Microvascular decompression (MVD) is accepted as the only aetiological surgical treatment for refractory classic trigeminal neuralgia (TN). There is therefore increasing interest in establishing the diagnostic and prognostic value of identifying neurovascular compressions (NVC) using preoperative high-resolution three-dimensional magnetic resonance (MRI) in patients with classic TN who are candidates for surgery.
MethodsThis observational study includes a series of 74 consecutive patients with classic TN treated with MVD. All patients underwent a preoperative three-dimensional high-resolution MRI with DRIVE sequences to diagnose presence of NVC, as well as the degree, cause, and location of compressions. MRI results were analysed by doctors blinded to surgical findings and subsequently compared to those findings. After a minimum follow-up time of six months, we assessed the surgical outcome and graded it on the Barrow Neurological Institute pain intensity score (BNI score). The prognostic value of the preoperative MRI was estimated using binary logistic regression.
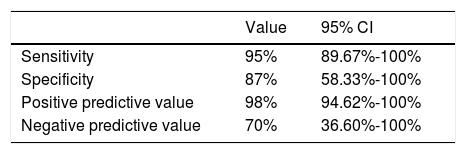
ResultsPreoperative DRIVE MRI sequences showed a sensitivity of 95% and a specificity of 87%, with a 98% positive predictive value and a 70% negative predictive value. Moreover, Cohen's kappa (CK) indicated a good level of agreement between radiological and surgical findings regarding presence of NVC (CK 0.75), type of compression (CK 0.74) and the site of compression (CK 0.72), with only moderate agreement as to the degree of compression (CK 0.48).
After a mean follow-up of 29 months (range, 6-100 months), 81% of the patients reported pain control with or without medication (BNI score I–III). Patients with an excellent surgical outcome, i.e. without pain and off medication (BNI score I), made up 66% of the total at the end of follow-up. Univariate analysis using binary logistic regression showed that a diagnosis of NVC on the preoperative MRI was a favourable prognostic factor that significantly increased the odds of obtaining an excellent outcome (OR 0.17, 95% CI 0.04-0.72; P=.02) or an acceptable outcome (OR 0.16, 95% CI 0.04-0.68; P=.01) after MVD.
ConclusionsDRIVE MRI shows high sensitivity and specificity for diagnosing NVC in patients with refractory classic TN and who are candidates for MVD. The finding of NVC on preoperative MRI is a good prognostic factor for long-term pain relief with MVD.
La descompresión microvascular (DMV) es aceptada como único tratamiento quirúrgico etiológico para la neuralgia del trigémino (NT) clásica refractaria al tratamiento médico. Por ello existe un creciente interés por establecer el valor diagnóstico y pronóstico de la identificación de compresiones neurovasculares (CNV) mediante resonancia magnética (RM) con secuencias tridimensionales de alta resolución en pacientes con NT clásica candidatos a cirugía.
MétodosEste estudio observacional incluye una serie consecutiva de 74 pacientes con NT clásica refractaria intervenidos mediante DMV. En todos los pacientes se realizó una RM tridimensional de alta resolución con secuencias DRIVE preoperatoria para diagnosticar la existencia de una CNV, así como su grado, origen y localización. Los resultados de la RM fueron analizados de forma «ciega» para los hallazgos de la exploración quirúrgica y posteriormente comparados con estos. Se realizó un seguimiento mínimo de 6 meses para comprobar los resultados quirúrgicos, que se clasificaron según la escala de dolor facial del Barrow Neurological Institute (BNI score). El valour pronóstico de la RM preoperatoria se analizó mediante una regresión logística binaria.
ResultadosLa RM preoperatoria con secuencias DRIVE demostró una sensibilidad del 95% y una especificidad del 87%, con un valor predictivo positivo del 98% y un valor predictivo negativo del 70%. Además se evidenció un buen grado de concordancia mediante el coeficiente kappa (CK) entre los hallazgos radiológicos y quirúrgicos respecto a la existencia de CNV (CK 0,75), al tipo de compresión (CK 0,74) y a la localización (CK 0,72), siendo del grado de concordancia moderado para el grado de compresión (CK 0,48).
Tras un seguimiento medio de 29 meses (rango 6-100 meses), el 81% de los pacientes presentaban un control del dolor sin o con medicación (BNI score I-III). Los pacientes con un resultado excelente del tratamiento, es decir aquellos sin dolor trigeminal y sin medicación (BNI score I) fueron el 66% al final del seguimiento. El análisis univariante mediante regresión logística binaria demostró que el diagnóstico de una CNV en la RM preoperatoria era un factor pronóstico favorable que incrementaba significativamente la probabilidad de obtener un resultado excelente (OR 0,17, IC del 95%, 0,04-0,72; p 0,02) o aceptable (OR 0,16, IC del 95%, 0,04-0,68; p 0,01) tras la DMV.
ConclusionesLa RM DRIVE presenta una elevada sensibilidad y especificidad para el diagnóstico preoperatorio de CNV en pacientes con NT clásica refractaria candidatos a tratamiento mediante DMV. El hallazgo de una CNV en el estudio de RM preoperatorio es un factor de buen pronóstico para la obtención de alivio del dolor a largo plazo con la DMV.
Trigeminal neuralgia (TN) is defined as “a sudden, usually unilateral, severe, brief, stabbing, recurrent pain in the distribution of one or more branches of the fifth cranial nerve,”1 and may be triggered by innocuous stimuli. It may manifest with no apparent cause (classical or primary TN) or as the result of some other disorder (secondary TN). According to the neurovascular compression (NVC) hypothesis, proposed by Gardner2 and subsequently developed by Jannetta3–5 based on his experience with microvascular decompression (MVD), TN is the result of vascular compression, generally by arteries, with continuous pulsation against the nerve eventually causing a traumatic lesion, resulting in demyelination.
Various studies have attempted to demonstrate the predictive value and accuracy of preoperative MRI in detecting possible NVC in patients with refractory classical TN who are eligible for MVD.6–17 While the indication of surgery is currently based solely on clinical symptoms, clearly demonstrating the presence of NVC may be a significant argument in favour of this treatment, particularly in cases of diagnostic uncertainty due to unusual presentations with constant pain, or in older patients who may benefit from MVD despite the surgical risk.14
On high-resolution, 3D T2-weighted volumetric MRI sequences, cerebrospinal fluid (CSF) shows high contrast against nervous tissues and tumours, without the need for gadolinium enhancement. The technique can also be used in conjunction with 2D or 3D contrast-enhanced T1-weighted sequences.14,18,19 Three-dimensional sequences enable multiplanar reconstruction with high spatial resolution. This technique, which includes 3D balanced fast field echo (bFFE), fast imaging employing steady-state acquisition (FIESTA), constructive interference in steady state (CISS), driven equilibrium radiofrequency reset pulse (DRIVE), and 3D fast recovery fast spin echo (FRFSE) sequences, shows high contrast between vascular and nervous structures, which appear hypointense, and CSF, which appears hyperintense; this contrast is observed even in sub-millimetre structures surrounding or within the CSF compartment.
This study aims to describe the usefulness of 3D T2-weighted DRIVE sequences in studying neurovascular relationships in the cerebellopontine angle (CPA) in patients with TN. To that end, we calculated the technique's sensitivity, specificity, and positive and negative predictive values, compared against findings from microsurgical exploration. We also assessed the correlation between detection of NVC in MRI studies and the medium- and long-term outcomes of MVD.
Patients and methodsWe performed an observational, analytical study including a consecutive series of patients treated surgically for TN at Hospital General Universitario Gregorio Marañón (Madrid, Spain) between January 2004 and January 2016. A database was created and populated with data from clinical histories, neuroimaging findings, surgical protocols, and intraoperative images. Data were gathered retrospectively from 2004 to 2009 and prospectively from 2010. Data were collected for all those patients who met the criteria of the third edition of the International Classification of Headache Disorders (beta version)20 for classical or idiopathic TN and for whom a minimum of 6 months’ follow-up data were available. A total of 98 patients underwent MVD for classical TN during the study period. All patients underwent a preoperative 3D T2-weighted MRI study with axial and coronal slices; this technique was introduced in 2004 but was not used systematically until 2009. Eighteen patients were therefore excluded from the sample for not undergoing this MRI study. We excluded a further 3 patients whose imaging studies were performed at another centre due to the uncertain quality of the images, and 3 patients who were followed up for less than the 6-month minimum period. Therefore, the final sample included 74 patients, with data collected retrospectively in 24 cases and prospectively in 50 cases.
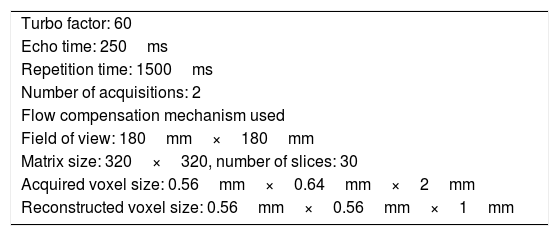
Two 1.5 T MRI scanners were used, one Intera and one Achieve (both from Philips Medical Systems; Best, Netherlands). Neurovascular relationships in the CPA were studied using a T2-weighted DRIVE sequence (parameters shown in Table 1).
DRIVE sequence parameters.
| Turbo factor: 60 |
| Echo time: 250ms |
| Repetition time: 1500ms |
| Number of acquisitions: 2 |
| Flow compensation mechanism used |
| Field of view: 180mm×180mm |
| Matrix size: 320×320, number of slices: 30 |
| Acquired voxel size: 0.56mm×0.64mm×2mm |
| Reconstructed voxel size: 0.56mm×0.56mm×1mm |
Acquisition on coronal and axial planes.
DRIVE: driven equilibrium magnetic resonance imaging.
In the surgical procedure described above, a retrosigmoid approach was used to perform microsurgical exploration of the CPA in order to identify potential NVC of the trigeminal nerve (Fig. 1).21 Where NVC was identified, the nerve was decompressed by vessel transposition and placement of a piece of Teflon® (BardPV; Tempe, AZ, USA) between the vessel and the nerve. Those patients in whom vascular compression was either not detected or was unclear underwent partial sensory rhizotomy of the inferior third of the trigeminal nerve.22 While it is not common for neuronavigation to be used in CPA surgery due to registration difficulties and the effect of cerebellar displacement on navigation reference points, there were specific cases, such as compression of the trigeminal nerve by vertebrobasilar dolichoectasia, in which presurgical planning employed 3D reconstructions generated with the BrainLab neuronavigation software (Feldkirchen, Germany) (Fig. 2).23
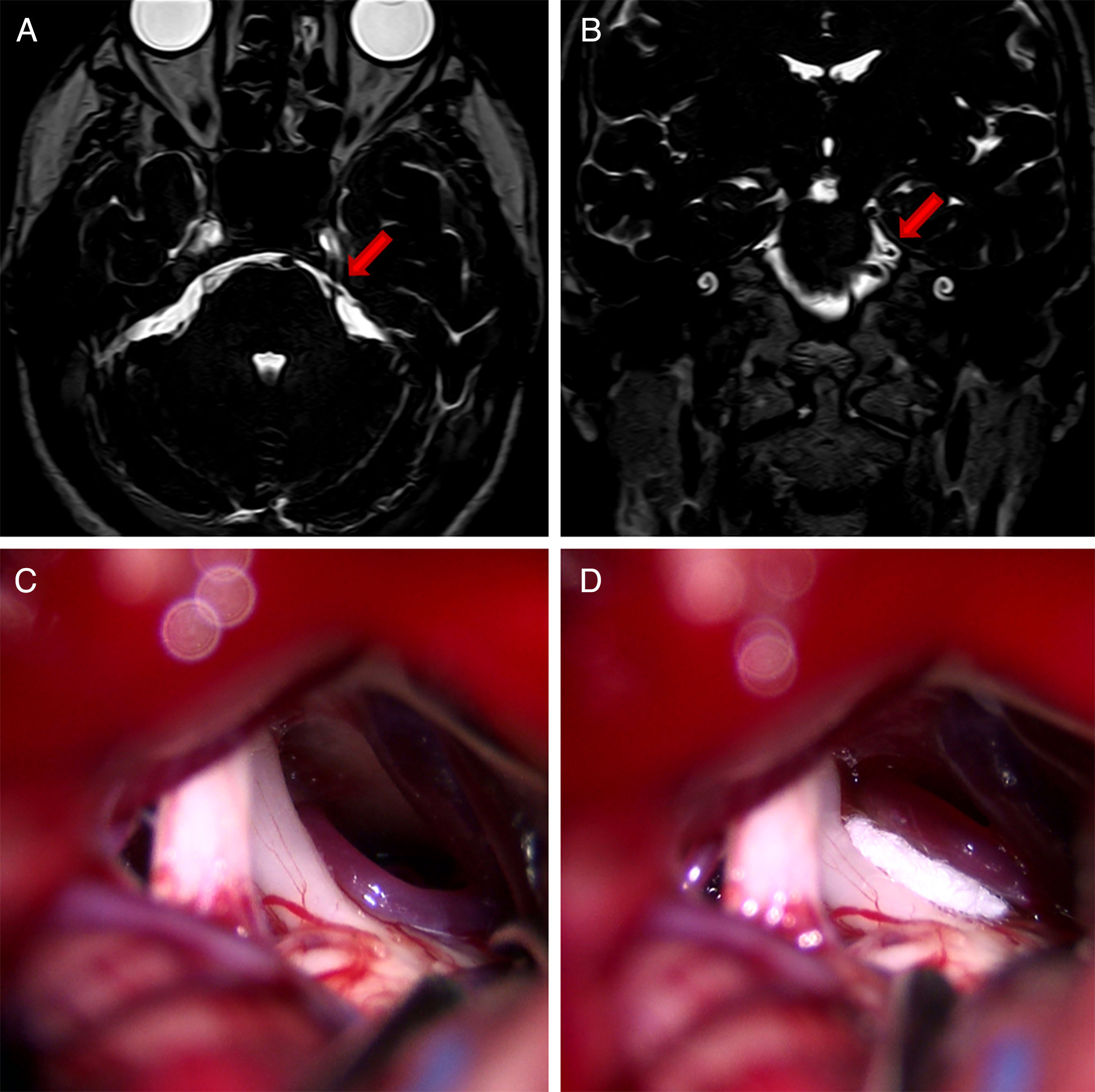
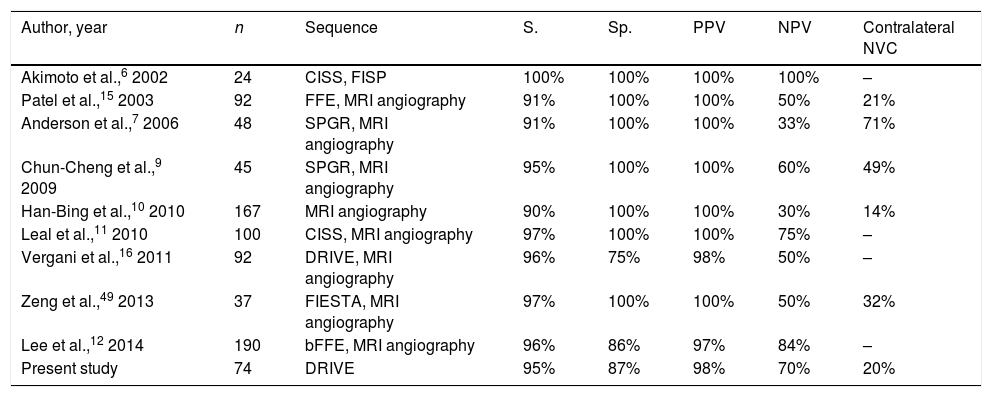
Axial (A) and coronal (B) DRIVE MRI sequences showing contact between the superior cerebellar artery (SCA) and the superior aspect of the left trigeminal nerve (arrow). (C) Microsurgical image of the left cerebellopontine angle showing compression of the trigeminal nerve by the SCA. (D) Decompression of the trigeminal nerve by placing a piece of Teflon®.
DRIVE MRI: driven equilibrium magnetic resonanace imaging.
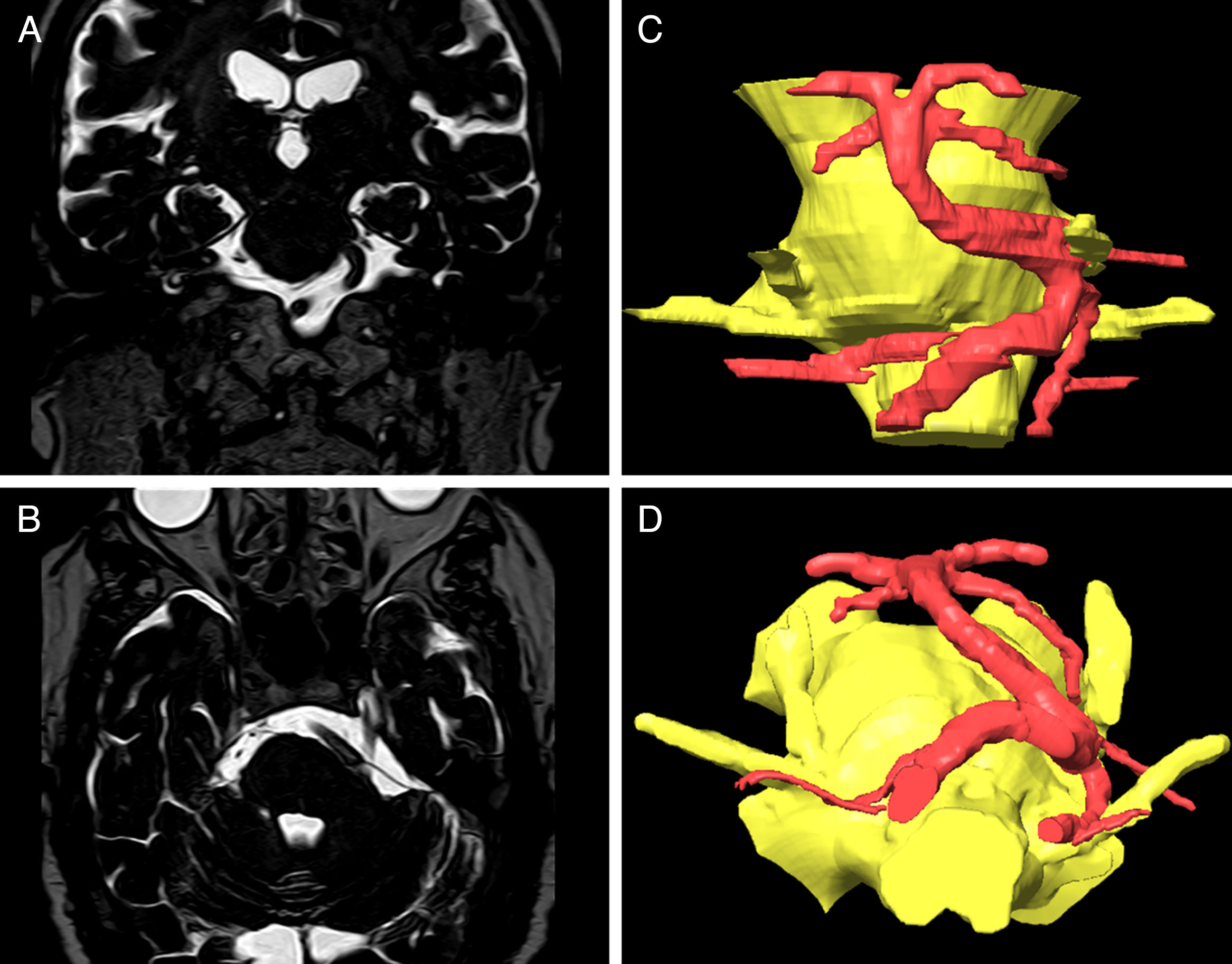
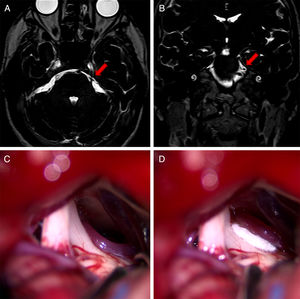
Coronal (A) and axial (B) DRIVE MRI sequences showing compression of the left trigeminal nerve due to vertebrobasilar dolichoectasia. The 3D reconstructions created using the BrainLab software for surgical planning show compression of the medial aspect of the trigeminal nerve.
DRIVE MRI: driven equilibrium magnetic resonanace imaging.
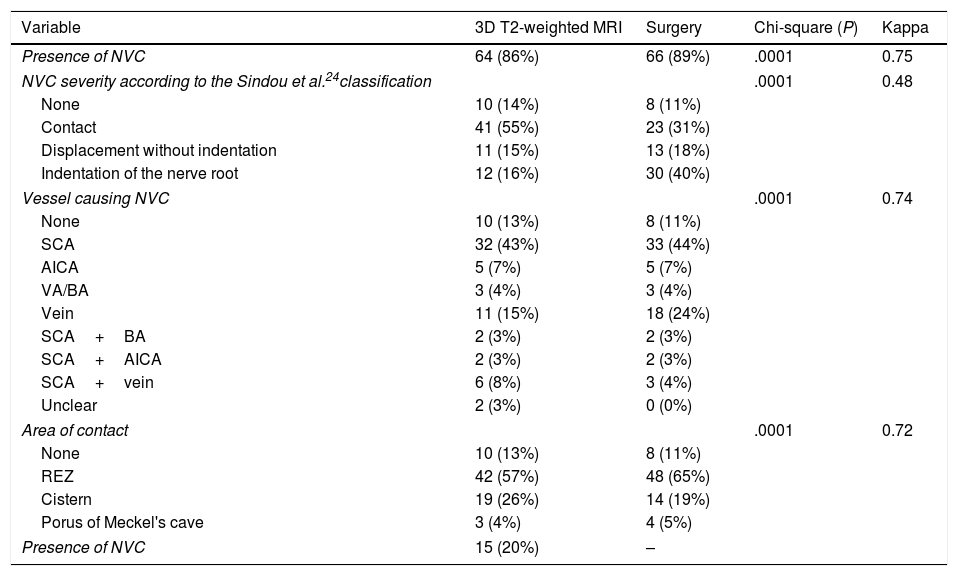
In the retrospective part of the study, FRJ gathered neuroimaging data before reviewing the operative reports; these therefore represent the observations of a researcher blinded to the results of the “gold standard” test, the microsurgical findings. Preoperative MRI scans were reviewed to identify high-resolution 3D T2-weighted DRIVE sequences. In cases where studies were performed at other centres, the equivalence and quality of the sequences were evaluated and the study was repeated where they were deemed inadequate. From 2010, 3D T2-weighted MRI studies were performed prior to surgery for all patients. NVC was defined as contact between the trigeminal nerve and a vascular structure in the CPA, with no visible layer of CSF separating them. We studied the following parameters: presence of NVC, degree of NVC, the vessel involved, and location of the NVC. Degree of NVC was defined according to the classification proposed by Sindou et al.24 (Table 2). Intraoperative findings from cases from before 2009 were gathered from operative reports and compared against intraoperative videos and images. After 2010, these data were entered into the database immediately after surgery.
MRI and surgical findings.
| Variable | 3D T2-weighted MRI | Surgery | Chi-square (P) | Kappa |
|---|---|---|---|---|
| Presence of NVC | 64 (86%) | 66 (89%) | .0001 | 0.75 |
| NVC severity according to the Sindou et al.24classification | .0001 | 0.48 | ||
| None | 10 (14%) | 8 (11%) | ||
| Contact | 41 (55%) | 23 (31%) | ||
| Displacement without indentation | 11 (15%) | 13 (18%) | ||
| Indentation of the nerve root | 12 (16%) | 30 (40%) | ||
| Vessel causing NVC | .0001 | 0.74 | ||
| None | 10 (13%) | 8 (11%) | ||
| SCA | 32 (43%) | 33 (44%) | ||
| AICA | 5 (7%) | 5 (7%) | ||
| VA/BA | 3 (4%) | 3 (4%) | ||
| Vein | 11 (15%) | 18 (24%) | ||
| SCA+BA | 2 (3%) | 2 (3%) | ||
| SCA+AICA | 2 (3%) | 2 (3%) | ||
| SCA+vein | 6 (8%) | 3 (4%) | ||
| Unclear | 2 (3%) | 0 (0%) | ||
| Area of contact | .0001 | 0.72 | ||
| None | 10 (13%) | 8 (11%) | ||
| REZ | 42 (57%) | 48 (65%) | ||
| Cistern | 19 (26%) | 14 (19%) | ||
| Porus of Meckel's cave | 3 (4%) | 4 (5%) | ||
| Presence of NVC | 15 (20%) | – | ||
AICA: anterior inferior cerebellar artery; BA: basilar artery; NVC: neurovascular compression; REZ: root entry zone; SCA: superior cerebellar artery; VA: vertebral artery.
Pain relief data were gathered from patient notes recorded during admission and consultations, and in telephone interviews conducted at discharge and at one month, 6 months, one year, 2 years, and at the end of the follow-up period. Pain relief was quantified using the Barrow Neurological Institute pain scale (BNI; Table 3), which classifies absence of pain without medication (BNI I) as excellent, and pain adequately controlled with medication (BNI I-III) as an acceptable outcome.25–27
Barrow Neurological Institute (BNI) pain intensity score.
| I | Excellent: no pain, no medication required |
| II | Good: occasional pain, no medication required |
| III | Acceptable: some pain, adequately controlled with medication |
| IV | Treatment failure: some pain, not adequately controlled with medication |
| M | Poor: severe pain or no pain relief |
In the statistical analysis, significance was set at P<.05; 95% confidence intervals (CIs) were calculated for the variables analysed. Kappa coefficients were calculated to estimate agreement between MRI and surgical findings. Agreement was considered to be weak for kappa values of 0-0.39, moderate for 0.4-0.59, good for 0.6-0.79, and very good for 0.8-1. The association between surgery outcome and presence of NVC was estimated using contingency tables and the Fisher exact test. Binary logistic regression was used to calculate relative risks and 95% CIs for MRI detection of NVC. Pain recurrence rates were calculated using the Kaplan-Meier estimator: recurrence of trigeminal pain refractory to medication (BNI IV or V) was regarded as treatment failure. We also estimated pain-free survival time with no pharmacological treatment (BNI score I). Differences in pain recurrence as a function of preoperative detection of NVC were analysed using the Mantel-Cox test. Statistical analysis was performed using the SPSS statistical software, version 15.0. We also used version 3.1 of the Epidat epidemiological analysis software to calculate the sensitivity, specificity, and positive and negative predictive values of MRI.
ResultsThe study included a series of 74 patients with classical TN who underwent preoperative studies with 3D T2-weighted MRI and were surgically treated with MVD between 2004 and 2016. Patients had a mean age of 61.1 years; 34% were older than 70 at the time of the intervention. The male:female ratio was 1:2.4. Patients were referred to our service following diagnosis of drug-resistant classical TN; mean disease duration was 7.3 years. At the time they were evaluated for surgical treatment, 74.3% of patients were receiving 2 or more antiepileptic drugs, which were either ineffective or were associated with intolerable adverse reactions. According to the classification proposed by Burchiel,28 pain was predominantly episodic in 88% of patients (TN type 1) and predominantly constant in 12% (TN type 2).
NVC was detected by the MRI study in 64 cases (86.5%), although in 41 of these patients (64%) the degree of compression was contact only (degree 1 severity), according to the classification proposed by Sindou et al.24 MRI-diagnosed NVC was arterial in 44 cases (69%), venous in 11 (17%), mixed in 7 (11%), and unclear in 2 (3%). Among cases of arterial NVC, the superior cerebellar artery was most frequently responsible for compression, with 42 patients (66%) showing involvement of this artery either alone or together with other vessels. Contact was located in the root entry zone in 42 patients (66%) and on the nerve's course through the cerebellopontine cistern in 19 (30%). The MRI study displayed vascular contact of the contralateral trigeminal nerve in 20% of patients; however, no patient reported bilateral facial pain.
Microsurgical exploration of the CPA identified NVC in 66 patients (89%), of whom 43 (65%) showed greater compression than contact alone (Sindou et al.24 degrees 2 and 3). This is a higher degree of NVC than that diagnosed with 3D T2-weighted MRI. Microsurgical findings also showed predominantly arterial and mixed NVC, which accounted for 45 (68%) and 3 (5%) cases, respectively. However, venous NVC was more frequent, observed in 18 patients (27%). The root entry zone was also the most common NVC location identified in surgical exploration (37 cases, 71%).
In the contingency table analysis, significant associations were observed between preoperative MRI observations and surgical findings for all parameters studied. Kappa values were greater than 0.7 for presence of NVC, vessel involved, and location; this indicates good agreement between both sets of findings. However, concordance for degree of compression (Sindou et al.24 classification) was moderate (kappa value of 0.48). These data are included in Table 2.
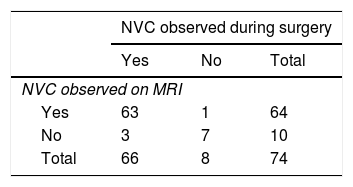
Contingency table analysis for MRI and surgical diagnosis of NVC (Table 4) shows that of the 64 cases diagnosed by MRI, one case was not confirmed by surgery; this patient therefore represents a false positive. In contrast, surgery detected NVC in 3 of the 10 patients found not to be affected according to MRI findings; these patients represent false negatives. Therefore, the sensitivity and specificity of 3D T2-weighted MRI were 95% and 87%, respectively. The positive predictive value was 98% (50 out of 51 cases were true positives) and the negative predictive value was 70% (7 out of 10 true negatives) (Table 5).
The most frequently performed surgical procedure was vessel transposition and decompression by placement of a piece of Teflon between the nerve and the compressing vessel. This procedure was performed in 63 cases (85%); partial sensory rhizotomy was also performed in 9 of these patients due to unclear compression, generally because of venous involvement. In the 8 patients in whom no NVC was observed during surgical exploration, we performed debridement of the nerve and partial sensory rhizotomy. Patients were followed up for a mean period of 29 months (range 6-100) and a median of 20. All patients included in the study were followed up for at least 6 months.
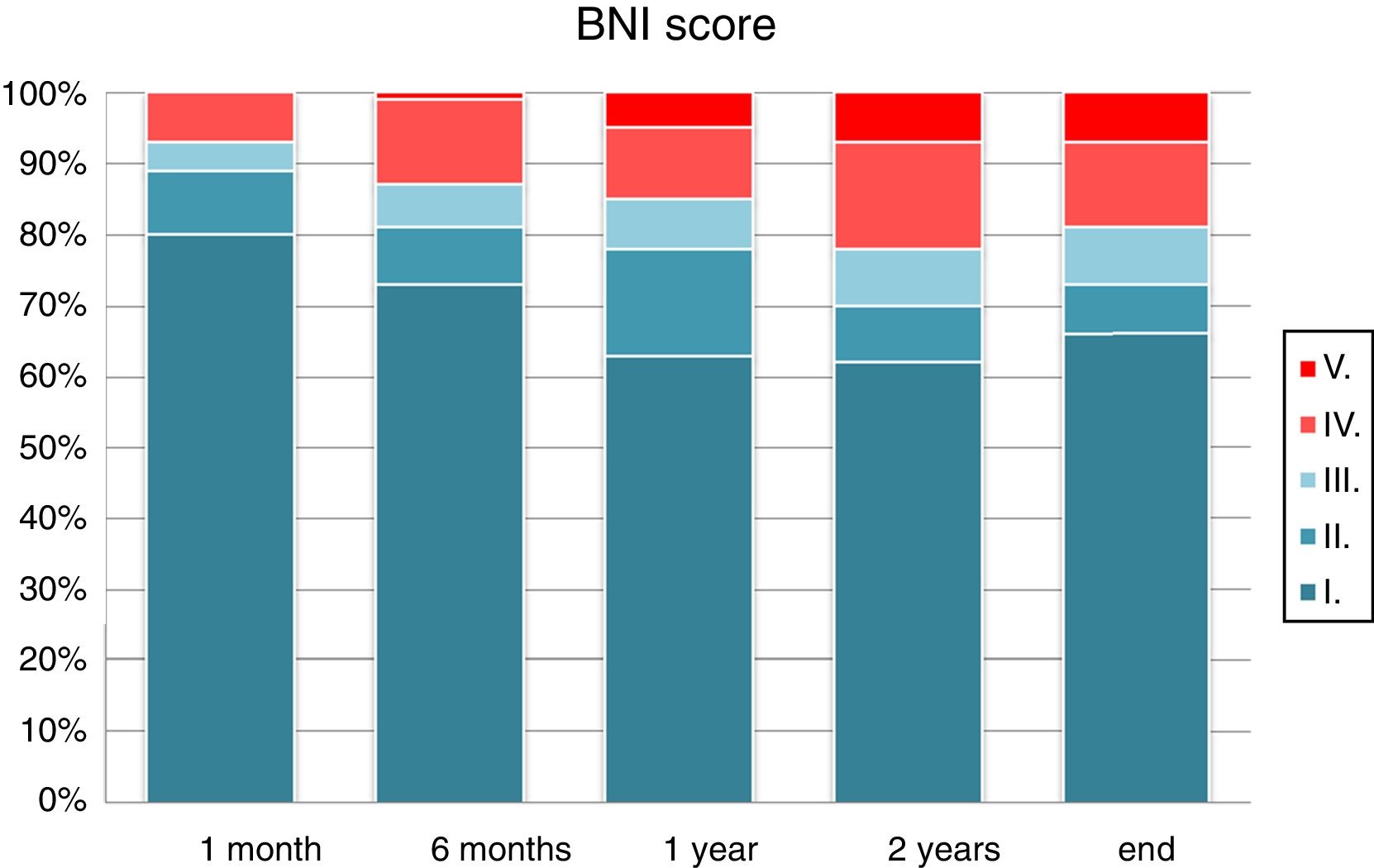
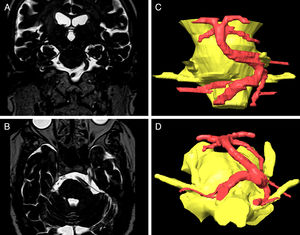
Visual analogue scores for pain decreased after surgery. Comparison of mean pain scores using the Wilcoxon test for non-normally distributed samples revealed a significant decrease in visual analogue scores at one month, 6 months, one year, 2 years, and at the end of follow-up (P<.0001). One month after surgery, 93% of patients presented an acceptable improvement in pain control with or without medication (BNI I-III). This rate fell to 85% at one year and 81% at the end of the follow-up period. The percentage of patients showing excellent outcomes (absence of trigeminal pain without the need for medication) was 80% at one month, 63% at one year, and 66% at the end of the follow-up period (Fig. 3). According to type of pain, 86.2% of the patients with Burchiel type 1 TN (predominantly episodic) and 44% of the patients with Burchiel type 2 TN (predominantly constant) showed satisfactory outcomes at the end of follow-up (P<.01).
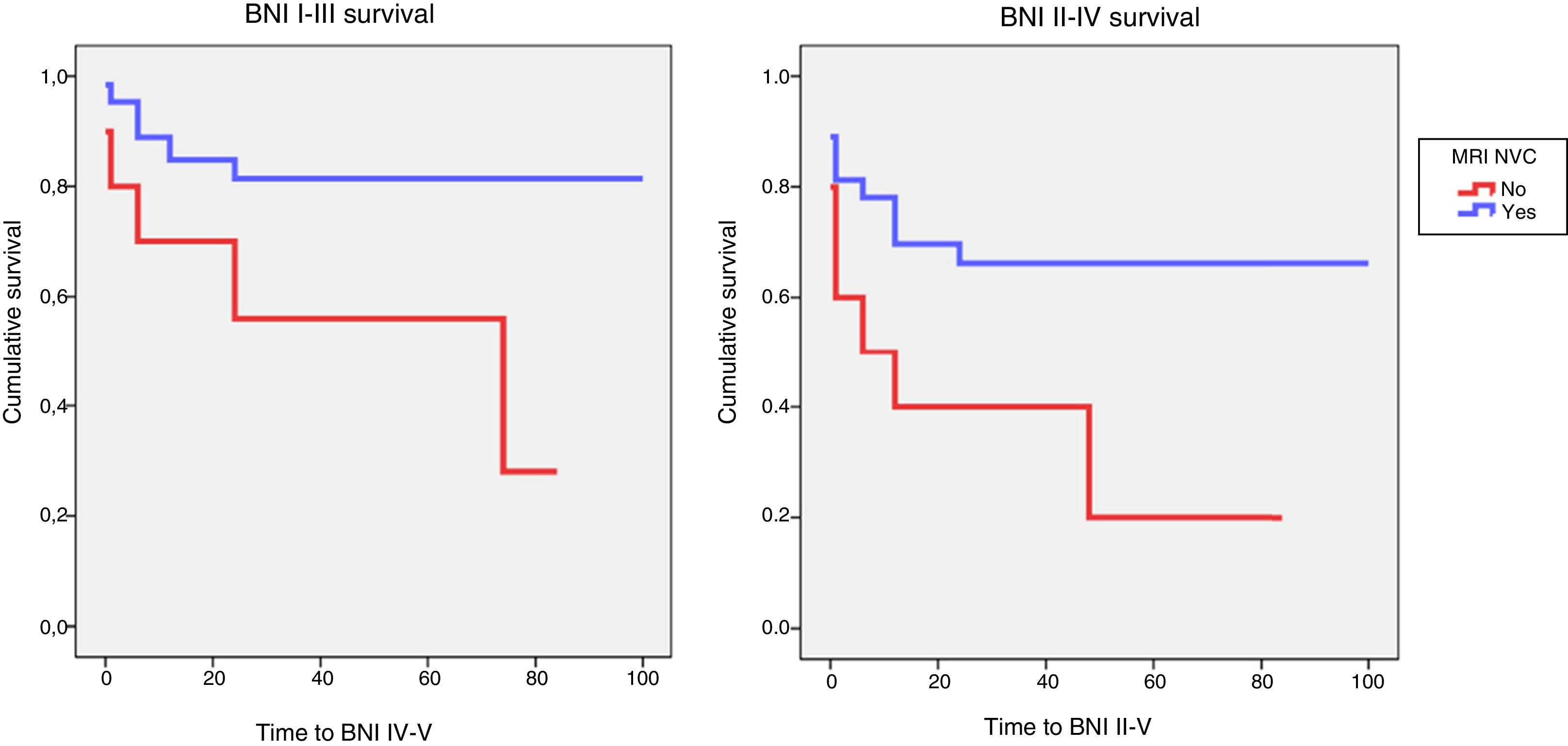
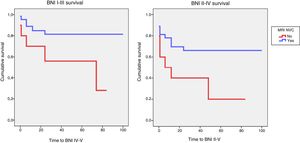
Identification of NVC on preoperative MRI was significantly associated with acceptable surgical outcomes (BNI I-III) (P=.02). A significant association was also observed between detection of NVC on MRI and excellent outcomes (BNI I) at the end of follow-up (P=.03). Logistic regression showed that patients whose preoperative MRI did not show NVC were 6 times less likely to achieve acceptable pain control at the end of follow-up (OR, 0.16; 95% CI, 0.04-0.68; P<.01). The probability of achieving pain freedom without medication was also 6 times lower in patients with no evidence of NVC in MRI studies (OR, 0.17; 95% CI, 0.04-0.72; P=.02). Kaplan and Meier curves showed lower survival rates for controlled pain (BNI I-III; P=.04) and absence of pain without medication (BNI I; P=.02) in this patient group (Fig. 4).
Kaplan-Meier survival analysis for controlled pain (BNI I-III) and absence of pain without medication (BNI I) as a function of MRI detection of neurovascular compression.
BNI: Barrow Neurological Institute pain intensity score; MRI NVC: neurovascular compression detected on magnetic resonance images.
Numerous studies have addressed the immediate and long-term outcomes of MVD in series of patients followed up for a mean of over 5 years; these studies report initial success rates ranging between 76% and 99%, long-term success rates of 62% to 89%, and relapse rates of 4% to 38%.29–39 Recent series report mortality rates below 1%, with rates of severe neurological complications below 5%.34,35,40
Before the systematic performance of MRI in patients with TN, space-occupying lesions were frequently found to be the cause of suspected classic TN during microsurgical exploration of the CPA. In their 1989 series of 252 surgical explorations, Bederson and Wilson30 report incidental detection of 2 meningiomas, 2 schwannomas, and 3 epidermoid cysts. The current recommendations of the American Academy of Neurology (AAN) and the European Federation of Neurological Societies (EFNS) include routine performance of neuroimaging studies in the diagnosis of TN in order to rule out causes of secondary TN.41 Given the fundamental role of NVC in classical TN pathophysiology and the fact that surgical decompression improves or fully resolves pain in most patients, there is growing interest in the use of MRI sequences to identify NVC in classical TN. Several MRI sequences can be used to visualise neurovascular relationships in the CPA. Older series use a variety of contrast-enhanced T1-weighted MRI angiography sequences, such as fast low angle shot (FLASH; Siemens) and spoiled gradient echo (SPGR, General Electric). These MRI angiography sequences show good contrast between vascular structures (hyperintense), nervous tissue (intermediate intensity), and CSF (hypointense). However, distinguishing arteries from veins represents a challenge, as does determining which specific vessel is involved in NVC.42–45 Meaney et al.46 analysed the diagnostic performance of MRI angiography, reporting sensitivity of 96% and specificity of 100% in a sample of 50 patients, comparing results to surgical findings. The researchers conclude that the technique is able to identify patients with greater likelihood of pain resolution following MVD.
Subsequent studies report the use of 3D T2-weighted sequences, also known as MR cisternography. In a study including 54 patients, Yoshino et al.47 used MRI angiography and CISS sequences to identify NVC caused by veins and small vessels, finding the latter technique to be significantly more sensitive. Following this trend, in more recent studies the preoperative analysis of NVC is fundamentally based on the analysis of 3D T2-weighted sequences; MRI angiography is used additionally in many cases.11,16,48 Using surgical exploration as the reference technique, various authors have reported sensitivity of 76%-97% and specificity of 75%-100%.7–12,15,16,49 Strong agreement has also been found between MRI and surgical findings, with kappa values of 0.7 to 0.9.6,7,9,11 Anderson et al.7 studied a series of 48 patients with 3D SPGR and time of flight (TOF) sequences, finding a sensitivity of 91% and a specificity of 100%. No false positives were found, although there were 4 false negatives. This represents a positive predictive value of 100% and a negative predictive value of only 33%. The authors report a kappa score of 0.8 for agreement between MRI and surgical findings. In that study, 71% of patients displayed contralateral compression; however, logistic regression analysis of the degree of compression revealed that the symptomatic side was 1.96 times more likely to have more severe compression (95% CI, 1.08-3.58).7 Other studies reporting high sensitivity and specificity often find no false positives, with positive predictive values consequently approaching 100%. Nonetheless, several studies do report false negatives, with negative predictive values ranging from 33% to 75%.9–12,16,49 The low rate of false events in general increases the variation in the false negative rate. However, this indicates a limitation in the resolution of MRI angiography and 3D T2-weighted MRI sequences. Analysis of false negatives often reveals cases of venous compression in close proximity to the brainstem, or compression by small vessels.
In our series, 3D DRIVE MRI sequences showed a sensitivity of 95% and a specificity of 87%. The presence of one false positive and 3 false negatives results in a positive predictive value of 98% and a negative predictive value of 70%. These results are comparable to those reported in studies using contrast-enhanced T1-weighted sequences, MRI angiography studies, and 3D T2-weighted sequences, either alone or in combination with other techniques (Table 6).7,9–12,15,16,49 MRI and surgical findings showed high levels of agreement for presence of compression (kappa score of 0.75), type of vessel involved (0.74), and location of compression (0.72) and moderate agreement for degree of compression (0.48). Leal et al.11 report kappa values of 0.79 for degree of compression, 0.82 for type of vessel, and 0.74 for location. While these scores indicate somewhat better agreement than that observed for our own series, we used a single MRI sequence, whereas that study used a combination of gadolinium-enhanced 3D T1-weighted, angiography, TOF, and CISS MRI sequences, with the associated increases in time and cost.11
Magnetic resonance imaging in the preoperative diagnosis of neurovascular compression in patients with trigeminal neuralgia.
| Author, year | n | Sequence | S. | Sp. | PPV | NPV | Contralateral NVC |
|---|---|---|---|---|---|---|---|
| Akimoto et al.,6 2002 | 24 | CISS, FISP | 100% | 100% | 100% | 100% | – |
| Patel et al.,15 2003 | 92 | FFE, MRI angiography | 91% | 100% | 100% | 50% | 21% |
| Anderson et al.,7 2006 | 48 | SPGR, MRI angiography | 91% | 100% | 100% | 33% | 71% |
| Chun-Cheng et al.,9 2009 | 45 | SPGR, MRI angiography | 95% | 100% | 100% | 60% | 49% |
| Han-Bing et al.,10 2010 | 167 | MRI angiography | 90% | 100% | 100% | 30% | 14% |
| Leal et al.,11 2010 | 100 | CISS, MRI angiography | 97% | 100% | 100% | 75% | – |
| Vergani et al.,16 2011 | 92 | DRIVE, MRI angiography | 96% | 75% | 98% | 50% | – |
| Zeng et al.,49 2013 | 37 | FIESTA, MRI angiography | 97% | 100% | 100% | 50% | 32% |
| Lee et al.,12 2014 | 190 | bFFE, MRI angiography | 96% | 86% | 97% | 84% | – |
| Present study | 74 | DRIVE | 95% | 87% | 98% | 70% | 20% |
bFFE: balanced fast field echo; CISS: constructive interference in steady state: DRIVE: driven equilibrium; FFE: fast field echo; FIESTA: fast imaging employing steady-state acquisition; FISP: fast inflow with steady-state precession; MRI: magnetic resonance imaging; NPV: negative predictive value; NVC: neurovascular compression; PPV: positive predictive value; S.: sensitivity; Sp.: specificity; SPGR: spoiled gradient-recalled.
Some studies include medium- to long-term follow-up of patients undergoing MRI and MVD in order to estimate the degree of association between detection of NVC on preoperative MRI and surgical outcomes. For instance, Vergani et al.16 found no significant association between negative MRI results (DRIVE and TOF MRI angiography) and poor surgical outcomes. In contrast, Han-Bing et al.10 assessed 144 patients with 3D TOF MRI angiography and found a significantly higher rate of improvement in patients with MRI findings indicating NVC than in patients with negative findings (94% and 69%, respectively; P<.01). In our study, detection of NVC on MRI was significantly associated with acceptable (BNI I-III, P=.02) or excellent outcomes (BNI I, P=.03) at the end of the follow-up period. Univariate analysis demonstrated that patients whose preoperative MRI studies did not show NVC were 6 times less likely to achieve acceptable pain control at the end of the follow-up period (OR, 0.16; 95% CI, 0.04-0.68; P<.01). The likelihood of achieving absence of pain without medication was also 6 times lower in patients with no evidence of NVC in MRI studies (OR, 0.17; 95% CI, 0.04-0.72; P=.02).
Despite these observations, various recent reviews underscore the lack of large, prospective, blinded case-control studies enabling accurate calculation of the sensitivity and specificity of preoperative MRI for detecting NVC in patients with TN.50,51 The 2008 AAN-EFNS recommendations advocate the use of preoperative MRI to rule out possible causes of secondary TN, but maintain that insufficient evidence is available to defend or refute its use for diagnosing NVC or identifying patients likely to respond well to MVD.41 In the absence of direct comparisons, a literature review found 3D T2-weighted sequences to be more accurate than MRI angiography.47,49,52 Our results are comparable to those from other studies using these 3D T2-weighted sequences in addition to MRI angiography.7,11,16,48,52 The high sensitivity and specificity values suggest that preoperative MRI is able to accurately predict the presence or absence of NVC. Despite this, most series report more false negatives than false positives. This seems to suggest that MRI findings indicating NVC ipsilateral to the reported pain should be considered highly predictive, and that MVD is more likely to be successful. However, negative MRI results do not preclude the possibility of trigeminal nerve compression being detected during the surgical procedure; such findings should therefore not be considered a contraindication for surgery, although they may alert the surgeon to the possibility of small-vessel or venous compression. Taking into account the possibility of less severe or venous compression, or even the absence of NVC, we may inform the patient about the prognostic implications.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ruiz-Juretschke F, Guzmán-de-Villoria JG, García-Leal R, Sañudo JR. Valor predictivo de la resonancia magnética para la identificación de compresiones neurovasculares en la neuralgia del trigémino. Neurología. 2019;34:510–519.