The limitations in performing functional activities in children and adolescents with cerebral palsy are important. The use of virtual reality systems is a new treatment approach that reinforces task-oriented motor learning. The purpose of this guide is to study the impact of the use of virtual reality systems in the improvement and acquisition of functional skills, and to evaluate the scientific evidence to determine the strength of recommendation of such interventions.
DevelopmentAll available full-text articles, regardless of their methodology, were included. The following databases were consulted: PubMed (Medline), PEDro, EMBASE (OVID-Elsevier), Cochrane Library, Medline (OVID), CINAHL, ISI Web Knowledge. An assessment was made of methodological quality, the level of scientific evidence, and the strength of recommendations using the tools: Critical Review Form for Quantitative Studies and the Guidelines for Critical Review Form for Quantitative Studies and U.S. Preventive Services Task Force. Finally, we included 13 articles and 97 participants were recruited. We obtained significant improvements in outcome measures that assessed postural control and balance, upper limb function, the selective joint control, and gait.
ConclusionsThe guide has some limitations: the limited number of patients enrolled, clinical diversity and age range, as well as the methodological quality of existing trials. Virtual reality is a promising tool in the treatment of children with cerebral palsy. There is strong scientific evidence of an acceptable recommendation for the use of virtual reality systems in the treatment of cerebral palsy.
Las limitaciones para realizar actividades funcionales en niños y adolescentes con parálisis cerebral son importantes. El empleo de sistemas de realidad virtual constituye un nuevo enfoque de tratamiento que refuerza el aprendizaje motor orientado a tareas. El objetivo del presente trabajo consiste en analizar qué repercusión tiene el empleo de sistemas de realidad virtual en la mejora y adquisición de habilidades funcionales; y evaluar la evidencia científica existente para determinar qué fuerza de recomendación tienen dichas intervenciones.
DesarrolloSe incluyeron todos los artículos disponibles a texto completo independientemente de su metodología. Se consultaron las siguientes bases de datos: Pubmed (Medline), PEDro, Embase (OVID-Elsevier), Cochrane Library Plus, Medline (OVID), CINHAL, ISI web Knowledge. Se evaluaron la calidad metodológica, el nivel de evidencia científica y la fuerza de las recomenda-ciones con las herramientas: Critical Review Form-Quantitative Studies and the Guidelines for Critical Review Form-Quantitative Studies y U.S. Preventive Services Task Force. Finalmente, se incluyeron 13 artículos y se reclutó a 97 participantes. Se obtuvieron mejoras significativas en medidas de resultado que evalúan el control postural y el equilibrio, la funcionalidad del miembro superior, el control selectivo articular y la marcha.
ConclusionesLa guía posee algunas limitaciones: número de pacientes reclutados, diversidad clínica y rango de edad; así como la calidad metodológica de los ensayos existentes. La realidad virtual es una prometedora herramienta en el tratamiento de ni¿nos con parálisis cerebral. Existe evidencia científica con fuerza de recomendación aceptable para el empleo de sistemas de realidad virtual en el tratamiento de la parálisis cerebral. © 2011 Sociedad Espa¿nola de Neurología. Publicado por Elsevier España, S.L. Todos los derechos reservados.
Cerebral palsy (CP) is described as a range of disorders of motor and postural development that cause functional limitations attributed to non-progressive lesions arising in the developing fetal or infant brain.1,2 It has traditionally been classified according to type of damage (spasticity, hypotonia, dyskinesia, or ataxia) and its topographical distribution (hemiplegia, diplegia, or tetraplegia). Until recently, there were no standardised methods for classifying cerebral palsy by subtype and severity of motor impairments. The Gross Motor Function Classification System (GMFCS) was developed to classify functional mobility in children diagnosed with cerebral palsy by level of motor function. It describes 5 levels ranging from level I, indicating children with minimal or no mobility dysfunction compared to the general population, to level V, including children who are totally dependent and need help moving around.1 Although CP is the most common physical disability in childhood, its precise global incidence and prevalence rates are not yet known.2 Thanks to population-based records, it is estimated that the prevalence of CP in developed countries is 2-2.5 cases per 1000 live births. The current survival probability is high, even for the most severe forms of CP, which results in increasing financial costs. A study in the USA estimated that direct costs (physician visits, hospital stays, assistive devices, and home modifications) and indirect costs (impact on work productivity) of CP in 2003 reached 11.5 billion dollars.3
Cerebral palsy and motor functionSkill development in children with CP is restricted by multiple factors that limit voluntary movements, whether manipulatory or mobility-related. These limitations are accompanied by postural constraints.4
Normal postural control requires combining sensory information from the visual, proprioceptive, and vestibular systems that provide information on the position and movement of the body and its surroundings. In normal postural control, this information must also be coordinated with motor actions. The proprioceptive system, also called the ‘sixth sense’, relays basic static (position) and dynamic (movement) information that enables us to know where our body is located in space. In the production of coordinated movement, proprioceptive feedback is critical for controlling muscles, limb segments during multi-joint movement, and movement trajectories. It also provides internal representations of the body that are essential for the acquisition and adaptation of motor skills.5
Postural control in patients with CP depends on the capacities of the neuromuscular and musculoskeletal systems. Their neuromuscular system has a restricted capacity for coordinating muscles in postural synergies, which gives rise to multiple dysfunctions in sequencing, in activation time for postural response, and in adapting posture to the setting. The main musculoskeletal dysfunction in these patients is body alignment. Lack of proper alignment between body segments leads to a change in body position with respect to the centre of gravity and the support base. As a result, these patients are unable to develop appropriate locomotion strategies.6,7
Virtual reality (VR) systems for rehabilitating children and adolescents with CP constitute a new treatment tool with multiple functional objectives.
Virtual realityVR is a computer technology providing artificial sensory feedback so as to allow individuals to experience activities and events similar to those they might encounter in real life.8,9 Individuals can develop motor skills in a three-dimensional virtual environment resembling the real world.8
The defining features of VR systems are interaction and immersion. ‘Interactivity’ with the computer refers to the presence of multiple sensory channels (sight, hearing, touch, and even smell); ‘immersion’ refers to an individual's level of involvement with the virtual environment.10,11 These two concepts together define ‘degree of presence’, that is, the feeling of being present in the situation. A high degree of presence is required in order to manipulate the cognitive processes involved in motor control.10,12 Therefore, patient-system engagement is stronger with systems that offers a higher level of immersibility.
VR has three key elements that are active in motor learning:
- -
Repetition. Plasticity is use-dependent. Repetition results in better learning of motor and functional skills.13
- -
Sensory feedback. Multisensorial stimulation is an essential part of rehabilitation for children with CP, since effects of the disease are systemic. Neural networks reach their full developmental state when patients work using different channels.14 Virtual environments provide massive and intensive sensorimotor stimulation, which is necessary to induce brain reorganisation.13
- -
Motivation of the subject. Subjects are motivated when the activities in their therapy programmes are presented in an enjoyable, attractive way.15
In paediatric neurorehabilitation, establishing flexible and personalised intervention programmes is crucial. VR can provide such personalised and flexible treatment by allowing us to integrate the child's preferences in the intervention programme, improve attention and motivation, and increase sensory feedback. These techniques increase engagement in therapy, which in turn results in more successful learning.11 Additionally, they provide structured and systematic intervention strategies, and the therapist is in complete control of the system to make any necessary changes and run specific sessions. The techniques also enable the development of telerehabilitation platforms through which therapists can monitor the patient's progress.16
One of the main problems in neurorehabilitation is application of the acquired skills to real-life situations. This is one of the most difficult objectives of these interventions, and strategies to maximise transferral therefore form part of the treatment plan. VR is designed to simulate real situations. It has a high degree of ecological validity (the extent to which an experiment reflects the real world), and this increases the probability that the acquired skills will transfer to the real world. VR offers safety in realistic environments that may be dangerous for children with CP in real life, thereby helping them develop confidence and self-efficacy in a safe environment and preparing them to approach the task in the real world.10
Virtual reality systems used in neurorehabilitationVR systems can be grouped in three major classes according to the type of human-computer interaction: gesture-based, feedback-focused, and haptic-based (or based on touch).10 The following systems have been used in neurorehabilitation:
- -
IREX® (Interactive Rehabilitation and Exercise Systems, Gesture Tek). This immersive VR system integrates the patients’ images in a virtual setting in which they can see themselves moving and interacting with virtual objects in real time. It can be used to design interactive exercise programmes for specific joints, combined movements, or the whole body. Since it does not require any additional devices, it provides complete freedom of movement.17,18
- -
Mandala Gesture Xtreme® (Vivid Group). Immersive VR system based on the movements of the user, who is transported to a virtual setting. This VR system enables free active movements without requiring any additional devices. Vivid Group has developed different software titles for the Mandala GX system: 5 entertainment titles, 7 educational titles, 9 sport titles, and 6 virtual theatre games. A multi-player mode is available.19–21
- -
CAVE® (Fakespace). The system includes a room with a floor and 3 walls (1 frontal and 2 lateral) onto which high-resolution 3D images are projected. This creates the illusion of being inside the virtual setting. CAVE is also an immersive VR system, and it is equipped with a device that measures postural reactivity by registering body movement. It can be used by several players.12,22
- -
BNAVE® (Balance Near Automatic Virtual Environment). This is an immersive system that projects stereoscopic images such that they occupy the patient's entire field of view. The patient is placed on a force platform in the centre of the virtual room. The data registered by BNAVE are head movements, centre of pressure of the foot, and electromyographic signals.15
- -
Head Mounted Displays® (HMD). This is the gold standard of immersive VR systems since it projects very high resolution images at close proximity to the eyes. It consists of a monocular or binocular device mounted on patients’ heads enabling them to monitor their movements. This way, the patient has the sensation of being part of the virtual scene.23 However, it has been criticised for restricting the wearer's movements, being heavy, causing dizziness and discomfort, and limiting the field of view.19–21
- -
Haptic systems. These systems use robots to create interaction between the user and the virtual reality setting. NJIT-RAVR®, GENTLE/s®, MIT-Manus®, PneuWREX®, Rutgers Master II-ND®, and Data Gloves® provide haptic effects during upper-limb activities in virtual environments. LOKOMAT® (Hocoma) and CAREN System® (Motek) are systems designed to facilitate gait training. Both can be integrated with VR since they present virtual locomotion scenarios displayed on a screen in front of the patient.13,24–26
- -
Low-cost systems. The following systems have been used in neurorehabilitation:
- 1.
Wii® (Nintendo). The Nintendo Wii video game console is an interactive motion-based device. Players are represented by avatars located within the virtual environment. A remote monitoring tool held in the hand measures the user's movements and transfers them to the screen. This tool detects changes in speed and orientation, and the system adjusts feedback according to these parameters. The Wii remote control provides haptic feedback, while the game display provides visual and auditory feedback. Wii has a multi-player mode and different levels of difficulty. It offers several applications which include Wii Sports® and Wii Fit® (Balance Board).27,28
- 2.
PlayStation® (Sony). EyeToy software® and the Move® navigation controller allow users to sample numerous virtual experiences.29–31
- 3.
Xbox® (Microsoft). Kinect technology® allows the user free range of motion by offering controller-free play. It employs a motion sensor that monitors the user's entire body.32
- 1.
The purpose of this clinical practice guide is to study the impact of using different VR systems to instil and improve functional skills in children and adolescents with CP, and evaluate available scientific evidence to determine the grades of recommendation for such interventions.
Materials and methodsSearch strategyWe conducted the literature search with no language restrictions for all articles published up to March 2011 and dating back to the year 2000.
Sources of information and keywords employed are listed below.
- -
Databases: Pubmed (Medline), PEDro, Embase (Ovid-Elsevier), Cochrane Library Plus, Medline (Ovid), CINAHL, and ISI Web of Knowledge.
- -
Search strategy: (1) cerebral palsy, (2) virtual reality, (3) cerebral palsy and virtual reality, (4) balance, (5) cerebral palsy and balance, (6) postural control, (7) cerebral palsy and postural control, (8) virtual reality and balance, (9) virtual reality and postural control, (10) somatosensory development, (11) cerebral palsy and somatosensory development, and (12) motor learning.
Additionally, we tracked the references cited by different trials and review articles9–11,16,33,34 that might be relevant for the purpose of this guide.
We contacted 2 authors to gain access to 2 articles19,35 that were not available in full-text format in the databases consulted for this study.
Inclusion criteria for this guideStudy typesThe purpose of this clinical practice guide was to collect studies with a high level of evidence. However, we included all articles addressing interventions in children with CP regardless of the level of evidence, since this topic is relatively new and the number of articles in the literature is low. All articles were reviewed and assessed critically using the appropriate tools.
Type of participantsWe included all articles describing participants who were exclusively children and/or adolescents aged 4 to 18 with cerebral palsy, regardless of motor diagnosis and level of dysfunction.
Type of interventionsWe included all interventions in the target population that involved either isolated use of VR systems for functional skill training, or a combination of a VR system and a complementary treatment. In this case we evaluated the improvement in performance after combined use.
Type of outcome measuresMeasures for assessing the participants had to belong to any of the following groups:
- -
Standardised and validated motor function assessment tools: Gross Motor Function Classification System (GMFCS),36 Canadian Occupational Performance Measure (COPM),37 Sitting Assessment for Children with Neuromotor Dysfunction (SACND),38 Bruininks-Oseretsky Test of Motor Proficiency (BOTMP),39 Pediatric Motor Activity Log (PMAL),40 Fugl-Meyer Assessment (FMA),41 Jebsen Hand Function Test (JHFT),42 Peabody Developmental Motor Scales (PDMS-2),43 Quality of Upper Extremity Skills Test (QUEST),44 Melbourne Assessment of Unilateral Upper Limb Function (MAUULF),45 Movement Assessment Battery for Children (MABC-2),46 and Standardized Walking Obstacle Course (SWOC).47
- -
Features of gait: spatial-temporal variables (speed, length of stride, symmetry), 1-Minute Walk Test,48 and 6-Minute Walk Test.
- -
Features of static position (weight distribution, joint alignment, symmetry).27,49
- -
Range of joint motion: goniometry.18
- -
Active involvement/muscle strength: biofeedback, surface electromyography (EMG).50
- -
Imaging-based diagnosis techniques: functional magnetic resonance imaging (fMRI).17,29
Two authors (E.M., F.M., both physiotherapists with experience in treating children with CP) independently screened the titles and abstracts delivered by the electronic search, in addition to annals of conferences and documents describing unpublished trials. Trials selected by each author were compared. The authors discussed any trials on which they did not agree. Articles that potentially met inclusion criteria were later gathered in full-text format. Their methodological quality was assessed by means of the Critical Review Form for Quantitative Studies and the Guidelines for Critical Review Form for Quantitative Studies.51 The U.S. Preventive Services Task Force (USPSTF)52 was used to examine the level of evidence and the strength of the recommendations for these studies. This was helpful for selecting the best evidence for use in decision-making in clinical practice.
A standardised extraction of different data was performed for each paper: sample size, age, sex, motor diagnosis and level of dysfunction of the participants (GMFCS), intensity of the intervention and complementary interventions, VR system used, assessment measures applied, and main outcomes.
ResultsDescription of studiesThe literature search of the selected databases identified 34 papers; 21 were excluded 17,19,23,25–27,49,53–66 since they did not meet inclusion criteria for this study (Table 1). Our review included 13 studies,18,20,21,29–31,35,50,67–71 with a total of 97 participants.
Excluded studies
| Reference | Reason for exclusion |
| Brütsch et al.17 | Presence of subjects with diagnoses other than CP. |
| Weiss et al.19 | Participants were adults. |
| Steele et al.23 | Study assessed pain modulation. No motor function assessment tools were used. |
| Koening et al.25 | Assessment of healthy subjects. |
| Baram et al.26 | Participants were older than the maximum age for inclusion. |
| Huber et al.27 | Update of an article already included.31 |
| Akhutina et al.49 | No standardised assessment measures were used. |
| Qiu et al.53 | Outcome measure designed by the authors. |
| Harris et al.54 | Study assessed motivation. No motor function assessment tools were used. |
| Reid et al.55 | Study assessed playfulness. No motor function assessment tools were used. |
| Miller et al.56 | No motor function assessment tools were used. |
| Reid et al.57 | Evaluation of self-efficacy. No motor function assessment tools were used. |
| Reid et al.58 | Evaluation of the person-environment experience. No motor function assessment tools were used. |
| Golomb et al.59 | Update of an article already included.31 |
| Brütsch et al.60 | Not limited to CP; participants had a range of different gait disorders. |
| Montesano et al.61 | No standardised motor function assessment measures were used. |
| Golomb et al.62 | Update of an article already included.31 |
| Kirshner et al.63 | No motor function assessment measures were used. |
| Guberek et al.64 | Evaluation of level of cooperation and satisfaction. No motor function assessment measures were used. |
| Odle et al.65 | Therapists were evaluated with questionnaires. Functional tasks were also assessed but no standardised test batteries were used. |
| Brien et al.66 | Update of an article already included.30 |
Participants’ dysfunctions represented all of the different topographical distributions. Regarding muscle tone, 67 of the participants had spasticity,20,21,29–31,50,67,68,70,71 1 had dyskinesia, 1 had ataxia,68 and 10 had hypotonia.29 Tone quality data were not available for 12 participants;18,35,69 the remaining 6 participants were healthy controls.30 In 7 studies, the level of dysfunction was assessed with the 5-level Gross Motor Function Classification System (GMFCS).20,21,29,30,50,68,70,71
The ages of the patients ranged from 4 to 17 years. With regard to sex, 53 participants were male and 34 were female. There is no data on the remaining 10 participants.20,21 The aspects addressed are listed below.
In 3 studies,18,20,70 changes in postural control and balance after VR system use were studied in a total of 10 patients. Six studies30,35,50,67,68,70 measured upper-limb functional improvements after VR use in 52 participants. Two studies30,71 evaluated the increase in selective joint torque control in 17 participants. Additionally, 2 studies50,68 assessed changes in different gait variables in 29 participants. In 2 articles,31,69 fMRI was used as an assessment tool for measuring changes occurring in the motor cortex of 4 participants after training motor skills of the upper limbs with VR systems. Another study30 compared conventional selective joint exercises with the same exercises performed with a VR system in 16 participants.
Three studies used additional means to complement treatment with simple VR systems. Kott et al.50 used a treadmill to train gait in a virtual environment; Cikajlo et al.71 designed an isokinetic dynamometer system; and Fluet et al.35 used a haptic robotic system (NJIT-RAVR®) to train hemiplegic upper-limb motor function. Telerehabilitation interventions were carried out in 2 studies.31,68 Lastly, in 5 studies18,20,35,67,70 VR was combined with additional treatments. Eight participants20,67,70 continued with physical and occupational therapies throughout the study period; 2 received physical therapy only,18 and 3 followed a specific therapy regimen that restricted movement in the unaffected upper limb.35
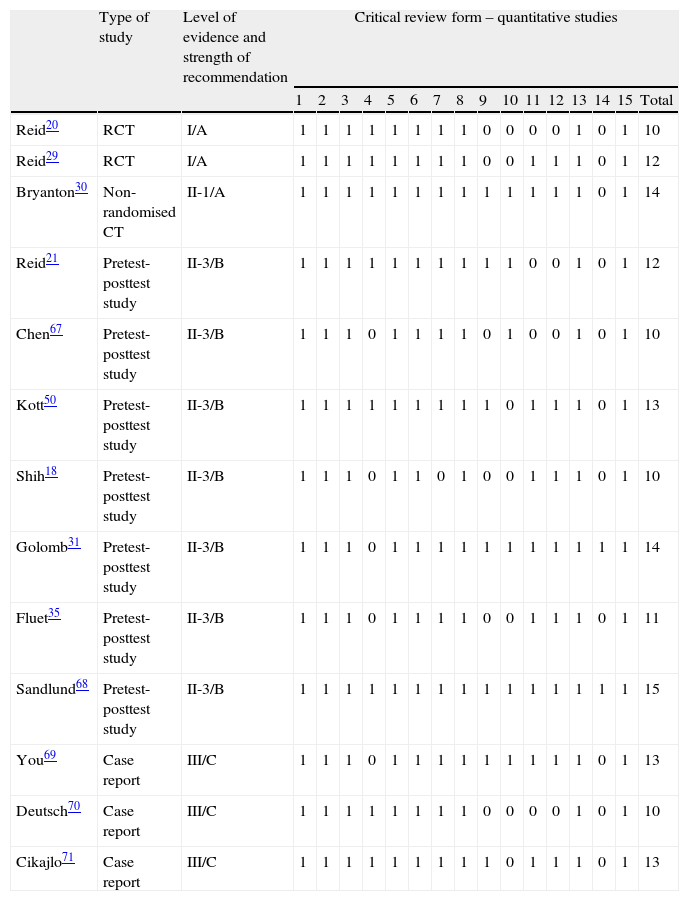
Methodological qualityThe score for the methodological quality of the studies ranged from 10 to 15 on the Critical Review Form for Quantitative Studies and the Guidelines for Critical Review Form for Quantitative Studies, which are 15-point scales. Two of the studies were randomised clinical trials,20,29 1 study was a non-randomised controlled trial,30 7 were pretest-posttest analyses,18,21,31,35,50,67,68 and 3 studies were case reports.69–71
The level of evidence of the articles ranged from I to III according to the USPSTF score, and strength of recommendation was either A, B, or C (Table 2).
Scores for methodological quality, level of evidence and strength of recommendation
| Type of study | Level of evidence and strength of recommendation | Critical review form – quantitative studies | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Total | |||
| Reid20 | RCT | I/A | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 10 |
| Reid29 | RCT | I/A | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 12 |
| Bryanton30 | Non-randomised CT | II-1/A | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 14 |
| Reid21 | Pretest-posttest study | II-3/B | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 12 |
| Chen67 | Pretest-posttest study | II-3/B | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 10 |
| Kott50 | Pretest-posttest study | II-3/B | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 13 |
| Shih18 | Pretest-posttest study | II-3/B | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 10 |
| Golomb31 | Pretest-posttest study | II-3/B | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| Fluet35 | Pretest-posttest study | II-3/B | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 11 |
| Sandlund68 | Pretest-posttest study | II-3/B | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| You69 | Case report | III/C | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 13 |
| Deutsch70 | Case report | III/C | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 10 |
| Cikajlo71 | Case report | III/C | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 13 |
RCT: randomised clinical trial.
Two studies were RCTs.20,29 In a study published in 2002, Reid20 assessed seated postural control in 6 participants, who achieved considerable improvements in postural tone, postural alignment, proximal stability, and balance. The author used the SACND but did not provide data on the results from the statistical analysis, which should therefore be interpreted with caution. Reid subsequently conducted a randomised controlled trial29 in which she correctly performed a statistical analysis of 31 patients using the same VR system. In this study, she assessed upper-limb function by means of standardised tools. Reid concluded that the results were statistically non-significant and unable to support a recommendation for VR intervention. Harter's Social Perception Profile for Children (SPPC) scale was the only tool that displayed significant differences between the two groups (P=.02 vs QUEST P=.43; COPM P=.12, P=.41).
Level of evidence II-1: strength of recommendation ABryanton et al.30 conducted a non-randomised controlled trial including 16 participants (10 with CP and 6 healthy controls) who exercised using a VR system. The purpose was to compare the effect of VR exercises and conventional exercises on selective ankle motor control. The results showed that patients in both groups were able to complete more repetitions of the conventional exercises (P<.04). However, average time to complete one repetition of a VR exercise was longer than for a similar conventional exercise (P<.01), patients with VR exercises achieved wider active ranges of motion (healthy controls: P<.03; CP: P=.09), and hold time was also longer for VR exercises. The reason for these differences is that VR systems propose task-oriented interventions.
Level of evidence II-3: strength of recommendation BSeven trials were pretest-posttest studies.18,21,31,35,50,67,68 In 4 of them, upper-limb motor function was assessed using VR interventions.21,31,35,67 All of these studies showed increased active function, better coordination, and a higher motion quality of the upper limbs, although no statistically significant changes were described. Only one article35 reports a decrease in movement time of 26% (P=.028) and an increase in trajectory length (P=.003). However, no significant differences were found for hand trajectory smoothness (P=.067). The study by Golomb et al.31 shows improvements in forearm bone health after VR telerehabilitation. The assessment tool, fMRI, yielded statistically significant results in motor cortex activation after intervention (P<.001). Pretest-posttest study by Shih et al.18 included 2 participants and assessed postural control and balance with a low-cost VR system. They obtained statistically significant differences (P<.01) in both participants between intervention and non-intervention phases for maintaining correct standing posture. The other 2 pretest-posttest studies50,68 evaluated gait. Participants showed considerable improvements in speed and length of stride (P=.02), skill development for GMFCS dimension E (P=.05),50 and increased motor performance (MABC P=.039). No significant changes were found in the 1-Minute Walk Test (P=.078) or the BOTMP test (P=.072)68.
Level of evidence III: strength of recommendation CThe 3 remaining articles69–71 are case reports. Results from You et al.69 pointed to increased use and quality of movement of the patient's affected upper limb during functional motor activities. The authors also assessed the sensorimotor cortex activation in a child with hemiparetic CP (P<.001). Deutsch et al.70 assessed postural control and ambulation functional mobility (in steps/metres travelled) in an adolescent with spastic diplegic CP. After the intervention, sway decreased by approximately 60%. Functional mobility increased during training from 15 steps/4m at baseline and remained at 250 steps/76.2m 12 weeks after training. Lastly, Cikajlo et al.71 assessed knee joint torque with isokinetic dynamometry in a patient training with a VR system. They found statistically significant results in task execution speed (P<.05), and surface EMG response was smoother and more powerful.
DiscussionThe purpose of this guide is to study how using VR systems affects the improvement and acquisition of functional skills in children and adolescents, and to evaluate available scientific evidence to determine the strength of recommendation of such interventions.
Although our aim was to collect studies with the highest levels of evidence possible, we had to include all articles discussing interventions in children with CP, regardless of the level of evidence, since this topic is relatively new and the number of articles in the literature is low. Results should therefore be interpreted with caution. We believe that all relevant studies were located using the search strategy and tracking the references cited by the articles and reviews identified by the search.
Our initial goal was to complete a systematic review, but it could not be carried out due to the scarcity of RCTs. We therefore used USPSTF to determine scientific evidence and strength of recommendation of VR interventions mentioned in the literature. The result is this evidence-based review, or clinical practice guideline.
The articles include patients with a wide range of ages (4 to 17) and different levels of dysfunction (GMFCS I to V), topographical distribution (hemiplegia, diplegia, and tetraplegia), and tone quality (spasticity, dyskinesia, ataxia, and hypotonia).
The 13 studies included in this guide show that VR training improves motor function in such areas as balance and postural control, upper-limb quality of movement, selective joint motor control, and gait. The highest level of evidence recommends VR training for balance and upper-limb function.
The same patients participated from beginning to end in all studies, and it is therefore possible to state that they all were suitable for inclusion in an intention-to-treat analysis. Most of the articles describe pilot studies and proof-of-concept clinical trials conducted with a view to increasing the sample size and developing more complex methodological designs in subsequent phases.
The articles included in this study used immersive and haptic VR systems which provide a considerable amount of sensory feedback. The literature suggests that greater immersion is associated with greater perceived reality of the experience, resulting in increased patient engagement in therapy. However, results do not allow us to establish a direct relationship between these two factors. The systems with the highest grades of recommendation are highly immersive (IREX®, Mandala® GX).
Overall, participants showed a strong commitment to therapy, with very few of them leaving the study. Only 2 studies reported drop-outs.31,68 Both were studies on telerehabilitation, suggesting that although telerehabilitation is a promising tool for making rehabilitation therapy more accessible, patients must be constantly supervised and therapists must modify interventions frequently so that they continue to engage patients, especially children and adolescents.
Numerous studies by Denise Reid54–58 on VR systems for patients with CP draw from theories of self-efficacy and motor learning to justify VR intervention. This type of therapy yields significant improvements in motivation, self-competence, and leisure and social opportunities for participants, since it lets them explore new experiences that would otherwise be prohibitively difficult or dangerous.
Regarding generalisation of learning, most trials included analyses performed before and after the training period. Postintervention assessment took place almost immediately after the intervention. Only 1 study included a follow-up.67 In this case, participants were assessed 4 weeks after the intervention, and gains had been partially maintained during follow-up. However, from these data we cannot state that improvements remain over time.
The literature does not provide conclusive data about whether VR intervention in children and adolescents with CP is superior to other rehabilitation therapies. Only 1 of the included studies compared conventional exercises with similar exercises performed in a VR environment. According to this study, the only advantage of VR systems is that it is task-oriented, resulting in patients feeling more motivated to complete the exercises.
This clinical practice guideline has some limitations that should be listed here. Our study included some studies of questionable methodological quality and low grades of recommendation. These studies also featured small sample sizes, considerable clinical diversity, and wide ranges of age, which has a large impact on mobility and therefore on the skills acquired by many participants. In consequence, the results must be interpreted critically and carefully.
ConclusionsThis clinical practice guide included articles with acceptable levels of recommendation addressing different VR interventions. However, it is essential to explore new lines of research that compare VR systems to other therapeutic procedures in order to justify the high cost of these systems. Such lines of research should be based on well-designed studies with a high methodological quality. Regarding the different types of VR systems, low-cost devices should be evaluated since they offer several advantages compared to devices specifically designed for therapeutic purposes: lower price, accessibility, good-quality technical support, easy update installation, and no need for additional modifications. Future studies will also have to establish well-defined selection criteria and make sure groups are homogeneous and similar in age, functional profile, topographical distribution, and tone quality. Additionally, studies should include functional outcome measures for assessing activities of daily living, and outcome measures on patients’ quality of life and treatment adherence. The main purpose of the above is to gain a deeper understanding of the transfer of the acquired motor skills, the impact of the intervention on social opportunities, and patients’ motivation and satisfaction.
Conflicts of interestThis study was carried out as part of the research project ‘Hybrid NeuroProsthetic and NeuroRobotic Devices for Functional Compensation and Rehabilitation of Motor Disorders’ (HYPER) within the framework of CONSOLIDER-Ingenio 2010 and the 6th Spanish National Plan for scientific research, development and technological innovation 2008-2011.
Please cite this article as: Monge Pereira E, Molina Rueda F, Alguacil Diego IM, Cano De La Cuerda R, De Mauro A, Miangolarra Page JC. Empleo de sistemas de realidad virtual como método de propiocepción en parálisis cerebral: guía de práctica clínica. Neurología. 2014;29:550–559.