Exposure to low doses of O3 leads to a state of oxidative stress. Some studies show that oxidative stress can modulate both the CNS and systemic inflammation, which are important factors in the development of Alzheimer disease (AD).
ObjectiveThis study aims to evaluate changes in the frequency of Th17-like cells (CD3+CD4+IL-17A+), the concentration of IL-17A in peripheral blood, and hippocampal immunoreactivity to IL-17A in rats exposed to low doses of O3.
MethodsOne hundred eight male Wistar rats were randomly assigned to 6 groups (n=18) receiving the following treatments: control (O3 free) or O3 exposure (0.25ppm, 4h daily) over 7, 15, 30, 60, and 90 days. Twelve animals from each group were decapitated and a peripheral blood sample was taken to isolate plasma and mononuclear cells. Plasma IL-17A was quantified using LUMINEX, while Th17-like cells were counted using flow cytometry. The remaining 6 rats were deeply anaesthetised and underwent transcardial perfusion for immunohistological study of the hippocampus.
ResultsResults show that exposure to O3 over 7 days resulted in a significant increase in the frequency of Th17-like cells and levels of IL-17A in peripheral blood. However, levels of Th17/IL-17A in peripheral blood were lower at day 15 of exposure. We also observed increased IL-17A in the hippocampus beginning at 30 days of exposure.
ConclusionThese results indicate that O3 induces a short-term, systemic Th17-like/IL-17A effect and an increase of IL-17A in the hippocampal tissue during the chronic neurodegenerative process.
La exposición a dosis bajas de O3 conduce a un estado de estrés oxidativo. Algunos estudios muestran que el estado de estrés oxidativo puede modular tanto el SNC como la inflamación sistémica, que son importantes para el desarrollo de la enfermedad de Alzheimer.
ObjetivoEvaluar la frecuencia de células tipo Th17, la concentración de IL-17A en plasma y la inmunorreactividad del hipocampo a IL-17A en ratas expuestas a dosis bajas de O3.
MétodosCiento ocho ratas Wistar machos fueron divididas en 6 grupos (n=18) con los siguientes tratamientos: control (sin O3) y O3 (0,25ppm, diario por 4h) durante 7, 15, 30, 60 y 90 días. De cada grupo se decapitaron 12 animales, se tomó una muestra de sangre periférica para aislar el plasma y las células mononucleares. La IL-17A plasmática se evaluó mediante LUMINEX y la frecuencia de células de tipo Th17 por citometría de flujo. Las ratas restantes se anestesiaron y se perfundieron para inmunohistoquímica en el hipocampo.
ResultadosMuestran que la exposición durante 7 días a O3 produce un aumento significativo en la frecuencia de células tipo Th17 y los niveles de IL-17A en sangre periférica. Sin embargo, existe una disminución de Th17/IL-17A en la periferia desde el día 15. También se encontró un aumento de IL-17A en el hipocampo desde los 30 días de exposición.
ConclusiónEl O3 produce un efecto sistémico a corto plazo de tipo Th17/IL-17A y un aumento de IL-17A en el tejido del hipocampo durante el proceso neurodegenerativo crónico.
Air pollution is one of the main health problems in densely populated and industrialised cities.1 Ozone is one of the main air pollutants and is produced by photochemical reactions in the lower atmosphere.2 Both acute and chronic inhalation of this gas have been reported to cause a state of oxidative stress.3 Chronic oxidative stress state is a critical factor in the development of neurodegenerative diseases.4
Previous studies by our working group have demonstrated that chronic exposure to low doses of ozone (0.25ppm) for 4hours daily induces oxidative stress and irreversible progressive neurodegeneration.5 In the hippocampus, this process is characterised by mitochondrial alterations leading to an energy deficit, endoplasmic reticulum stress, alterations to the Golgi apparatus and nucleus, glial activation and proliferation, cell death by apoptosis and necrosis, and intracellular accumulation of beta-amyloid 1-42 (Aβ1–42), in addition to loss of brain repair and memory and learning impairment; this process is similar to that observed in humans during the development of Alzheimer disease (AD).6
Another crucial factor in the development and progression of neurodegeneration is the loss of regulation of the inflammatory response. The inflammatory response is normally restorative and self-limited. However, in chronic degenerative diseases this response is characterised by abnormal production of proinflammatory cytokines, chemokines, growth factors, reactive oxygen species (ROS), and reactive nitrogen species (RNS), as well as by adaptive immune cell and microglial activation,7 creating a vicious circle that prevents the self-limitation of the response.
In the context of the adaptive immune response, Th17 cells play a significant role in host defence, but have frequently been associated with various inflammatory diseases, including such neurodegenerative diseases as AD.8 One of the most characteristic cytokines of this cell subpopulation is interleukin-17A (IL-17A), which transduces signals that bridge the innate and the adaptive immune systems, since it influences a wide variety of cell lines (macrophages, denditric cells, endothelial cells, etc.) and induces the expression of several inflammatory mediators9 (cytokines, chemokines, and growth factors).
An animal model of Aβ1–42-induced AD revealed that peripheral Th17 cells infiltrate the brain parenchyma due to alterations in the blood-brain barrier.10 Furthermore, elevated hippocampal expression of the Th17 cytokines IL-17A and IL-22 and high concentration of these proteins in the cerebrospinal fluid and blood have been reported in rat models of AD. These findings provide robust evidence of the participation of the Th17 response in neuroinflammation in AD.
It is important to highlight that the inflammatory process has been observed not to affect the central nervous system (CNS) exclusively in AD. There is considerable evidence suggesting that systemic inflammation plays a significant role in AD pathogenesis11; therefore, CNS and systemic inflammation may not be considered in isolation. Furthermore, low-grade systemic inflammation may have a significant impact on the CNS12, and harmful effects are likely to be more acute in patients with AD.
The aim of this study was to analyse changes to the Th17 cell frequency (CD3+, CD4+, IL-17A+) in peripheral blood, plasma IL-17A concentration, and the presence of IL-17A in the hippocampi of rats chronically exposed to low doses of ozone.
Material and methodsAnimalsWe used 108 male Wistar rats weighing 250-300g, housed in individual acrylic cages with free access to food and water (NutriCubo, Purina; USA). Animals were kept in a vivarium with controlled temperature and humidity. Care and handling of the animals complied with the National Institutes of Health Guidelines for Animal Treatment and with the official Mexican regulations (NOM-062-SSA-2-2002). The study was approved by the research ethics committee of the School of Medicine at Universidad Nacional Autónoma de México.
General procedureRats were randomly assigned to 6 experimental groups (n=18 per group). The control group was exposed to an ozone-free air flow for 4hours daily for 30 days; groups 2, 3, 4, 5, and 6 were exposed to ozone (0.25ppm) for 4hours daily for 7, 15, 30, 60, and 90 days, respectively.
Ozone exposureRats were placed inside a chamber with a diffuser connected to an ozone generator with variable output (5L/s); this procedure has been described in previous studies.13 To summarise, the generator uses filtered air to produce ozone. Ozone concentration was proportional to air intensity and flow. We used the Ozone Switch Model OS-06 monitor (Ecosensors; Germany) to measure the ozone concentration inside the chamber. To expose the control group to ozone-free air, the same chamber was connected to an ozone-free air flow.
After 2hours of exposure to air or ozone, 12 animals from each group were decapitated and blood from the carotid artery was collected in 4.0-mL Vacutainer tubes (BD Vacutainer; NJ, USA) with 7.2mg of ethylenediamine tetraacetic acid for quantification of Th17 cell frequency and IL-17A levels. The remaining 6 rats in each group were deeply anaesthetised with pentobarbital sodium (50mg/kg) and administered perfusions of 4% paraformaldehyde. Brains were removed and placed in the same fixative solution for 24hours at 4°C. Conventional histological methods were subsequently used to obtain paraffin-embedded tissue. Brains were cut into 5-μm sagittal slices, and the hippocampal sections were used in the immunofluorescence study.
Processing of peripheral blood samplesBlood samples were centrifuged at 2500rpm for 10minutes. Plasma was separated and stored at −70°C. Peripheral blood mononuclear cells (PBMC) were isolated according to the Ficoll-Hypaque protocol.14 Following separation of PBMC, surface staining was performed with FITC Mouse Anti-Human CD3 and PE-Cy™5 Mouse Anti-Human CD4 monoclonal antibodies (BD Pharmigen; San Diego, CA, USA). Samples were incubated for 30minutes at 4°C in the dark and then washed for 5minutes in 1mL of PBS at 3000rpm. The cell membrane was permeabilised using the Cytofix/Cytoperm commercial kit (BD Biosciences; San Diego, CA, USA); cell labelling was then conducted with the PE anti-mouse IL-17A antibody (eBioscience; San Diego, CA, USA).
Labelled cells were resuspended in 0.2mL of 3% paraformaldehyde for subsequent counting by flow cytometry.
Plasma interleukin-17A levelsPlasma samples were thawed at room temperature to be analysed with a Bio-Plex® MAGPIX™ Multiplex Reader (Bio-Rad), using a commercial kit to quantify 9 analytes (Milliplex MAP Rat Cytokine/Chemokine Magnetic Bead Panel, Merck; Billerica, MA, USA). Standard curves were prepared with premixed standards included in the kit. The mean fluorescence intensity of each sample was obtained automatically with the Milliplex® Analyst 5.1 software (Life Science Research).
Frequency of IL-17-producing CD4+ T cellsMarked cells were assessed by flow cytometry. The data obtained were exported to the FlowJo 10.2 software (Treestar; Ashland, OR, USA).
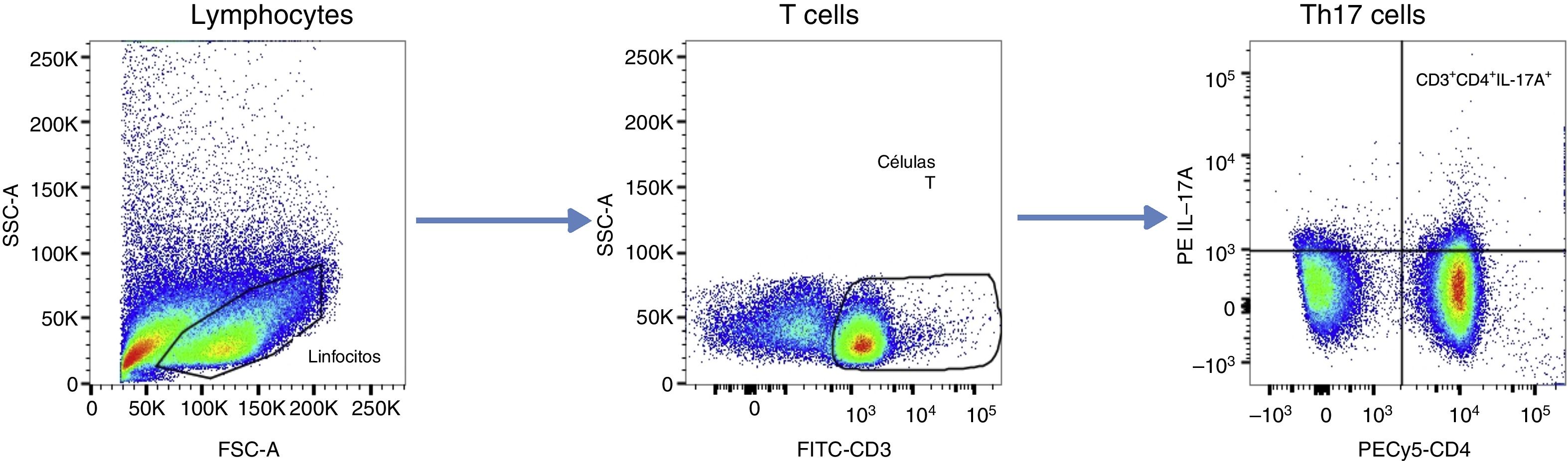
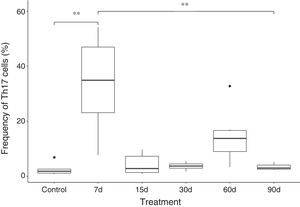
To set the size of the graph windows, we used the “fluorescence minus one” control. Data are presented as cell frequency (%), according to the analysis algorithm shown in Fig. 1. We considered CD3+, CD4+, and IL-17A+ to be “IL-17A-producing CD4+ T cells” or type Th17 cells.
Flow cytometry analysis algorithm. After doublet discrimination, lymphoid cells were selected and T cells identified based on CD3 expression. The frequency of IL-17A-producing CD4+ T cells was quantified. Windows were set using the “frequency minus one” control. Data are presented as cell frequencies (%).
Hippocampal tissue specimens were deparaffinised with xylene and rehydrated. Sections were then washed with phosphate buffered saline (PBS) solution (50mM, NaCl 0.15M, pH 7.4), and were incubated for 30minutes in 2% bovine serum albumin (fatty acid free, fraction V from MP Biomedical, LLC, Darmstadt, Germany) to avoid non-specific binding. Samples were impregnated with 0.2% Triton™ X-100 in PBS for 10minutes and were subsequently incubated overnight at 4°C with rabbit polyclonal Anti-IL-17A antibody. After the slides were washed, samples were incubated with Alexa Fluor® 488-conjugated donkey anti-rabbit IgG antibody. Samples were placed on glass slides with Vectashield mounting medium (Vector Laboratories; Burlingame, CA, USA). Representative brain sections from each group were examined with an Axioimager A2 microscope (Zeiss; Jena, Germany) and were photographed with an Axiocam HRm camera (Zeiss, Jena, Germany), controlled with the Zen software (Zeiss, Jena, Germany).
Statistical analysisStatistical analysis was performed using R 3.3.2 for Mac. The Shapiro–Wilk test was used to test for normal distribution.
Given that data were non-normally distributed, we used the non-parametric Kruskal–Wallis test to identify differences between groups and the Tukey test for post hoc analysis.
Pearson correlation coefficients were calculated to identify the covariance between the frequency of Th17 cells and serum IL-17A concentration.
Statistical significance was set at P<.05.
ResultsFrequency of Th17 cells in peripheral bloodIn order to determine whether there is an association between the levels of secreted IL-17A and Th17 cell frequency, we calculated the Pearson correlation coefficient, obtaining a correlation of r=0.3780812 (t34=2.3813, P=.02299); this suggests a weak positive correlation.
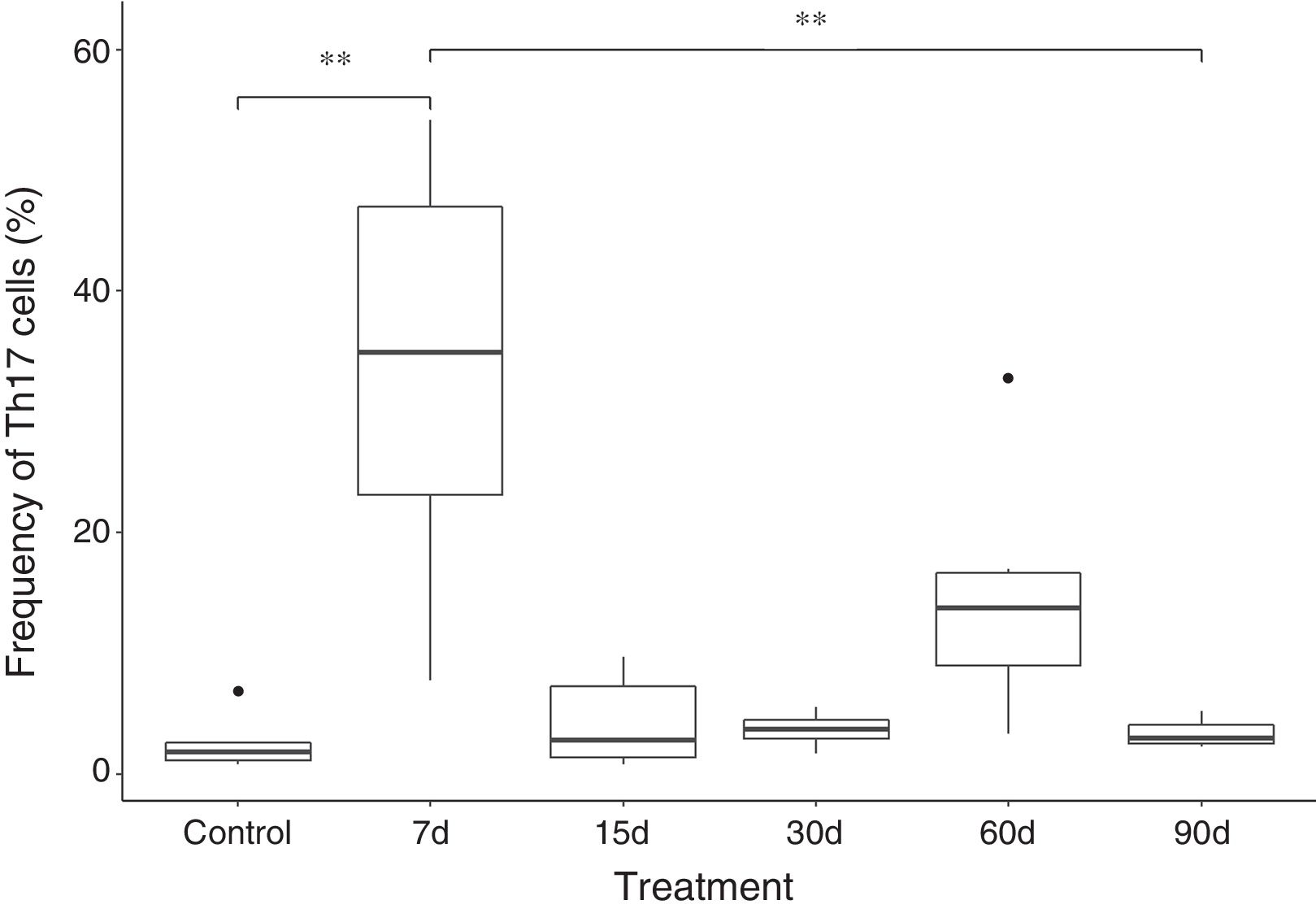
Our findings show that the frequency of Th17 cells increases approximately 10 times in the group exposed to ozone for 7 days, when compared with the control group and the group exposed for 90 days. The increase in this cell population seems to be a short-term effect, since after 7 days of exposure, there is a decrease in the frequency of IL-17A-producing CD4+ T cells. However, an increase was also observed in the number of Th17 cells after 60 days of ozone exposure (this difference was not significant) (Fig. 2).
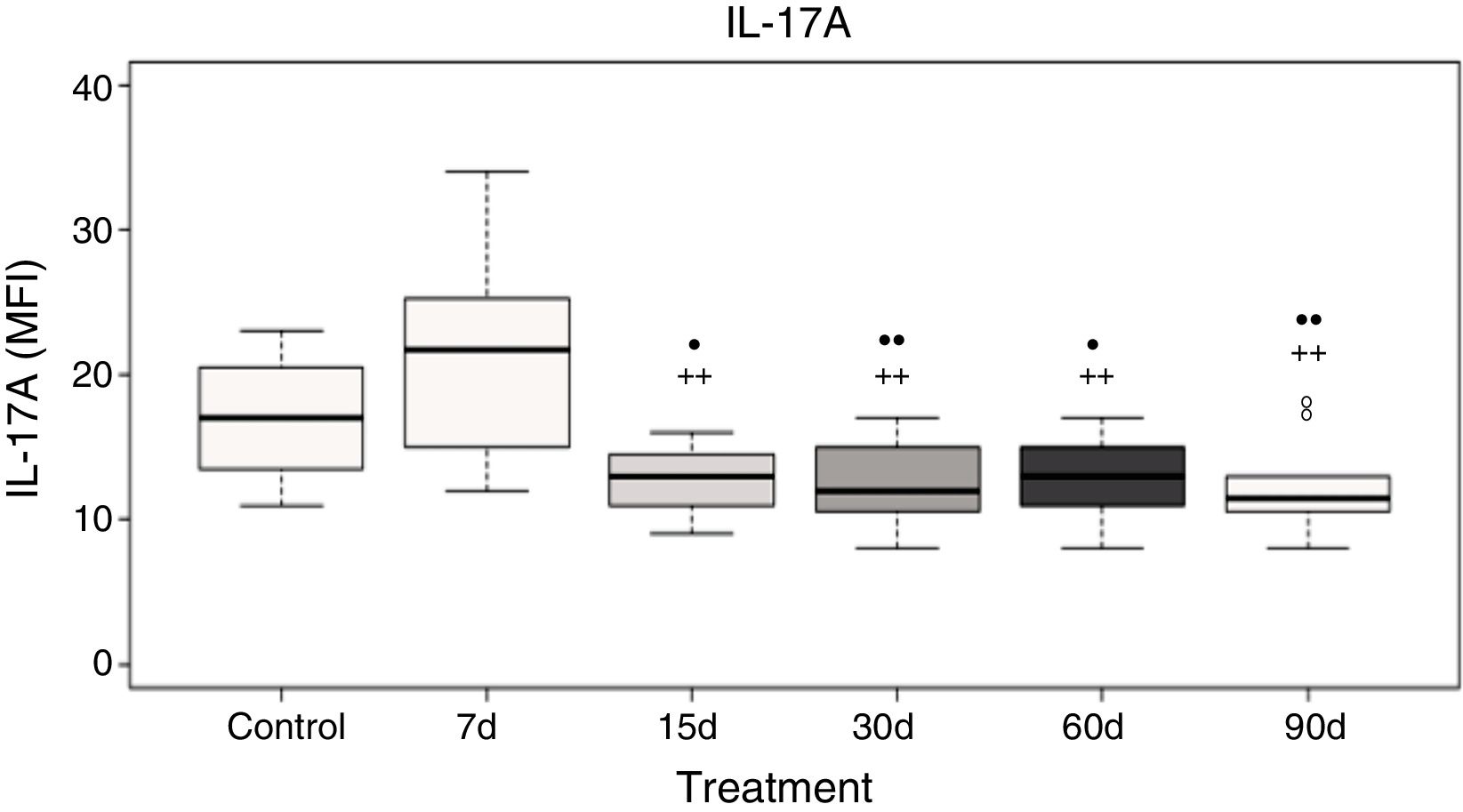
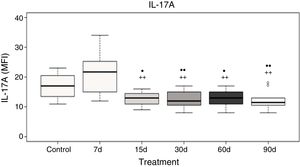
Plasma interleukin-17A levelsData analysis showed no significant differences in IL-17A after 7 days of ozone exposure when compared with the control group; however, there was a significant increase in IL-17A levels after 7 days of ozone exposure when compared to the groups exposed to ozone for 15, 30, 60, and 90 days (P<.05). Furthermore, groups exposed to ozone for 15, 30, 60, and 90 days show a significant decrease in IL-17A levels when compared with the control group (P<.05) (Fig. 3).
Plasma IL-17A levels in rats exposed to ozone (for 7, 15, 30, 60, and 90 days) vs controls exposed to an air flow for 30 days.
• and •• indicate a statistically significant difference with respect to the control group (P<.05 and P<.01, respectively); + and ++ indicate a statistically significant difference with respect to the group exposed to ozone for 7 days (P<.05 and P<.01, respectively). MFI: mean fluorescence intensity.
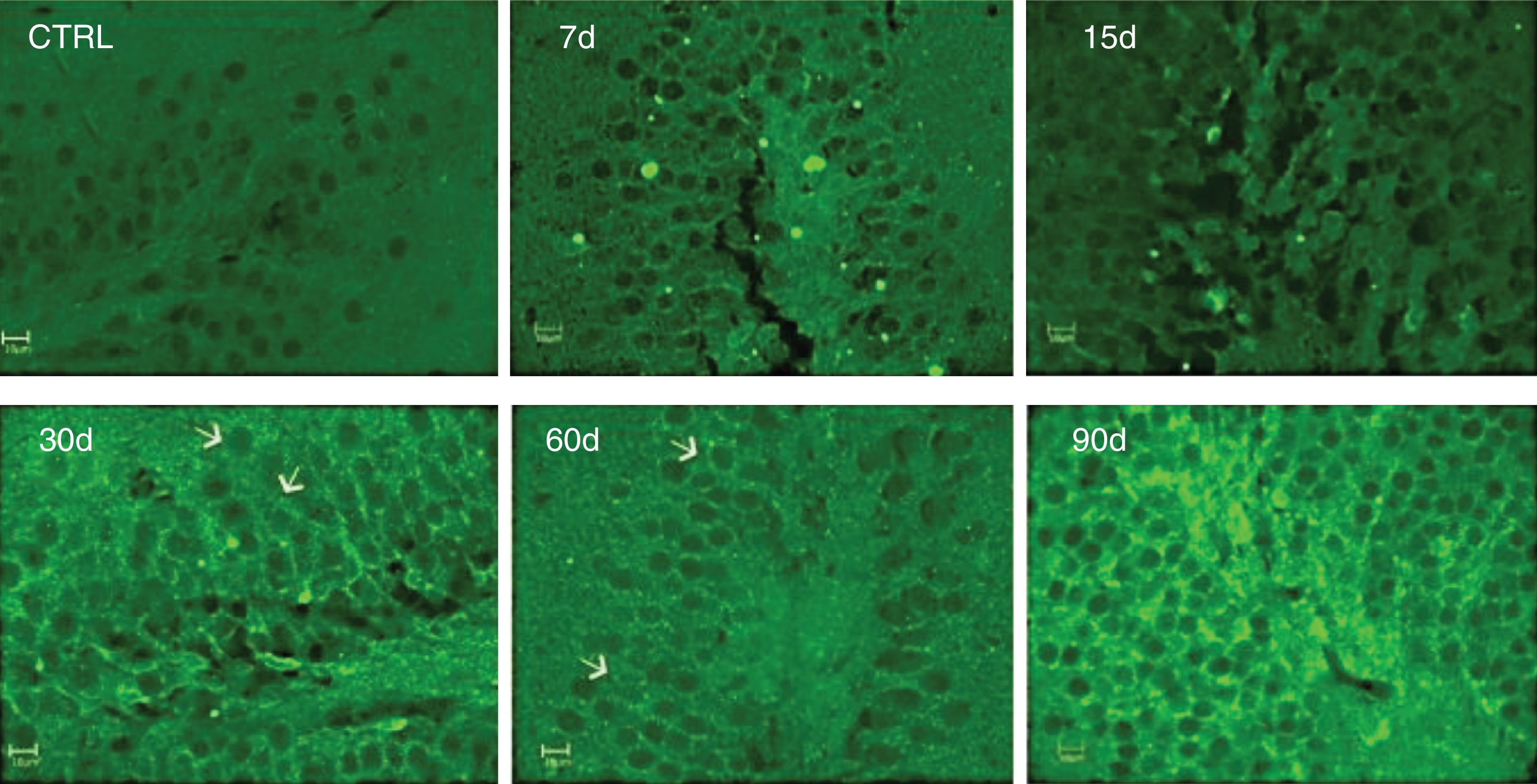
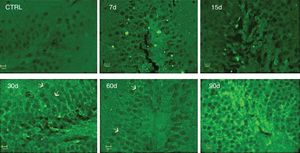
Immunoreactivity to IL-17A was assessed by immunofluorescence. We observed increased IL-17A in hippocampal tissue after 30, 60, and 90 days of ozone exposure (Fig. 4).
Effect of the exposure to low doses of ozone on hippocampal immunoreactivity to IL-17A. Animals were exposed for 7, 15, 30, 60, and 90 days. Images show the dentate gyrus magnified at 40×. Note that immunoreactivity to IL-17A increases considerably after 30, 60, and 90 days of exposure. Arrows indicate immunoreactivity to IL-17A.
Significantly, immunoreactivity to IL-17A was observed in neurons of the dentate gyrus.
DiscussionChronic exposure to low doses of ozone causes an oxidative stress state; according to the exposure time, this leads to progressive neurodegeneration, which becomes irreversible from 30 days of exposure.15 Neurodegeneration is accompanied by metabolic alterations16, changes to intracellular signalling pathways,17 and loss of regulation of the inflammatory response at the tissue level18; however, the systemic changes associated with this process are still unclear.
We detected an increase in plasma IL-17A in rats exposed to low doses of ozone for 7 days, and a decrease in IL-17A from 15 days to 90 days of exposure. A similar response occurs at the systemic level when analysing the response of Th17 lymphocytes; our results also show an increase in the frequency of Th17 lymphocytes, followed by a decrease in this cell subpopulation as neurodegeneration progresses in the hippocampus of rats exposed to ozone. IL-17A levels and Th17 lymphocyte frequency in peripheral blood increase while hippocampal damage is still reversible. However, when we observe hippocampal immunoreactivity to IL-17A, increased IL-17A expression is observed after 30, 60, and 90 days of exposure (when progressive neurodegeneration becomes irreversible).5
While CD4+ T cells may be the main source of IL-17A, we found a weak positive correlation between plasma IL-17A and the frequency of Th17 cells; in other words, other cell populations may be involved in the production of this cytokine. Similarly, an association is reported between subacute exposure to ozone (0.3ppm for 24-72h) and increased IL-17A messenger RNA expression in mouse lungs,19 with γδ T cells being the main cytokine producing cells.
The results of our study suggest that the first observable effect after exposure to low doses of ozone is a systemic Th17/IL-17A response that may be induced by the production of ROS after ozone inhalation.20 In this case, inflammatory response originates in a system that is still in redox balance, where antioxidants may still protect against oxidative agents.21 However, prolonged and persistent production of ROS, causing a state of oxidative stress, inhibits the proliferation of T cells and leads to cell death.22 Therefore, it is likely that chronic exposure to ozone may lead to decreased proliferation of IL-17A-producing cells in the peripheral blood. However, as the hippocampal tissue is already damaged, signs of cell injury may be responsible for inducing IL-17A secretion in different types of cells, including hippocampal neurons. This would potentially explain the increased levels of this cytokine in the hippocampus at 30, 60, and 90 days of exposure.
Our results suggest that long-term exposure to low doses of such air pollutants as ozone is associated with chronic degenerative diseases, since it produces a state of chronic oxidative stress in the body. This state may alter the regulation of inflammatory response pathways, which leads to an increased Th17 response. Our results suggest that this response is initially non-specific and systemic; however, once tissues are damaged and injury is progressive and irreversible (as has been observed in the hippocampus after 30 days of ozone exposure) the inflammatory response becomes tissue-specific and involves the action of such resident cells as neurons.
Better understanding of the events enabling transition from a predominantly systemic Th17/IL-17A inflammatory response to a local response will provide greater insight into the immunopathogenesis of AD, which will enable us to identify potential molecular targets to prevent the development of the disease.
FundingThis research project was funded by the General Directorate of Academic Personnel Affairs (IN22114, awarded to SRA).
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Dr Yolanda López-Vidal for her valuable assistance in performing the flow cytometry analysis, and Dr Nancy Mejía from UNAM's Research Support Network for her assistance in the statistical analysis in our study. We would also like to thank Gabino Morgonio-Pérez for helping us with the handling of the laboratory animals and in sampling.
Please cite this article as: Solleiro-Villavicencio H, Rivas-Arancibia S. La respuesta sistémica Th17/IL-17A aparece antes del proceso neurodegenerativo en el hipocampo de ratas expuestas a bajas dosis de ozono. Neurología. 2019;34:503–509.