To describe baseline and procedural characteristics and clinical outcomes of isolated striatocapsular infarct (iSCI) after mechanical thrombectomy in patients with large-vessel occlusion of the anterior cerebral circulation and its clinical outcome.
MethodsWe performed a longitudinal study including all patients treated with mechanical thrombectomy at our centre between 2015 and 2017; patients were divided into 2 groups (iSCI and non-iSCI) according to whether they presented iSCI in a control CT scan at 24 hours.
ResultsOf the 83 patients identified, 22.9% developed an iSCI. There were no statically significant differences in baseline characteristics or in reperfusion times. Patients presenting iSCI showed better collateral circulation and better reperfusion rates in the bivariate analysis. No significant difference was observed for mortality at discharge or at 3 months, or for functional prognosis at 3 months.
ConclusionsEven if successful reperfusion is achieved, iSCI is a common sequela, independently of reperfusion time, especially in patients with good collateral circulation.
Describir las características basales y procedimentales asociadas al desarrollo de infarto estriatocapsular aislado (IECa) tras la trombectomía mecánica (TM) en los pacientes con oclusión de gran vaso de la circulación anterior, así como su pronóstico.
MétodosEstudio longitudinal en el que se identificaron los pacientes tratados con TM en nuestro centro entre 2015 y 2017 y se dividieron en dos grupos en función de la existencia o no de un IECa (grupo IECa y grupo nIECa) en la TC de control a las 24 h.
ResultadosDe los 83 pacientes tratados con TM, un 22,9% desarrollaron un IECa. No hubo diferencias estadísticamente significativas en cuanto a las características basales ni en cuanto a los tiempos de reperfusión. Los pacientes con IECa tuvieron mejor colateralidad y mejores tasas de reperfusión en el análisis bivariante. No hubo diferencias en la mortalidad al alta ni a los 3 meses, ni en el pronóstico funcional a los 3 meses.
ConclusionesAunque se consiga una reperfusión exitosa, el IECa es una secuela frecuente e independiente del tiempo de reperfusión, especialmente en aquellos pacientes con buena colateralidad.
The striatocapsular area is made up of a group of anatomically independent but physiologically related structures, including the caudate nucleus, putamen, internal capsule, and globus pallidus, among others. Furthermore, these structures present strong anatomical and functional connections with the cerebral cortex, diencephalon, and brainstem. Together, they perform a wide range of functions (movement control, learning, cognition, memory, and language, among others); therefore, lesions to the striatocapsular area may cause numerous neurological dysfunctions, which may manifest clinically as extensive lesions with cortical involvement potentially leading to significant disability.1 The striatocapsular area is supplied by a group of arteries including the recurrent artery of Heubner (a branch of the anterior cerebral artery) and the lenticulostriate arteries (branches of the M1 segment of the middle cerebral artery [MCA]).2 Unlike the cortical region, these arteries are not supplied by collateral branches and do not anastomose with other arteries.2–4 This makes the region especially susceptible to ischaemia.
Mechanical thrombectomy (MT) represents a new paradigm in the treatment of acute stroke secondary to anterior circulation large artery occlusion. However, the aforementioned lack of collateral supply limits the ability of MT to salvage ischaemic tissue of the striatocapsular region in cases of occlusion proximal to the orifices of the lenticulostriate arteries.2,5
The aim of this study is to analyse the prevalence of isolated striatocapsular infarct (iSCI) in patients undergoing MT in order to determine the baseline characteristics and parameters associated with this entity and to describe its functional prognosis.
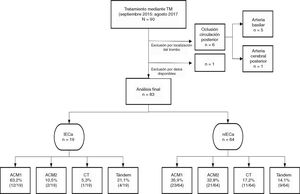
Patients and methodsWe conducted a longitudinal study that prospectively collected data on all MTs (with or without intravenous thrombolysis) performed between September 2015 and August 2017 at our centre (a reference hospital and the only one equipped to perform MT in Aragon); patients belonged to our hospital or to others in the autonomous community of Aragon. We included patients with a diagnosis of stroke due to occlusion of the intracranial internal carotid artery, the M1 or M2 segment of the MCA, or tandem occlusion (simultaneous occlusion of the extracranial internal carotid artery and ipsilateral MCA). We excluded patients who underwent MT due to posterior circulation occlusion. We identified those patients in whom iSCI was detected in a 24-hour follow-up CT scan, and performed a descriptive analysis of the study population.
We subsequently performed a comparative analysis between the case group (iSCI group), including patients with follow-up CT scans displaying iSCI after MT, and the control group (non-iSCI group), including those patients not showing this imaging finding. We performed a statistical analysis of the baseline characteristics (age, sex, hypertension, diabetes, dyslipidaemia, smoking, alcohol consumption, atrial fibrillation, history of carotid artery disease, baseline NIHSS score, use of intravenous fibrinolysis, strokes with unknown time of onset) and procedural characteristics (reperfusion times, degree of collateral circulation, and degree of reperfusion).
To analyse outcomes (in-hospital mortality, mortality at 3 months, and functional prognosis at 3 months), we excluded patients from the non-iSCI group not presenting lesions in the follow-up brain CT scan and those with malignant infarction, as both findings strongly influence prognosis. We then compared the iSCI group with each of the defined subgroups:
- •
iSCI group vs remaining non-iSCI group ([iSCI + cortical] + isolated cortical lesion).
- •
iSCI group vs (iSCI + cortical).
- •
iSCI group vs isolated cortical lesion.
iSCI was defined as a hypodense region limited to the striatocapsular area, shown in the follow-up brain CT scan (24 h after MT), measuring more than 20 mm in diameter without affecting any other brain area; successful reperfusion was defined as a TICI score of 2b or 3. Reperfusion was considered to have failed in patients with a TICI score of 0/1. We used the modified Tan scale6 to analyse the degree of collateral circulation: scores of 2-3 were considered as “good collateral circulation,” with scores of 3 indicating “excellent collateral circulation.” The reperfusion times used were time from symptom onset to reperfusion (TSOR) and time from symptom onset to groin puncture (TSOP). We used the modified Rankin Scale (mRS) to assess functional prognosis, and defined scores ≤ 2 as favourable.
Data analysisData were processed using the SPSS 16 and Office 2007 statistical software. Normal distribution of continuous variables was analysed using the Kolmogorov–Smirnov test. We used the arithmetic mean and the standard deviation as measures of central tendency and dispersion for normally-distributed quantitative variables. For the inferential analysis, we used the t test. For non–normally distributed quantitative variables, we used the median as a measure of central tendency, the interquartile range as a measure of dispersion, and the Mann-Whitney U test for the inferential analysis. Qualitative variables are expressed as frequencies and percentages, and were compared using the chi-square test in the inferential analysis. The significance threshold was set at P < .05. We performed a multivariate analysis using binary logistic regression and included statistically significant variables, as well as those believed to have a significant influence on the dependent variable, in the bivariate analysis.
ResultsStudy populationWe identified a total of 83 patients treated with MT, with 45.8% (38/83) being men. Median age was 74 (65-82) years and median baseline NIHSS score was 17 (13-21). Median TSOR and TSOP were 240 (158-285.7) and 315 (240-367.5) minutes, respectively. Time of onset was unknown in 23.4% of patients (18/77), and 65.8% (54/82) underwent intravenous fibrinolysis in addition to MT. Clinical onset consisted of total anterior circulation infarct in 85.5% of patients (71/83).
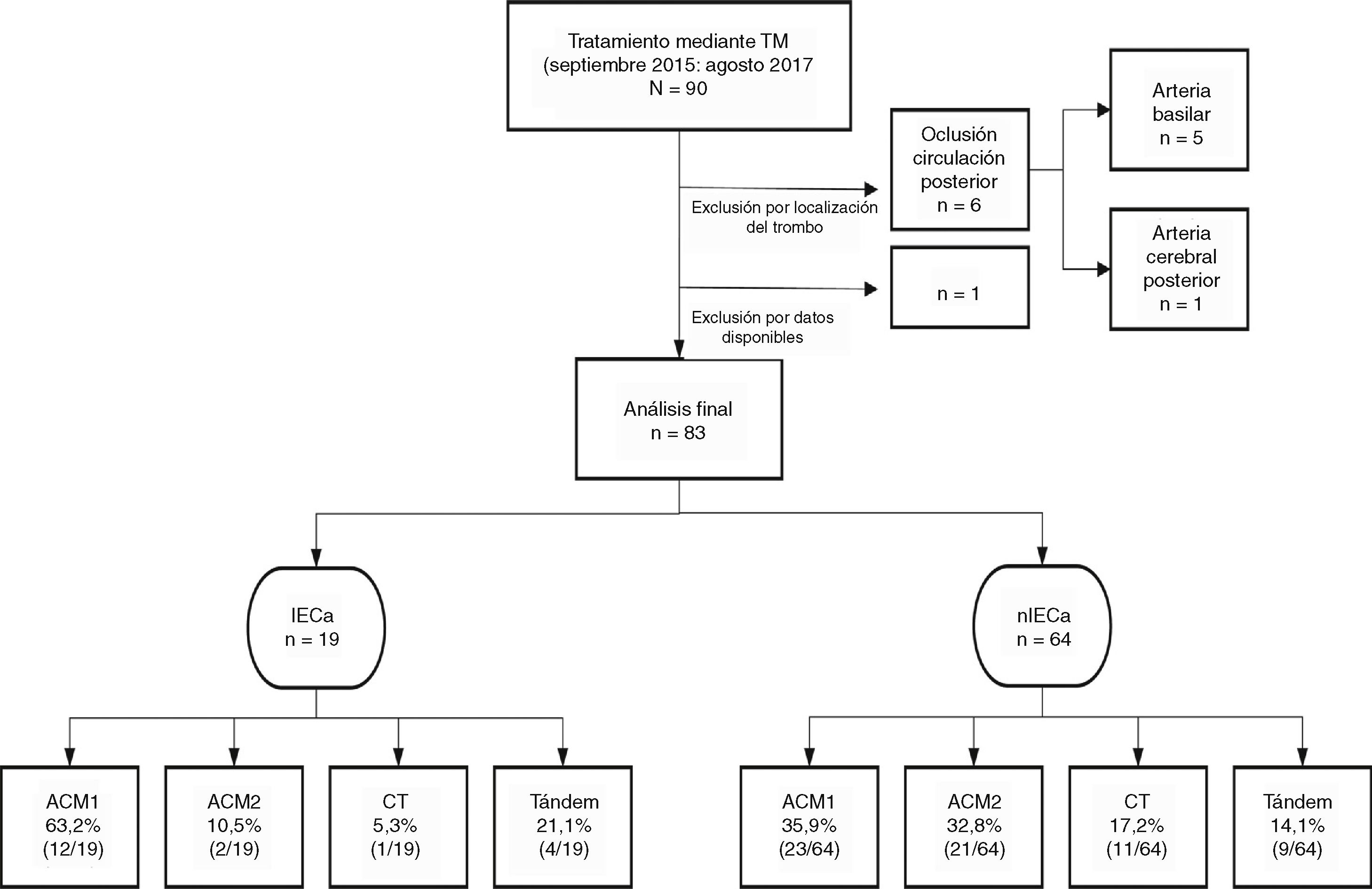
An iSCI was identified in 22.9% (19/83) of cases in the 24-hour follow-up CT scan. In most of the patients who developed iSCI, the thrombus was located in the M1 segment of the MCA (63.1%), as shown in Fig. 1.
Sample distribution by thrombus location.
M1 MCA: segment M1 of the middle cerebral artery; M2 MCA: segment M2 of the middle cerebral artery; TCA: terminal carotid artery; iSCI: isolated striatocapsular infarct; non-iSCI: non-isolated striatocapsular infarct; MT: mechanical thrombectomy.
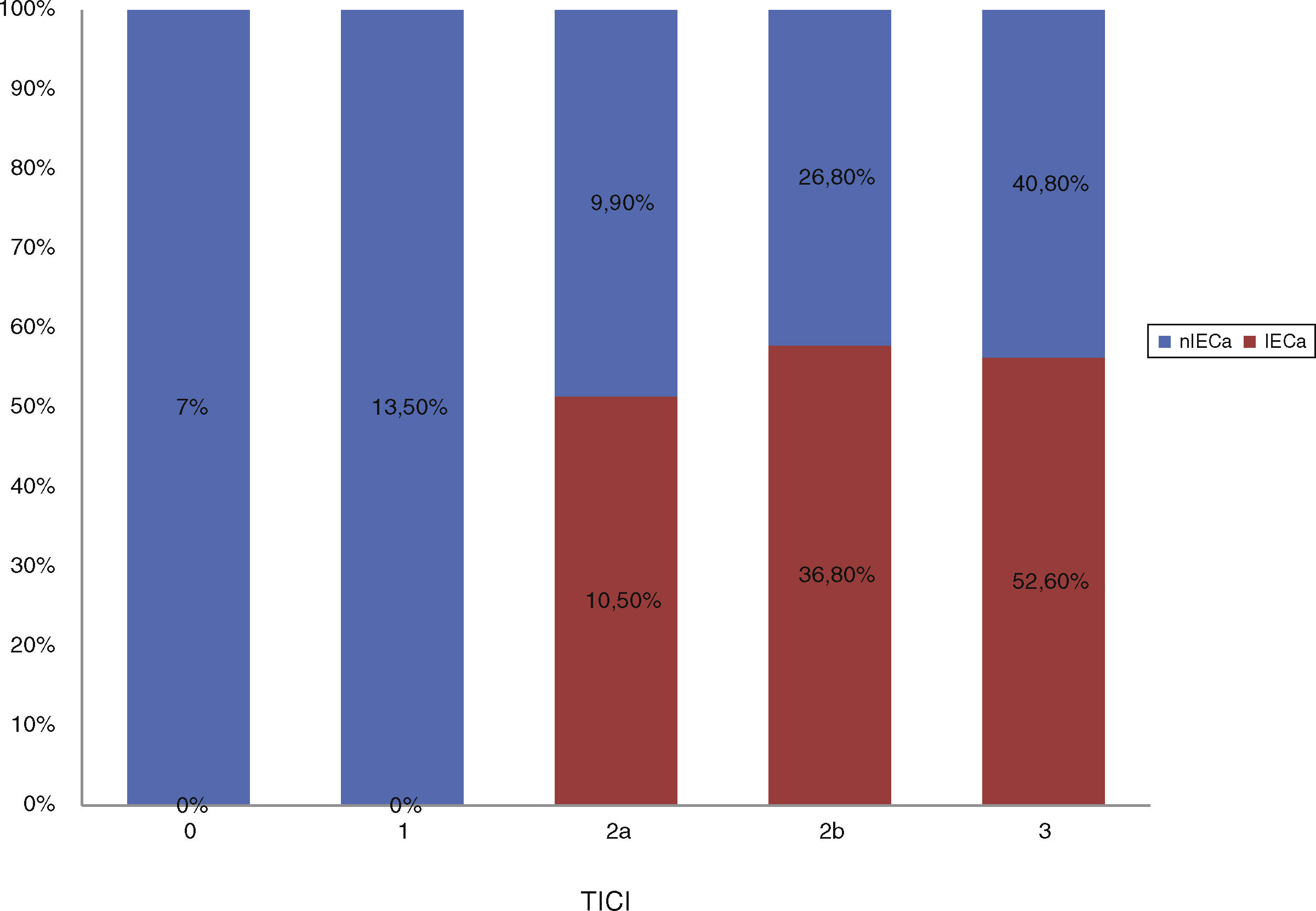
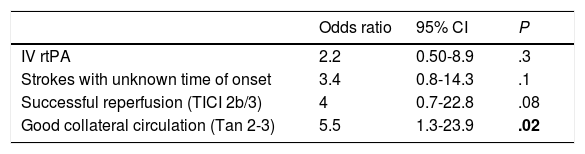
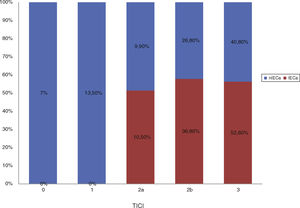
Baseline characteristics showed no statistically significant differences between groups, as shown in Table 1. After comparing procedural characteristics (Table 2), we observed that patients with iSCI presented significantly higher rates of successful reperfusion (TICI 2b/3: 89.5% vs 67.2%; P = .04) and a higher degree of collateral circulation (Tan 2-3: 83.3% vs 47.3%, P < .01; and Tan 3: 33.3% vs 7.27%, P = .007). iSCI was not observed in any of the patients who achieved no degree of reperfusion (TICI 0/1: 0% vs 23.4%; P = .013), as shown in Fig. 2. There were no significant differences between groups in the administration of fibrinolytic drugs (77.8% vs 62.5%; P = .02). iSCI was somewhat more frequent in cases of unknown time of onset (36.8% vs 18.9%; P = .1), with a trend towards significance in the multivariate analysis (OR = 3.4; 95% CI, 0.8-1.43; P = .08). TSOR and TSOP showed no differences between groups (median of 240 vs 246 min and median of 330 vs 312 min for iSCI and non-iSCI, respectively). The multivariate logistic regression analysis revealed that good collateral circulation (Tan 2-3) was associated with iSCI (OR = 5.5; 95% CI, 13-23.2); however, successful reperfusion (TICI 2b/3) was not found to be associated (OR = 4; 95% CI, 0.7-22.8; P = .1), as shown in Table 3.
Baseline characteristics of our sample.
| Baseline characteristics | iSCI (n = 19) | Non-iSCI (n = 64) | P |
|---|---|---|---|
| Age in years, median (IQR) | 71 (65.8) | 74 (63–82) | .5 |
| Male sex, % (n) | 36.8 (7) | 48.5 (31) | .3 |
| Hypertension, % (n) | 57.9 (11) | 60.9 (39) | .5 |
| Diabetes, % (n) | 10.5 (2) | 15.6 (10) | .4 |
| Dyslipidaemia, % (n) | 26.3 (5) | 40.6 (26) | .2 |
| Smokers, % (n/N) | 16.6 (3/18) | 23.2 (14/60) | .4 |
| Alcohol consumption, % (n/N) | 11.1 (2/18) | 8.3 (5/60) | .5 |
| Atrial fibrillation, % (n) | 42.1 (8) | 56.1 (32/57) | .2 |
| History of carotid artery disease, % (n) | 15.8 (3) | 15.6 (10) | .6 |
| Baseline NIHSS score, median (IQR) | 18 (13-23) | 17 (13-20.7) | .1 |
| IV rtPA, % (n/N) | 77.8 (14/18) | 62.5 (40) | .2 |
| Strokes with unknown time of onset, % (n/N) | 36.8 (7) | 18.9 (11/58) | .1 |
iSCI: isolated striatocapsular infarct; non-iSCI: non-isolated striatocapsular infarct; NIHSS: National Institute of Health Stroke Scale; IQR: interquartile range; IV rtPA: intravenous recombinant tissue plasminogen activator.
Procedural characteristics.
| iSCI (n = 19) | Non-iSCI (n = 64) | P | |
|---|---|---|---|
| TSOP (min), n = 58, median (IQR) | 230 (149.2–270) | 246 (158–296.2) | .8 |
| TSOR (min), n = 57, median (IQR) | 330 (255–375) | 312 (237.5) | .5 |
| Excellent collateral circulation (Tan 3), % (n/N) | 33.3 (6/18) | 7.27 (4/55) | .01 |
| Good collateral circulation (Tan 2-3), % (n/N) | 83.3 (15/18) | 47.27 (26/55) | .007 |
| Failed reperfusion (TICI 0/1), % (n/N) | 0 (0) | 23.43 (15/64) | .01 |
| Successful reperfusion (TICI 2b/3), % (n/N) | 89.5 (17) | 67.18 (43/64) | .04 |
Statistically significant values are shown in bold (P < .05).
iSCI: isolated striatocapsular infarct; non-iSCI: non-isolated striatocapsular infarct; IQR: interquartile range; TICI: Thrombolysis In Cerebral Infarction scale; TSOP: time from symptom onset to groin puncture; TSOR: time from symptom onset to reperfusion.
Multivariate analysis using binary logistic regression.
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| IV rtPA | 2.2 | 0.50-8.9 | .3 |
| Strokes with unknown time of onset | 3.4 | 0.8-14.3 | .1 |
| Successful reperfusion (TICI 2b/3) | 4 | 0.7-22.8 | .08 |
| Good collateral circulation (Tan 2-3) | 5.5 | 1.3-23.9 | .02 |
Statistically significant values are shown in bold (P < .05).
95% CI: 95% confidence interval; IV rtPA: intravenous recombinant tissue plasminogen activator; TICI: Thrombolysis In Cerebral Infarction scale.
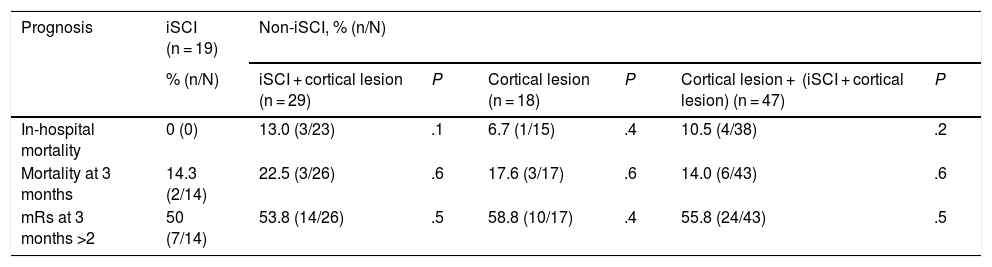
No statistically significant differences were found in the mortality rate at discharge, mortality at 3 months, or the mRS at 3 months when comparing the iSCI group with the other subgroups (Table 4).
Study results.
| Prognosis | iSCI (n = 19) | Non-iSCI, % (n/N) | |||||
|---|---|---|---|---|---|---|---|
| % (n/N) | iSCI + cortical lesion (n = 29) | P | Cortical lesion (n = 18) | P | Cortical lesion + (iSCI + cortical lesion) (n = 47) | P | |
| In-hospital mortality | 0 (0) | 13.0 (3/23) | .1 | 6.7 (1/15) | .4 | 10.5 (4/38) | .2 |
| Mortality at 3 months | 14.3 (2/14) | 22.5 (3/26) | .6 | 17.6 (3/17) | .6 | 14.0 (6/43) | .6 |
| mRs at 3 months >2 | 50 (7/14) | 53.8 (14/26) | .5 | 58.8 (10/17) | .4 | 55.8 (24/43) | .5 |
iSCI: isolated striatocapsular infarct; mRs: modified Rankin Scale; non-iSCI: non-isolated striatocapsular infarct.
iSCI was defined as an ischaemic infarct greater than 20 mm in diameter in the area supplied by more than one lenticulostriate artery.7
Although arteriosclerosis of the MCA may lead to iSCI in a limited number of cases, these infarcts are fundamentally caused by simultaneous occlusion of multiple adjacent perforating arteries. This blockage is caused by an occlusion of the proximal MCA, in the terminal internal carotid artery, or a tandem occlusion compromising circulation in the lenticulostriate arteries.4
Thus, the pathogenesis of iSCI is different from that of lacunar infarcts, as the latter are caused by occlusion of a single lenticulostriate artery, mainly due to lipohyalinosis, leading to an infarct measuring ≤ 15 mm in diameter.6 In our sample of patients with iSCI, the thrombus was located in one of the mentioned sites in 89.5% of the cases, and in the M2 segment of the MCA in only 2 cases (10.5%), although we believe that the thrombus may have migrated distally after an infarct in the striatocapsular area.
Before the introduction of reperfusion therapies in the management of acute stroke, iSCI was observed in a limited number of cases (from 0.01% to 6%, depending on the registry).1,4 This is due to the fact that iSCI formation requires successful reperfusion to prevent ischaemia in the cerebral cortex and a subsequent total infarct.
Intravenous fibrinolysis presents some limitations in the treatment of large artery occlusion,4,8,9 and MT has dramatically changed the management of acute stroke in these situations, showing higher effectiveness than intravenous fibrinolysis alone.10–14 As MT achieves higher reperfusion rates, it seems logical to expect it to be more frequently associated with iSCI (up to 22.9% in our sample).
Furthermore, MT presents limited capacity to prevent ischaemia in the striatocapsular area in the event of occlusion proximal to the orifices of the lenticulostriate arteries.4 This occurs because the striatocapsular area is highly vulnerable to ischaemia due to its scarce collateral supply, unlike the peripheral cortical region.2–4 Due to this absence of collateral circulation, ischaemia in the striatocapsular area essentially depends on the location of the thrombus, with the subsequent involvement of the perforating arteries.2,5 However, preservation of the peripheral cortical region will depend both on the degree of collateral circulation and the reperfusion time, thus avoiding the development of a cortical infarct before claudication of these collaterals.
Our study shows a strong association between the degree of collateral circulation and the development of iSCI in patients who underwent MT due to occlusion of the anterior circulation; this is consistent with the pathogenesis of iSCI and findings reported in the literature.4
The association between successful reperfusion (TICI 2b/3) and iSCI was significant in the bivariate analysis. Furthermore, no case of iSCI was found among those patients with failed reperfusion (TICI 0/1), as these patients presented total infarcts with cortical involvement; reperfusion is a necessary condition for the development of iSCI.
Therefore, a patient with an occlusion proximal to the orifices of the lenticulostriate arteries will develop an iSCI if the peripheral brain tissue is preserved by the collateral system and the occlusion is cleared, whether spontaneously or through intravenous fibrinolysis, MT, or both.
No statistically significant association was found between reperfusion times (TSOP or TSOR) and the development of iSCI, as mentioned above.4 This highlights the relevance of the collateral circulation in preserving the cortical penumbra in an “all or nothing” process, by which patients with insufficient collateral circulation will develop an infarct of cortical tissue before reperfusion is achieved, whereas those with good collateral circulation will develop it later, ultimately presenting an iSCI if the flow in lenticulostriate arteries is blocked for a prolonged period of time. Therefore, we know that infarct volume depends not only on time, but also on the competency of the collateral circulation.15
The lack of association between reperfusion times and the development of iSCI supports the findings reported by the DAWN and DEFUSE 3 clinical trials, in which selected patients with good collateral circulation (slow infarct progression) may benefit from MT beyond 6 hours after symptom onset.16,17 In the light of this, we believe that expanding the treatment window in this type of patient may lead to an increase in the incidence of iSCI, which will become increasingly relevant.
iSCI was more frequent in patients with stroke of unknown time of onset, with a trend towards statistical significance in the multivariate analysis. This fact may also be related to the findings of the previously mentioned clinical trials and to the fact that in the patients selected for MT at our centre, peripheral tissue remained viable due to the good collateral circulation, which would make this tissue salvageable even after the classic therapeutic window of 6 hours.
Regarding prognosis, we did not find statistically significant differences in mortality rate at discharge, mortality at 3 months, or mRS score at 3 months, after comparing the iSCI group with the other groups.
Although an association was observed between iSCI and higher rates of successful reperfusion, which helps to preserve the whole brain neocortex, our data reflect similar outcomes in the different groups. This may be explained by the complex connectivity of the structures linking the striatocapsular area with the brain cortex and the thalamus; lesions circumscribed to this area may therefore cause multiple symptoms. Thus, while an important brain area is salvaged by reperfusion therapies, the sequelae of iSCI may be incapacitating and may occasionally behave like extensive lesions with cortical involvement, or cause different neuropsychological dysfunctions.1,18 This fact becomes especially relevant as half of patients presenting an acute stroke with good functional outcomes show cognitive alterations and depression at 3 years after stroke; these factors are not assessed in the mRS.19
Our study has some limitations. Firstly, it presents the typical limitations of a retrospective study. Secondly, the sample size is small, which limits the statistical power of our analysis. Lastly, although we are aware that brain MRI is more sensitive than CT for detecting possible infarcts extending beyond the striatocapsular area, the resources available in our setting prevented us from using the technique early in the patients under study.
ConclusionsThe incidence of iSCI is considerable in patients undergoing MT for anterior circulation large artery occlusion, and higher than that reported by series published before the implementation of the new strategies for brain reperfusion.
The high vulnerability of the striatocapsular area to ischaemia leads to the formation of striatocapsular infarcts in patients with proximal occlusions of the anterior circulation, despite adequately reperfusion, especially in patients whose cortical tissue remains viable due to good collateral circulation. Cortical penumbra in these patients will persist in the long term; therefore, development of an iSCI does not depend on reperfusion times, once infarction of the area has occurred.
Furthermore, we should mention that an iSCI can be as incapacitating as a total infarct, despite reperfusion of the whole cerebral cortex; therefore, it is essential to achieve reperfusion before an infarct occurs in the striatocapsular area.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sancho Saldaña A, Tejada Meza H, Serrano Ponz M, Aladrén Sangrós JÁ, Navasa Melado JM, Seral Moral P. et al. Incidencia, pronóstico y factores asociados al infarto estriatocapsular aislado tras trombectomía mecánica. Neurología. 2022;37:250–256.