patients with neurodegenerative or progressive diseases. In all of them, after implantation of the device, there is a significant reduction both of the dosage and the number of the drugs used previously, thus objectifying a period of clinical stability we call “honeymoon” with a median duration of 14.4 months (IQ: 8.3–25.8) and a better control of muscle tone disorders. The complication rate in the immediate response and subsequent follow-up was acceptable, which supports it being an effective therapy and safe in pediatric population as it is in adults. Further prospective studies are needed to confirm and corroborate these data.
Conflict of interestsThe authors declare that they have no conflict of interest.