The purpose of this review is to update and summarise available evidence on environmental risk factors that have been associated with risk of Parkinson's disease (PD) or Alzheimer disease (AD) and to discuss their potential mechanisms.
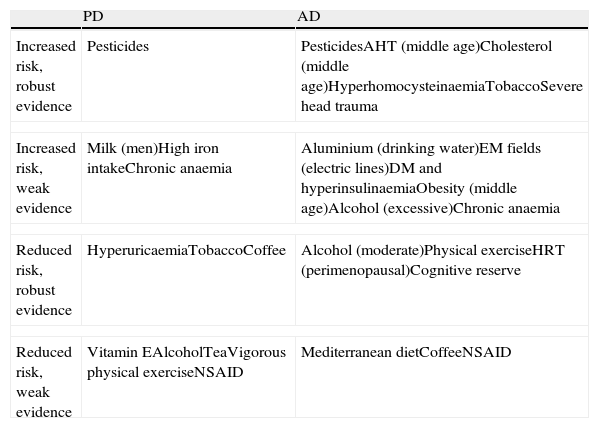
DevelopmentEvidence consistently suggests that a higher risk of PD is associated with pesticides and that a higher risk of AD is associated with pesticides, hypertension and high cholesterol levels in middle age, hyperhomocysteinaemia, smoking, traumatic brain injury and depression. There is weak evidence suggesting that higher risk of PD is associated with high milk consumption in men, high iron intake, chronic anaemia and traumatic brain injury. Weak evidence also suggests that a higher risk of AD is associated with high aluminium intake through drinking water, excessive exposure to electromagnetic fields from electrical grids, DM and hyperinsulinaemia, obesity in middle age, excessive alcohol consumption and chronic anaemia. Evidence consistently suggests that a lower risk of PD is associated with hyperuricaemia, tobacco and coffee use, while a lower risk of AD is associated with moderate alcohol consumption, physical exercise, perimenopausal hormone replacement therapy and good cognitive reserve. Weak evidence suggests that lower risk of PD is associated with increased vitamin E intake, alcohol, tea, NSAIDs, and vigorous physical exercise, and that lower risk of AD is associated with the Mediterranean diet, coffee and habitual NSAID consumption.
ConclusionsSeveral environmental factors contribute significantly to risk of PD and AD. Some may already be active in the early stages of life, and some may interact with other genetic factors. Population-based strategies to modify such factors could potentially result in fewer cases of PD or AD.
Esta revisión pretende actualizar y resumir la evidencia disponible sobre los factores de riesgo ambientales que se han asociado a riesgo de enfermedad de Parkinson (EP) o de Alzheimer (EA) y discutir sus posibles mecanismos.
DesarrolloHay evidencia consistente de mayor riesgo de EP asociado a pesticidas y de mayor riesgo de EA asociado a pesticidas, hipertensión y colesterol en edad media, hiperhomocistei-nemia, tabaco, traumatismo craneoencefálico grave y depresión. Hay evidencia débil de mayor riesgo de EP asociado a consumo elevado de leche en hombres, ingesta alta de hierro, ane-mia crónica y traumatismo craneoencefálico grave, y de mayor riesgo de EA asociado a ingesta elevada de aluminio en agua potable, alta exposición a redes eléctricas, DM e hiperinsuline-mia, obesidad en edad media, consumo excesivo de alcohol y anaemia crónica. Hay evidencia consistente de menor riesgo de EP asociado a hiperuricemia, tabaco y café, y de menor riesgo de EA asociado a consumo moderado de alcohol, ejercicio físico, terapia hormonal sustitutiva perimenopáusica y buena reserva cognitiva; hay evidencia débil de menor riesgo de EP asociado a mayor consumo de vitamina E, alcohol, té y AINE y a ejercicio físico vigoroso, y de menor riesgo de EA asociado a dieta mediterránea, café y consumo crónico de AINE.
ConclusionesDiversos factores ambientales contribuyen significativamente al riesgo de EP y EA. Algunos de ellos podrían actuar ya desde etapas tempranas de la vida o interaccionar con otros factores genéticos. Estrategias poblacionales de modificación de estos factores podrían potencialmente evitar algunos casos de EP o de EA.
The most common neurodegenerative diseases are Parkinson's disease (PD) and Alzheimer disease (AD). A small percentage of cases develop due to known genetic mutations, and discovering these variants is contributing to our knowledge of the pathophysiology of PD and AD. However, the vast majority of cases are attributed to the action and interaction of a host of genetic and environmental influences acting as susceptibility factors or triggers. Numerous epidemiological studies have linked different environmental factors to lower or higher levels of risk for these diseases. These studies are very heterogeneous and results have been more or less consistent depending on the factor examined and on study design. The purpose of this article is to review the risk factors linked to PD and AD and their potential pathophysiological mechanisms, and to present a summary of available evidence collected through a Medline literature search of systematic reviews and meta-analyses of the most significant epidemiological studies published in the past 10 years. The main risk factors for PD and AD are listed in Table 1.
Most important risk factors for PD and AD
| PD | AD | |
| Increased risk, robust evidence | Pesticides | PesticidesAHT (middle age)Cholesterol (middle age)HyperhomocysteinaemiaTobaccoSevere head trauma |
| Increased risk, weak evidence | Milk (men)High iron intakeChronic anaemia | Aluminium (drinking water)EM fields (electric lines)DM and hyperinsulinaemiaObesity (middle age)Alcohol (excessive)Chronic anaemia |
| Reduced risk, robust evidence | HyperuricaemiaTobaccoCoffee | Alcohol (moderate)Physical exerciseHRT (perimenopausal)Cognitive reserve |
| Reduced risk, weak evidence | Vitamin EAlcoholTeaVigorous physical exerciseNSAID | Mediterranean dietCoffeeNSAID |
Interest in the link between pesticides and PD was awakened in the 1980s when parkinsonism was observed among users of a synthetic opioid bearing traces of MPTP. Pesticides destroy dopaminergic (DA) neurons, which is why they are used in animal models of PD.1 Some case-control studies (CC) have linked exposure to pesticides in general with a higher risk of PD while others found no association; the combined odds ratio (OR) was 1.94 in a meta-analysis (MA) of 19 CC studies that did not show a dose-response relationship.2 Four prospective studies showed a consistent association (relative risk [RR] 1.7-1.9). Herbicides and insecticides, examined separately, showed either elevated risk or no association, whereas fungicides examined separately showed no association. A handful of studies have linked risk of PD to agricultural work, living in a rural environment, and consuming well water; however, these factors cannot be shown to be independent since they are frequently associated not only with each other, but also with pesticide use.1 A systematic review (SR) of 21 CC studies and 3 cohort studies3 also showed a consistent association between pesticide exposure and risk of AD. Robust evidence points to an association between pesticide exposure and elevated risk of PD and AD. This risk cannot be explained by chance and biases alone, even though levels of pesticide exposure are often low and difficult to measure and confounders are present. However, these are observational studies, so the relationship cannot be shown to be causal. Furthermore, the action mechanism is not well understood; despite availability of animal models, conditions are not comparable because the doses and routes of administration differ from those in human cases of exposure.
MetalsParkinsonism has been described in cases of high exposure to manganese or to lead. Researchers have found higher levels of iron, copper, and zinc in the substantia nigra of PD patients than in controls,1 and these metals are also present in amyloid deposits.4 Metals participate in the formation of senile plaques and tangles, as well as in the processes of oxidation, calcium homeostasis, and neuronal death. Aluminium is particularly important because it has been linked to AD epidemiology. It is a neurotoxin that inhibits more than 200 biological functions, can induce formation of neurofibrillary tangles, and is present at high levels in the brains of patients with AD. Epidemiological studies, most of which were carried out using surveys on occupational exposure to metals, generally do not show any associations between PD and occupational exposure to lead, copper, iron, mercury, zinc, or manganese.1 While low-quality studies have not detected any links between AD and lead or aluminium exposure, 9 out of 13 studies have established an association with consuming drinking water with high levels of aluminium. This association was also confirmed by a 15-year prospective study.5 Aluminium in water only accounts for a small part of an individual's total consumption, but it is readily absorbed through this channel. In summary, there is no convincing evidence that exposure to metals causes PD or AD, and only consumption of water with elevated aluminium levels has been associated with a higher risk of AD.
Solvents and other toxinsExposure to solvents may cause neuropsychiatric symptoms. One study using magnetic resonance spectroscopy found neural damage in the lentiform nucleus in subjects exposed to high levels of hydrocarbonate solvents.6 However, most epidemiological studies do not show an association between occupational exposure to solvents and risk of PD or AD,1,3 and neither do they consistently show associations between exposure to other toxins and PD risk in the few studies that examine this subject.1
Electromagnetic fieldsEpidemiological studies performed in recent years point to an increase in leukaemia incidence among children living in close proximity to broadcast relay stations or high-voltage power lines. The biological mechanism by which electromagnetic (EM) fields may be harmful to health is unknown, and animal studies have delivered negative results.7 One SR concluded that there is no link between occupational exposure to EM fields and risk of PD.8 In contrast, a meta-analysis of very heterogeneous studies showed an association with higher risk of AD in both 9 CC studies (combined OR, 2.03) and 5 prospective studies (combined RR, 1.62). Risk was especially high in men exposed to high levels (≥0.5μT) of extremely low-frequency EM fields (power lines).9 A recent study in Switzerland also found higher mortality associated with AD in individuals living less than 50m from high-voltage power lines, and that mortality was correlated with distance.10
ProfessionsCertain types of work may affect risk levels due to occupational exposure to toxins and viruses, or due to the level of physical or mental exercise. Higher or lower levels of risk of PD have been described for different professions, but confounders and inconsistent data are present.1 No studies on the risk of AD are available.
High blood pressureThe combined effects of ageing and vascular risk factors cause vascular degeneration, oxidative stress, mitochondrial dysfunction, and neurodegeneration.11 There have been few studies on the potential association between arterial hypertension (AHT) and risk of PD, and results are not consistent. One CC study showed a lower risk of PD (OR 0.43) among patients with hypertension,12 whereas a single prospective study with a mean follow-up time of 18.8 years found a higher level of risk (HR 1.62) in hypertensive women only.13 In contrast, many studies have consistently shown that AHT in middle-aged individuals (aged 40-64) is associated with a higher level of risk of AD (RR 1.61). This is not the case in the elderly, in whom hypotension is associated with an elevated risk of AD.14,15 These results have been confirmed by SRs of prospective studies. A Cochrane review and meta-analysis16 of 4 randomised clinical trials (RCT) showed no differences in dementia incidence between hypertensive individuals treated with antihypertensives or placebo after a mean follow-up time of 3 years. The study design did not permit an assessment of the drug's efficacy for preventing dementia. Another meta-analysis of RCTs found a slightly lower incidence of dementia (HR 0.87) among treated patients. To conclude, there is no consistent evidence associating AHT with risk of PD. There is consistent evidence that AHT in middle-aged (not elderly) individuals is associated with an elevated risk of AD, but it has not been clearly shown that treating AHT would reduce the AD incidence rate.15
Diabetes mellitusDiabetes mellitus (DM) may affect the risk of PD or AD through several mechanisms: ischaemic cerebrovascular disease in the context of metabolic syndrome; toxic effects of hyperglycaemia on neurons; and insulin resistance associated with hyperinsulinaemia, which has a vasoactive effect and alters the metabolism of amyloid beta (Aβ).11 The evidence of an association between DM and PD is inconclusive: 5 CC studies showed no association, and out of 4 prospective studies, 2 pointed to higher risk of PD with DM, 1 found no association, and the last found lower risk.1 Regarding AD, some evidence points to a higher risk among patients with DM and subjects with high insulin levels but not DM. According to a review of 8 prospective studies, 3 studies identified a higher risk of AD in DM, whereas 5 found no association (combined RR of 1.39).17 The Cardiovascular Health Study found a synergic effect with the APOE*4 allele such that risk of AD in patients with type 2 DM (DM2) and the APOE*4 allele was higher than for each separate risk factor. It was also higher than additive risk: RR=1.62 in DM2, RR=2.50 in bearers of APOE*4, and RR=4.99 in DM+APOE*4.18 The WHICAP study in women found that high insulin levels were associated with a higher risk of AD (HR 1.7, upper vs lower quartile).19 High levels of C-peptide (a hyperinsulinaemia marker) were associated with a higher 10-year risk of dementia in non-diabetics (OR 3.2, upper vs lower quartile).20 No RCTs have analysed the effect of treating DM2 on the incidence of AD.21
Cholesterol and statinsStatins have anti-inflammatory and antioxidant properties, decrease Aβ deposition, and exert a protective effect on DA neurons in animal models. Three CC studies also show a slight association between high cholesterol and lower risk of PD; 4 prospective studies indicate slightly lower PD risk in a few subgroups (for example, only in women, or only in men aged 71-75 with high-LDL levels). One study showed elevated risk among patients younger than 55.22 On the other hand, retrospective studies seem to show lower PD incidence among individuals treated with statins. Studies consistently show an association between AD and elevated cholesterol in middle-aged but not elderly individuals, according to an SR of 18 prospective studies.23 In contrast, statin use does not reduce risk of AD according to a meta-analysis of CC and cohort studies.24 Two RCT have also shown that taking statin drugs in old age does not decrease the risk of AD.25
Body mass indexObesity is a vascular risk factor that may contribute to neurodegenerative processes. According to one longitudinal study in women, higher body mass index was associated with smaller hippocampal volume.26 The few studies to have evaluated the potential link between body mass index and risk of PD have delivered contradictory results. Regarding risk of AD, a meta-analysis of 15 prospective studies23 showed that high body weight (RR 1.35), obesity (RR 2.04), and low body weight (RR 1.96) in middle-aged individuals (not elderly) were associated with a higher risk of disease, as with AHT and high cholesterol.
DietCertain food products have been associated with different degrees of PD risk in CC studies, but there are no clear associations. Curiously, a higher risk of PD (RR 1.8 upper vs lower quintile) is associated with milk consumption in men only, according to 2 prospective studies. The reason is unknown, and this tendency has not been observed with other dairy products or with calcium or vitamin D consumption.1 Several prospective studies have indicated that the Mediterranean diet in general is associated with slower cognitive decline and reduced risk of AD, but evidence is insufficient to make specific dietetic recommendations.27,28 Omega-3 fatty acids have shown a neuroprotective effect in animal models of PD.29 However, a single CC study found no relationship between omega-3 consumption and risk of PD.12 Diets rich in DHA, a fatty acid with antioxidant properties, reduce amyloid disease and improve cognition in animal models of AD.30 Some epidemiological studies suggest that increased consumption of fish and omega-3 reduce risk of dementia, but the results are inconsistent and have only been examined over the short term. The Rotterdam study found no differences in risk of AD among subjects with diets including or excluding fish after a mean follow-up time of 10 years.31 A Cochrane SR found no RCTs either confirming or refuting the efficacy of omega-3 fatty acids for preventing AD.32 No associations have been established between consumption of certain vitamins and minerals and the risk of PD or AD, except that PD risk is slightly lower with higher vitamin E consumption, and PD risk is slightly higher for individuals with more elevated iron consumption, according to some studies.1 Generally speaking, dietary factors do not seem to play a crucial role.
Uric acidUric acid is a powerful antioxidant with a potentially neuroprotective effect. In a meta-analysis of 3 prospective studies, high uric acid levels were associated with a lower risk of PD (RR 0.80) and slower rate of clinical progression. A history of gout is also associated with a lower risk of PD according to one CC study (OR 0.69) and another prospective study (RR 0.7). A phase 2 RCT with inosine, a precursor that increases uric acid levels, is currently underway.1 Case control studies have delivered inconsistent results since serum or CSF uric acid levels may vary substantially among AD patients.33
HyperhomocysteinaemiaHomocysteine is considered a neurotoxin since it destroys DA neurons in vitro, induces motor impairment in rats, elicits intracellular calcium increase, inhibits hippocampal neurogenesis in adults, causes blood-brain barrier dysfunction, generates reactive oxygen species, activates microglia, increases amyloid precursor protein (APP) and tau phosphorylation, and increases Aβ in the brain and plasma. The role it may play in PD is unclear, since levodopa increases its level. Hyperhomocysteinaemia was not found to be associated with risk of PD in 3 prospective studies,34 but 18 out of 22 prospective studies and several CCs link it to increased risk of AD.35 Normalising high homocysteine levels using B-vitamin supplementation in healthy older subjects did not result in improved cognitive function according to one study.36
TobaccoNicotine stimulates DA neurons, inhibits alpha-synuclein fibril formation, and lessens symptoms of PD.1 It may also improve cognitive function by stimulating nicotinic receptors. On the other hand, tobacco use accelerates cerebral atrophy, decreases perfusion, increases oxidative stress, and causes silent infarctions and inflammation. Autopsies of smokers have revealed fewer senile plaques but more neurofibrillary changes than in non-smokers.37 Tobacco use is the environmental factor showing the most consistent inverse association with PD in CC and prospective studies.1 PD risk is especially lower in active smokers (RR 0.27-0.56 compared to non-smokers) and in former smokers (RR 0.5-0.80), and it is related to dose and time since cessation. It has been suggested that this association may be due in part to information bias, to shorter lifespan in smokers, to smokers being under-represented among prevalent PD cases, or to subjects with PD being less likely to smoke due to common genetic factors. However, twin studies have showed that non-affected twins smoked more than affected twins, and that the children of 2 smoking parents had a lower risk of PD than children of 2 non-smoking parents (RR 0.73). Several RCTs with nicotine patches have failed to lessen symptoms in patients with PD. In contrast, smoking was associated with a higher risk of AD, with an OR of 1.59 in active smokers vs non-smokers according to a meta-analysis of 23 prospective studies with no difference between former smokers and non-smokers.37
AlcoholAlcohol may contribute to oxidative stress. In excess, it may cause temporary or permanent cognitive damage, and it has been associated with cerebral atrophy. However, it has also shown a neuroprotective and antioxidant effect in animal models of PD and cell cultures.38 Most CC and prospective studies of alcohol, especially beer, have shown a non-significant tendency towards a lower risk of PD, with OR <1.1 A twin study showed that the non-affected patient drank more than the affected patient, as was the case for tobacco use. The association is more pronounced in AD. Moderate alcohol consumption was associated with a lower risk of AD (RR 0.57) in a meta-analysis of 23 longitudinal studies.38 In the Cardiovascular Health Study, having 1 to 6 drinks weekly was associated with a lower risk of AD (OR 0.46 vs no alcohol); no benefits were associated with higher levels of consumption and AD risk increased among individuals who had more than 14 drinks per week (OR 1.22). Most studies show wine to be beneficial, although some present positive results from other types of drinks. It is therefore possible for alcohol consumption to show a slight association with lower risk of PD, although we cannot rule out the residual confounding effects of tobacco or coffee. Moderate alcohol consumption is clearly associated with a lower risk of AD. In summary, observational studies support moderate alcohol consumption (≤1 drink daily for women and ≤2 daily for men) as a means of reducing the risk of cardiovascular disease and cognitive impairment with no significant adverse effects.
Coffee and teaAdenosine A2A receptor antagonists lessen parkinsonism in animal models and RCTs. Caffeine, an A2A antagonist, is considered to have a neuroprotective effect since it was shown to inhibit MPTP toxicity in animal models of PD1 and reduce production of Aβ in AD models.39 Green tea has shown an anti-apoptotic effect in cellular models. Almost all studies of coffee in PD have shown a protective effect1 (RR 0.69 coffee vs no coffee) according to a meta-analysis of 8 CC studies and 5 prospective studies. This benefit is not enjoyed by women undergoing hormone replacement therapy (HRT) because of an interaction. Fewer studies have examined tea; results have generally pointed to a lower risk of PD or no association. In a study conducted in Singapore, consumption of large quantities of black tea was associated with a lower risk of PD. Since lower risk remained after adjusting for caffeine content, it is thought that benefits are due to a different compound. Evidence is less robust for AD, and data regarding tea consumption are very scarce. An SR with 2 CCs and 2 cohort studies showed a lower risk of AD among coffee drinkers; heterogeneity was high (combined RR 0.7).40 The CAIDE study found that consumption of 3 to 5 cups daily by middle-aged subjects was associated with a 64% decrease in risk of dementia or AD in old age.
Physical exercisePhysical exercise improves DA neuronal function and decreases toxin-induced DA neuronal loss in mouse models of PD. It also improves learning, neurogenesis, and hippocampal volume, and reduces Aβ load in AD models; this may be due to increased expression of neurotrophic factors.41 The few CC studies to have been carried out include confounders and generally show no associations between physical exercise and risk of PD. Three prospective studies indicated a lower risk of PD (RR 0.7, upper vs lower quintile of MET hours/week) in men who engaged in vigorous exercise. No studies have found links to moderate exercise or recreational activities.1 Evidence for AD, however, is quite consistent. An SR of 16 prospective studies showed a lower risk of AD (RR 0.55) in more physically active groups, and higher risk of AD (RR 1.82) among sedentary groups.15 It is unclear whether this association arises because subjects with better cognitive function are more active, nor do we know which type of exercise, and of what duration and intensity, is the most beneficial. Some RCTs have shown that elderly sedentary subjects who begin to exercise display increased mental processing speed, but whether this reduces the incidence or slows the development of AD has not been studied.
InfectionsThe infectious disease hypothesis was proposed in 1963, based on an outbreak of lethargic encephalitis that caused parkinsonism in the early 20th century. The disease was attributed to the virus that caused the 1918 flu pandemic. At a later time, a higher incidence of PD was discovered among individuals born during the flu pandemic years between 1890 and 1930. Intrauterine exposure to bacterial toxins induces loss of DA neurons in rats,1 and even peripheral infections may accelerate neurodegeneration by activating microglia.42 CC studies have examined antibodies against different viruses in serum and CSF without finding differences between PD patients and controls in most cases.1 In some studies, having had measles as a child is associated with a lower risk of PD, while diphtheria and croup are associated with higher risk. Researchers have also suggested a link between AD and central nervous system or systemic infections, with HSV1, C. pneumoniae, and spirochaetes as the possible aetiological agents; however, evidence is limited and inconsistent.43 Deposits of Aβ have been observed in mice that inhale C. pneumoniae. Aβ and phosphorylated tau also accumulates in neurons infected with HSV-1 in vitro, and antibodies and antigens have been discovered in areas of the brain that have been damaged by AD.44 Generally speaking, the available evidence is insufficient to draw conclusions about the relationship between infections and PD or AD.
Non-steroidal anti-inflammatory drugsAn inflammatory response is present in neurodegenerative diseases, either as the cause or the result of neurodegeneration. Non-steroidal anti-inflammatory drugs (NSAIDS) protect the brain from MPTP-induced neuronal loss in animal models of PD,1 reduce deposits of Aβ and tau, and elicit behavioural improvements in mouse models of AD.45 On the other hand, neuritic plaques have been found in larger quantities in long-term NSAID users.46 Prolonged use of NSAIDs was associated with a lower risk of PD according to a meta-analysis of 3 cohort studies (RR 0.74), and another MA of 2 cohorts and 5 CCs (OR 0.85 for NSAID, OR 0.75 for ibuprofen). Acetylsalicylic acid has not been shown to be beneficial.1 Likewise, prolonged use of NSAIDs was also linked to a lower risk of AD in prospective studies (RR 0.79) and non-prospective studies (OR 0.51) according to an SR.47 One meta-analysis found a lower risk of dementia among users of ibuprofen or naproxen, although this was not the case when cognitive impairment was analysed as the main variable.48. Numerous RCTs with NSAIDs or COX-2 inhibitors have failed to demonstrate a neuroprotective effect in patients with established AD. The ADAPT49 study recently showed that prolonged use of NSAIDs (more than 2-3 years) reduces AD incidence.
Head traumaHead trauma may trigger an inflammatory cascade that interferes with cell repair mechanisms. Disruption of axonal transport leads to accumulation of APP in both animals and humans. Autopsy studies in subjects with a history of head trauma have reported high levels of senile plaque and neurofibrillary tangles. Head trauma is also likely to diminish the cognitive reserve.50 Nine CC studies found a higher risk of PD (OR 1.4-11.7) among subjects with a history of head trauma, but these results do not agree with those from another 13 CC studies and a handful of prospective studies.1 A bias is present in that patients with chronic illness are more likely to recall minor head trauma events. However, twin studies have shown that subjects with a history of head trauma were at more risk for PD, and more likely to experience early onset of symptoms. Increased risk of AD (RR 2.32-4.51) has also been observed among soldiers with a history of severe head trauma, but this tendency has not been confirmed by other studies.48 Mild head trauma is not associated with AD.
Oestrogen and hormone replacement therapyThe DA depletion caused by toxins is decreased by β-oestradiol in both in vitro and animal models.51 DA depletion is less pronounced in females. Oestrogens are antioxidants that stimulate the growth and survival of cholinergic neurons, induce non-amyloidogenic metabolism of APP, and increase cholinergic activity.52 HRT has been associated with a lower risk of PD in some studies, but not the majority.51 Many of these studies were not adjusted for tobacco use or other confounders. While caffeine generally reduces risk of PD, it is linked to increased risk in patients on HRT due to interactions with oestrogen.1 High levels of oestradiol were associated with a 43% increase in risk of dementia among women in the Rotterdam follow-up study on chronic diseases.53 Use of HRT has not consistently delivered evidence of improved cognition and lower risk of dementia among women older than 65.54 The effect of HRT on the risk of AD depends on whether oestrogens are used alone or in combination with progestogens, and the age at which they are used. In the WHIMS study carried out in women aged 65 and older, risk of AD was higher (HR 1.76) for those on combined HRT (oestrogens and progestogens) and no effect was observed in women treated with oestrogens alone; risk was only lower for patients who started treatment a few years earlier.55 Researchers therefore believe that there is a perimenopausal window during which oestrogens will exert a protective effect, and that they will actually increase risk in subjects older than 65.56 An RCT is underway to confirm this hypothesis.
Calcium channel antagonistsSustained activation of calcium channels in DA cells may accelerate ageing and predispose the subject to PD. In AD, Aβ alters calcium levels in neurons, which in turn accelerates Aβ deposition. Some dihydropyridines act more selectively on L-type calcium channels (LTCC), which are highly expressed in neurons. In animal models, nimodipine protects subjects from MPTP neurotoxicity while isradipine protects them from DA degeneration. Other calcium channel antagonists (CCA), such as flunarizine or cinnarizine, may cause parkinsonism since they also exert an anti-dopaminergic effect.57 Isradipine limits the neurotoxic damage caused by Aβ in cell cultures and animal models.58 Nimodipine, despite its effects on LTCC, stimulates Aβ secretion through another mechanism. A CC study demonstrated lower risk of PD among CCA users, while another CC study and 2 prospective studies found no associations.59 The effect of isradipine on patients with PD is currently being evaluated. Some epidemiological studies suggest that LTCC antagonists decrease risk of AD or inhibit disease progression, but large RCTs with nimodipine have found no benefits for AD patients. Evidence that CCA leads to reduced risk of PD or AD is inconsistent.
AnaemiaOne CC study recorded higher prevalence of chronic anaemia among PD patients than in controls (OR 2). Low haemoglobin levels have also been linked to a greater risk of dementia (HR 1.94) in 2 prospective studies, and to increased risk of dementia in women in 1 of 2 CC studies.60 While several hypothesis have been suggested in an attempt to explain this association, data are limited and no conclusions may be drawn.
Thyroid disordersThe clinical symptoms of hypothyroidism may be confused with parkinsonism. One CC study found no associations between hypothyroidism and PD.61 Thyroid hormones participate in Aβ regulation. The relationship between thyroid alterations and risk of AD is a complex one; hyperthyroidism has been linked to increased risk of AD, and so has subclinical hypothyroidism to a lesser extent.62
Cognitive reserveA good cognitive reserve lets an individual function at a normal level despite neurodegenerative changes. An SR including 22 longitudinal studies found lower risk of dementia among subjects with a high educational level, success in the workplace, high IQ, and mentally stimulating recreational activities (OR 0.54), whereas subjects with a low cognitive reserve were at increased risk (OR 1.85). A different SR also found a higher risk of AD among individuals with a low educational level in 13 CC studies (RR 2.4) and 6 cohort studies (RR 1.59). The Kungsholmen project provided an index of different social components (marital status, friendships, children, other people in the household) and found that risk of dementia decreased as scores on the index increased.63 Cognitive interventions in healthy elderly subjects improve certain measures of cognitive function over the short term, according to a Cochrane SR of 36 RCTs; however, the effects of individual interventions on AD risk have not been evaluated.15
Depression and stressEpidemiological studies show that history of depression and the number of depressive episodes are associated with increased risk of AD, but the mechanism is unknown. It has also been suggested that depression may be a prodromal phase of the disease, but this is unlikely; depression may be present many years before the onset of AD, and an autopsy study showed that depressive symptoms were not be related to level of pathology and did not modify the relation between the pathology and clinical AD.64 In another autopsy study, chronic psychological stress was associated with a higher probability of dementia or cognitive impairment late in life, but not with Alzheimer's disease.65
Certain polymorphisms (SNPs) of genes involved in the metabolism of DAs or external toxins seem to modulate the effect of certain risk factors. However, a multi-centre CC study found no significant interactions between SNPs in 15 such genes and exposure to solvents, pesticides, and metals.66 Interactions between genes and the environment have been studied less in AD than in PD.4 Epidemiological studies have described interactions between the APOE*4 genotype and cholesterol, alcohol, tobacco, and social factors.
Experimental studies in animal models of PD suggest that certain environmental risk factors for developing PD may already be at work in the first years of life, or even during fetal development.67 Toxins may cause static or progressive damage, as in the case of acute MPTP exposure, which results in progressive neuronal loss and a permanent inflammatory reaction. Animal studies show that prior exposure to a toxin increases susceptibility to greater damage, which is why a second toxin or ageing may trigger selective nervous system degeneration. Murine models have also shown that intrauterine infections may interfere with the development of fetal DA neurons due to inflammation mediated by TNF-α in the amniotic fluid. Epidemiological data suggest that increased risk of intrafamilial PD may be due not only to genetic factors, but also to shared environmental factors. A population study in families with PD found an RR of 6.7 for siblings of a PD patient, 3.2 for children, and 2.7 for nieces and nephews. Since risk levels were similar between children and nieces/nephews and different for siblings, this suggests that the similarity of risk for the first 2 cohorts is the result of simultaneous exposure to similar environmental factors.68
The relative importance of environmental factors was underlined by a recent study estimating that half of all AD cases can be attributed to 7 potentially modifiable risk factors,15 provided that those factors are independent. The study calculated that the factors contributing the most to risk of AD are, in order of importance, low cognitive reserve, tobacco use, sedentary lifestyle, obesity, AHT in middle age, and DM. Each factor contributes between 2% and 20% to the risk of AD. RCTs are currently underway to evaluate how employing multidimensional strategies to reduce risk factors (physical and cognitive exercise; nutrition) may impact AD incidence. For now, effective strategies may include public awareness campaigns aimed at increasing cognitive reserve in the population, anti-smoking campaigns, and movements to promote physical exercise and healthy habits.
ConclusionsDifferent environmental factors contribute significantly to the risk of PD and AD. Some may be already active in the early stages of life, and environmental factors may interact with genetic factors. Population-wide strategies targeting these factors may provide a means of preventing certain cases of PD or AD.
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Campdelacreu J. Enfermedad de Parkinson y enfermedad de Alzheimer: factores de riesgo ambientales. Neurología. 2014;29:541–549.
This study was presented orally at the 12th Annual Meeting of the Study Group on Occupational Neurology, 63rd Annual Meeting of the Spanish Society of Neurology, 18 November 2011.