Cerebral embolism caused by a myxoma in the left atrium is a rare but usually fatal event. Atrial myxomas represent 50% of all primary cardiac tumors; 75% of them are located in the left atrium.1 Myxomas usually appear between the ages of 30 and 60. Pedunculated myxomas, which have an irregular morphology, are the type of myxoma most likely to cause embolism since they are mobile.2 Transoesophageal echocardiography is a safe, accurate, and non-invasive means of diagnosing these myxomas.3 Treatment with intravenous fibrinolysis or rescue mechanical thrombectomy is based on previously published cases.4,5 However, no guidelines or studies provide reliable evidence supporting use of these techniques.
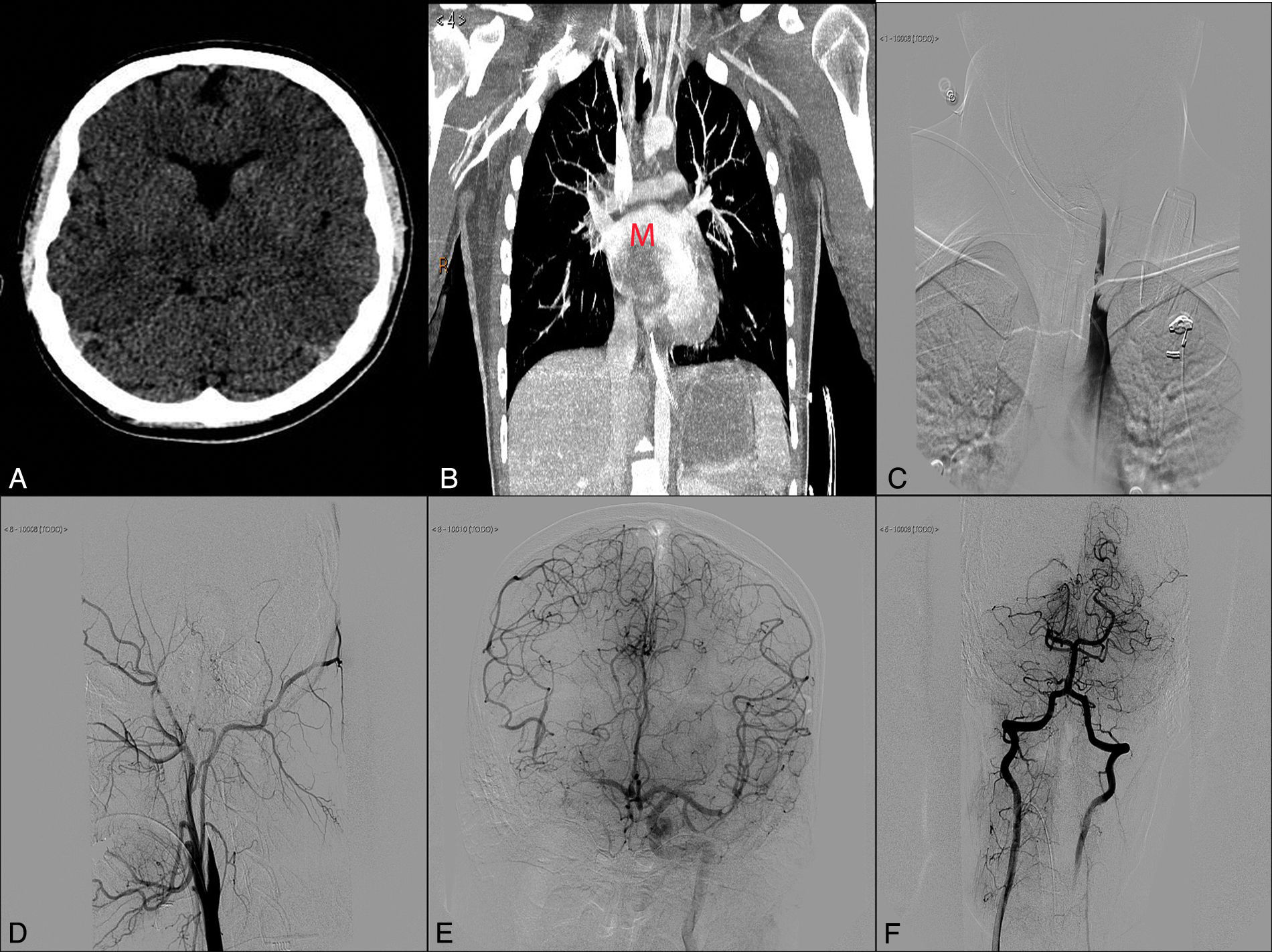
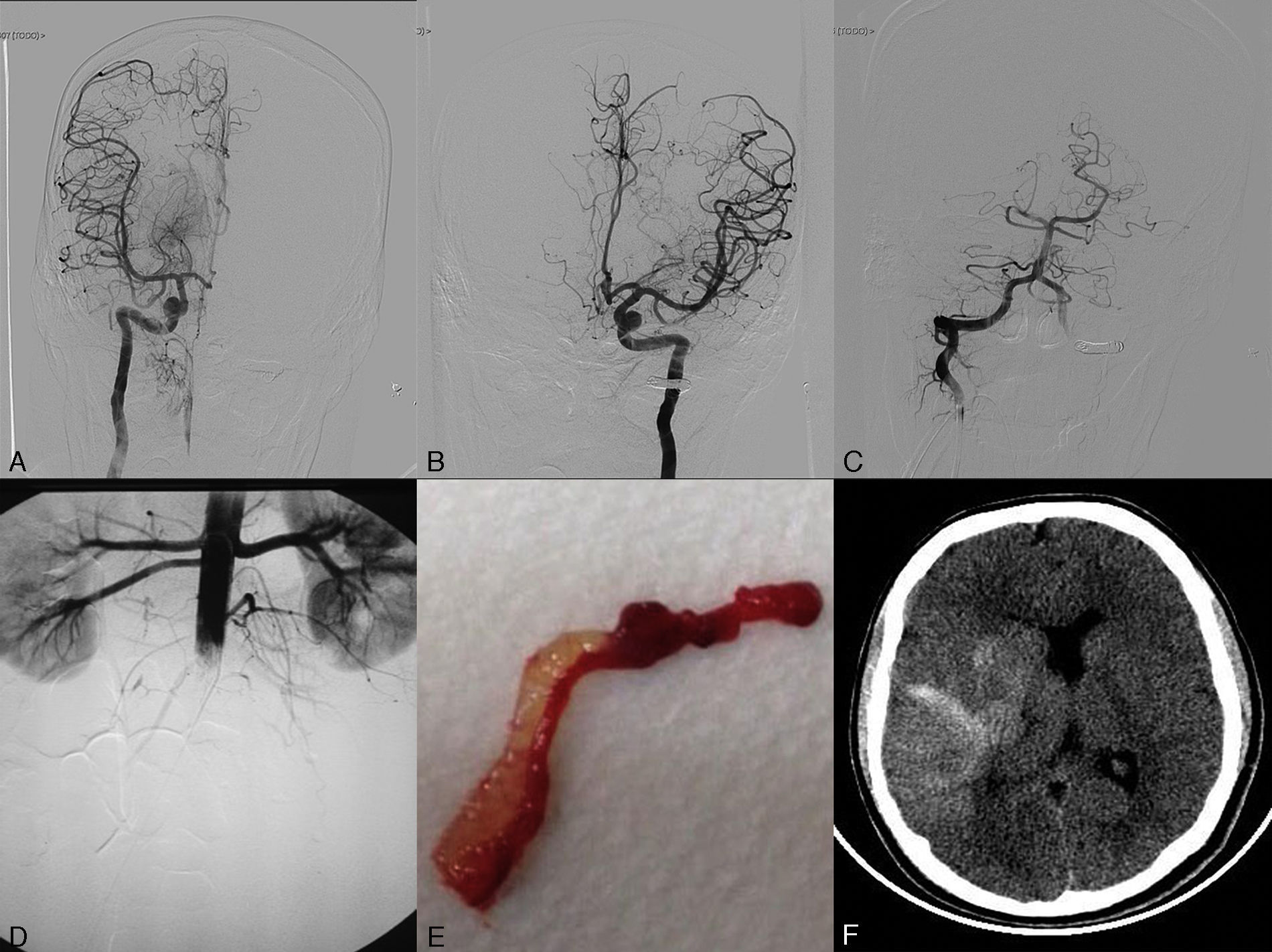
We present the case of a 17-year-old adolescent who was admitted to our hospital's emergency department due to sudden loss of consciousness while he was exercising. He had no known drug allergies, history of drug use, or any other relevant medical history. The general examination showed a blood pressure of 110/60mmHg, 110bpm, and no fever. Cardiac auscultation revealed a rhythmic heart sound with a systolic murmur that could be heard in all areas and radiated to both carotid arteries. In the neurological examination, the patient scored 32 on the NIHSS (2+2+2+2+2+3+3+4+3+4+0+2+0+2+1). Time from symptom onset to assessment by a neurologist was 45minutes. The emergency department performed a complete blood count, which yielded normal results, and an ECG, which revealed sinus tachycardia at 108bpm and no signs of acute ischemia. A simple cranial CT scan showed no anomalies (Fig. 1). A CT angiography performed before the intravenous fibrinolysis showed occlusion of the distal segment of the right internal carotid artery (ICA) and stenosis of the distal basilar artery, affecting the exit of the right posterior cerebral artery (PCA). In view of these findings, we decided to administer intravenous fibrinolysis; our patient's guardians signed the informed consent form. At this point, door-to-needle time was 50minutes. While receiving fibrinolytic treatment, the patient underwent a transthoracic echocardiography which revealed a large pedunculated mass with irregular borders in the left atrium. A few days later, a thoracic CT scan confirmed the presence of an intracardiac mass (Fig. 1). After thrombolytic treatment, our patient scored 26 on the NIHSS and we opted to conduct a rescue mechanical thrombectomy. Door-to-femoral puncture time was 130minutes. When the patient arrived at the interventional neuroradiology department, his lower limbs were pale, cold, and livid. As we were unable to register his blood pressure, we administered norepinephrine before starting the procedure. An angiography study revealed occlusion of the proximal left subclavian artery (SA) due to a thrombus which penetrated the origin of the left vertebral artery (VA). It also revealed occlusion of the right ICA, right middle cerebral artery (MCA), and the proximal segment of the right PCA, with a partially recanalised thrombus in the M1–M2 segment of the left MCA (Fig. 1). To address the multiple occlusions, we performed thrombectomies in the following order: right ICA, right MCA, and left MCA (2 passes in each) with a Solitaire FR device (4× 20). We also coiled the left VA at V3–V4 below the left posterior inferior cerebellar artery to prevent new embolic events in the basilar artery due to the thrombus in the left SA (Fig. 2). An angiography study conducted after embolectomy revealed occlusion of the abdominal aorta (Fig. 2), both iliac arteries, both femoral arteries, and the left SA (Fig. 1). The patient subsequently underwent thromboendarterectomy of the abdominal aorta, the iliac and femoral arteries, and the axillary and humeral circumflex arteries, all of which achieved partial recanalisation. Despite the above procedures, a neuroimaging study taken 24hours after admission revealed diffuse hemorrhagic transformation in the right temporoparietal area affecting the basal ganglia and causing a mass effect leading to a midline shift of approximately 10mm. An ischaemic area was also found in the territory of the right PCA (Fig. 2). The patient died due to multiple systemic complications. The samples of the material removed during mechanical thrombectomy confirmed a myxoma as the source of the emboli (Fig. 2).
(A) Simple cranial CT scan (axial slice). (B) Thoracic CT-angiography showing an intracardiac mass (M). (C) Thrombus in the left subclavian and vertebral arteries. (D) Artery angiogram (lateral projection) showing occlusion of the right internal carotid artery. (E) Left internal carotid artery angiogram (anteroposterior projection) displaying a thrombus at the bifurcation of the M2 segment of the left middle cerebral artery with considerable pial collateral circulation to the right hemisphere. (F) Angiogram of the right vertebral artery with retrograde flow from the left vertebral artery and occlusion of the P1 segment of the right posterior cerebral artery.
(A) Angiogram of the right internal carotid artery (anteroposterior projection) showing complete recanalisation after the second pass. (B) Left internal carotid artery angiogram (anteroposterior projection) showing complete recanalisation of the middle cerebral artery after the second pass. (C) Left vertebral artery angiogram (anteroposterior projection) showing coil occlusion. (D) An infrarenal abdominal aorta angiogram (anteroposterior projection) conducted after embolectomy revealed occlusion. (E) Thrombus with myxomatous material removed in the embolectomy. (F) Simple, non-contrast CT scan (axial slice) at 24hours. The image reveals hemorrhagic transformation in the right temporoparietal area.
Systemic embolism is a potential complication of atrial myxomas, especially those located in the left atrium; emboli travel to the cerebral arteries and are the cause of 0.5% of all ischaemic strokes.6 Emboli usually consist of tumor tissue or thrombi that form on the surface of the myxoma. The effectiveness of intravenous fibrinolysis varies, and it may depend on the composition of the thrombus; for example, thrombi of myxomatous tissue are more difficult to break down.7 Likewise, as fibrinolysis may break down any thrombi on the surface of the myxoma, the treatment may increase the risk of embolism. Lastly, some authors state that patients with cardiac myxoma receiving intravenous systemic thrombolytic therapy are at high risk for hemorrhage due to the high incidence of preexisting brain microaneurysms.8 Regarding use of mechanical thrombectomy in these patients, recently published cases report good rates of recanalisation and functional improvement.9,10 In one of these cases, the authors used a stent retriever plus a Penumbra® aspiration pump to prevent distal migration of the thrombus, and achieved recanalisation of the occluded segment.9
Rescue endovascular treatment, which was shown to be effective in recent studies, was not successful in our case.11–14 Patients under 18 will require an aetiological diagnosis as quickly as possible because of lack of evidence on the safety and effectiveness of intravenous fibrinolysis and rescue mechanical thrombectomy for this age group. Given that the cause of ischaemic symptoms in young patients may contraindicate fibrinolysis, they will need quick screening tests to help prevent any potential complications of fibrinolytic treatment. In any case, diagnostic tests should never delay reperfusion treatment.
In our case, performing a heart ultrasound before intravenous fibrinolysis would have revealed the cause of multiple embolism and helped in therapeutic decision-making. Intravenous fibrinolysis was not fully effective, which was probably due to the composition and aggressiveness of the source of embolism. This treatment could have been omitted in favor of thrombectomy with aspiration devices that may have extracted myxomatous material more easily. This approach would have been a more effective means of reperfusing occluded vessels.
Given the low incidence of this type of stroke, establishing diagnostic and treatment criteria is a complex task. The final decision resides with the neurologist or neuroradiologist responsible for each specific case. Multicentre studies and clinical trials should be conducted in this population to provide scientific evidence on treatment of this entity.
FundingThis study has received no public or private funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Zapata-Arriaza E, Pardo-Galiana B, González-García A, Montaner Villalonga J. Trombólisis intravenosa y trombectomía en paciente joven con ictus isquémico por mixoma auricular desconocido: ¿hay suficiente evidencia en los recientes ensayos clínicos de reperfusión para estos casos? Neurología. 2017;32:404–407.
This study has not been presented at any scientific conferences.