The growth hormone (GH) has been reported as a crucial neuronal survival factor in the hippocampus against insults of diverse nature. Status epilepticus (SE) is a prolonged seizure that produces extensive neuronal cell death. The goal of this study was to evaluate the effect of intracerebroventricular administration of GH on seizure severity and SE-induced hippocampal neurodegeneration.
MethodologyAdult male rats were implanted with a guide cannula in the left ventricle and different amounts of GH (70, 120 or 220ng/3μl) were microinjected for 5 days; artificial cerebrospinal fluid was used as the vehicle. Seizures were induced by the lithium–pilocarpine model (3mEq/kg LiCl and 30mg/kg pilocarpine hydrochloride) one day after the last GH administration. Neuronal injury was assessed by Fluoro-Jade B (F-JB) staining.
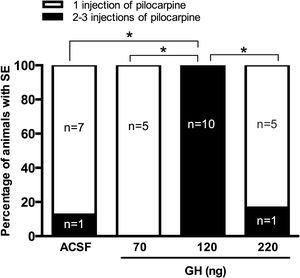
ResultsRats injected with 120ng of GH did not had SE after 30mg/kg pilocarpine, they required a higher number of pilocarpine injections to develop SE than the rats pretreated with the vehicle, 70ng or 220ng GH. Prefrontal and parietal cortex EEG recordings confirmed that latency to generalized seizures and SE was also significantly higher in the 120ng group when compared with all the experimental groups. FJ-B positive cells were detected in the hippocampus after SE in all rats, and no significant differences in the number of F-JB cells in the CA1 area and the hilus was observed between experimental groups.
ConclusionOur results indicate that, although GH has an anticonvulsive effect in the lithium–pilocarpine model of SE, it does not exert hippocampal neuroprotection after SE.
La hormona de crecimiento (HC) es un factor que favorece la supervivencia neuronal en el hipocampo ante agresiones de diversa naturaleza. El status epilepticus (SE) es un tipo de crisis epiléptica de larga duración que produce muerte neuronal. El objetivo de este estudio fue evaluar el efecto de la administración intracerebroventricular de HC en la severidad de las convulsiones y la neurodegeneración hipocampal debida al SE.
MetodologíaA ratas macho adultas se les implantó una cánula guía en el ventrículo lateral izquierdo y se les microinyectaron diferentes cantidades de HC (70, 120 o 220ng/3μl) durante 5 días; como vehículo se inyectó líquido cefalorraquídeo artificial. Las convulsiones se generaron con el modelo de litio-pilocarpina (3mEq/kg LiCl y 30mg/kg clorhidrato pilocarpina) un día después de la última inyección de HC. La neurodegeneración se identificó con la tinción de Fluoro-Jade B (F-JB).
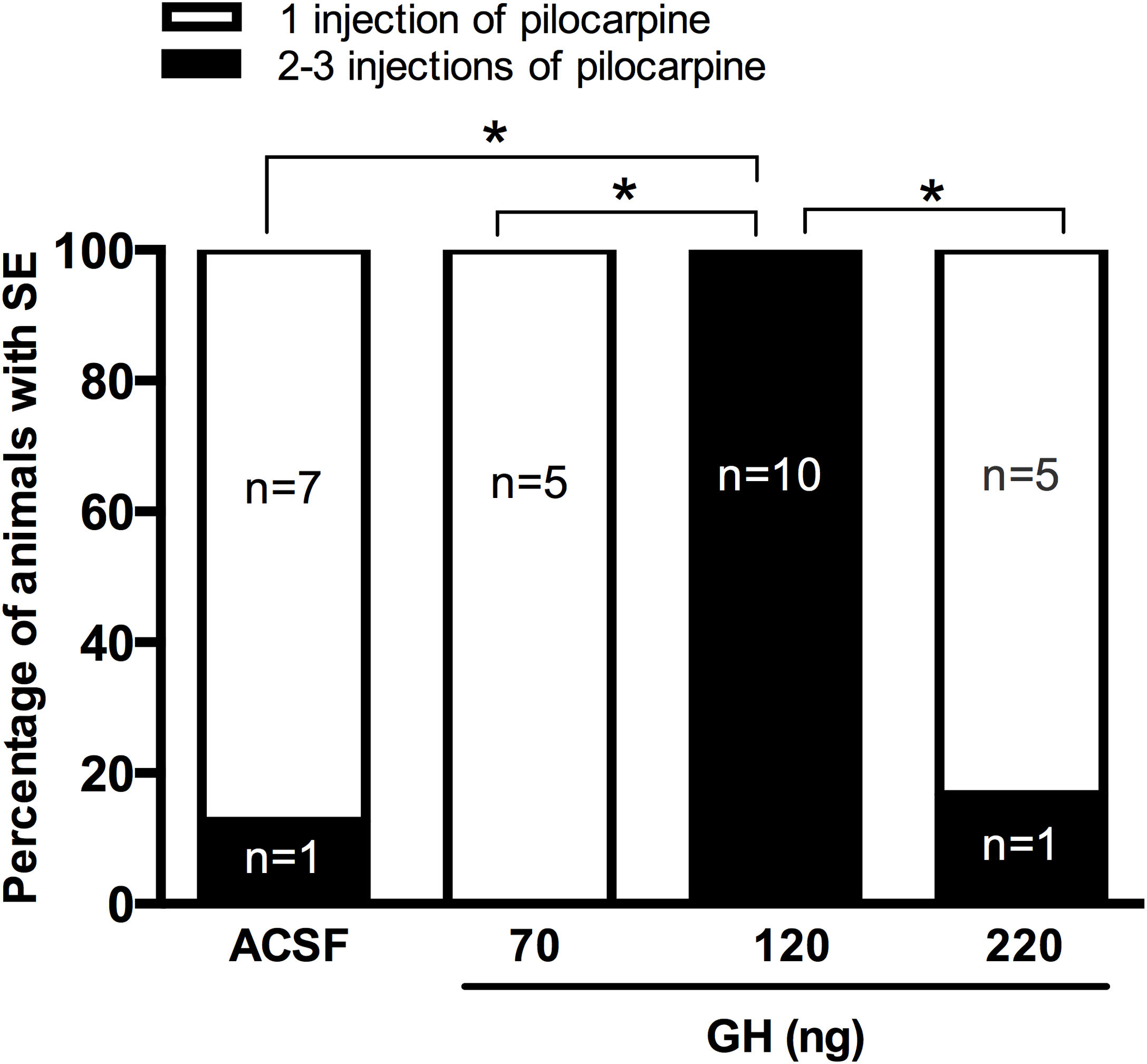
ResultadosLas ratas a las que se les inyectaron 120ng de HC requirieron 2 o 3 inyecciones de pilocarpina para desarrollar SE, en comparación con el resto de los grupos experimentales que requirieron solo una aplicación del convulsivante. Los registros EEG de la corteza prefrontal y parietal confirmaron que la latencia a las crisis generalizadas y al SE fue mayor en dicho grupo experimental. Todas las ratas con SE presentaron células positivas al FJ-B en el área CA1 e hilus del hipocampo, y no se identificaron diferencias entre los tratamientos.
ConclusiónNuestros resultados muestran que, aunque la HC tiene un efecto anticonvulsivante, una vez que se ha desarrollado el SE no promueve neuroprotección en el hipocampo.
The growth hormone (GH) is produced by the adenohypophysis and its most reported functions include body growth and lipid and protein metabolism (Moller and Jorgensen, 2009). In the Central Nervous System (CNS), GH promotes cell proliferation and survival of neurons and neural progenitors,1,2 brain growth, myelination, neuronal arborization, glial differentiation3,4 and spinogenesis.5 Interestingly, GH has been reported as an endogenous relevant factor in the hippocampus where it is locally secreted and regulated by age, sex and stress as well as by insults of different nature.6–9 Thus, exogenous GH administration exerts neuroprotective effect in this brain structure against aging, hypoxia-ischemia and sleep deprivation insults.3,10–12
On the other hand, status epilepticus (SE) is a non-self-limited type of epileptic seizure characterized by an enduring epileptic state.13 In humans and experimental rats, SE produces neuronal cell death in different brain regions including the hippocampus, the piriform cortex, the mediodorsal thalamic nucleus, the amygdala, and the cerebellum.14–16 Although some alterations in the serum levels of GH have been reported during seizures,17,18 information regarding the effect of GH on epilepsy or its possible neuroprotective effect on SE-induced neuronal damage is limited. In a study by Kato, intrahippocampal administration of GH in mice enhanced the progression of amygdala kindling, whereas pegvisomant, an antagonist of GH receptors, delayed it.19 However, it has not been explored whether GH could protect against neuronal damage induced by SE. Therefore, the main goal of this study was to evaluate the effect of intracerebroventricular (i.c.v.) administration of GH on hippocampal neuronal injury in the lithium–pilocarpine model of SE. We performed a detailed evaluation of the GH effect on seizure severity considering that this may affect the subsequent neuronal damage. This study expands our knowledge on the effects of GH in the hippocampus, and more specifically on epilepsy.
Materials and methodsAnimalsThis study was carried out in strict accordance with the Mexican guidelines on the care and use of laboratory animals (NOM-062-ZOO-1999), the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and under the ARRIVE guidelines.20 Internal Committee for the Care and Use of Laboratory Animals of the Instituto de Ciencias de la Salud at Universidad Veracruzana approved this protocol (CICUAL-ICS 2017-0002). All efforts were made to minimize the number of animals used and their suffering. Male rats Wistar were obtained from the facilities of the Centro de Investigaciones Cerebrales (Universidad Veracruzana) or the Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz. Rats were housed in groups of 4 and kept in environmental conditions of temperature and humidity (20–28°C and 40–70% RH, respectively), with light–dark cycles of 12/12h (07:00–19:00) and free access to water and food.
Surgical proceduresAll surgical procedures were performed entirely in aseptic conditions and under anesthesia with isoflurane (1.5–2%, Vedco, Inc., USA). Rats were stereotaxically implanted with a stainless-steel guide cannula (gauge 21, C313G/SPC Plastics One, Roanoke, VA, USA) in the left lateral ventricle (AP=1.4mm, ML=2mm, DV=−4.3mm, relative to bregma).21 For rats that underwent Electroencephalogram (EEG) recording, two nail-shaped stainless steel electrodes were also implanted on the prefrontal (PFC) and parietal cortices (PC). The body temperature of the rats was always controlled with a temperature regulation system (FHC brand, model 41-90-D8) and after the surgery, rats were rehydrated with glucose saline (equivalent to 5% body weight, s.c.). An analgesic (Meglumine, 2.5mg/kg s.c., for 2 days) and an antimicrobial (Enrofloxacin, 5mg/kg s.c., for 5 days) were applied in the postoperative period. Rats underwent experimental protocols 5 days after surgery. Only rats correctly implanted in the left lateral ventricle were included in the study.
Intracerebroventricular microinjection of growth hormoneFor the dose–response curve experiments, the GH (Lilly laboratories) was administered through the guide cannula using a microinjector connected to an insulin syringe (BD ultra-Fine, 1ml), which was placed in a microperfusion pump (Pump 11 Elite, Harvard Apparatus) with a flow rate of 0.3μl/min. Three different concentrations of GH were tested: 70ng/3μl (n=5), 120ng/3μl (n=10) and 220ng/3μl (n=6). The control group received 3μl of ACSF as the vehicle (n=8). Each rat was treated with a single dose of GH or the vehicle, which was injected daily during five consecutive days. SE was induced the following day after the last GH administration and the behavioral manifestation of seizures was evaluated. For the EEG recordings, two additional groups of rats were injected with 120ng/3μl (n=5) or vehicle (n=8) as described before.
Induction of status epilepticus with lithium–pilocarpineAll rats were treated with lithium chloride (3mEq/kg i.p.) and 24h later, they were injected with pilocarpine hydrochloride (30mg/kg s.c.) to induce SE or saline solution for control rats (volume of administration 1ml/kg). If SE was not induced after sixty min of the first pilocarpine injection, the animals were given one or two additional doses of pilocarpine to generate it. The seizures were stopped 1h after the SE onset with diazepam (10mg/kg i.p.) and rats were rehydrated with glucose saline. The behavioral manifestation of seizures in the presence and absence of GH were video-recorded and evaluated according to the Racine scale.22 The parameters evaluated included the number of injections of pilocarpine required for induction of SE and seizure latency, duration and frequency. At the end of these experiments, rats were processed as indicated below for brain histology.
EEG recordings were performed using an analog EEG amplifier Grass Model 7 (Astro Med., Warwick, USA, 2002). Signals were amplified and band-pass filtered (1–100Hz), acquired and digitally stored for later detailed analysis with GAMMA, Grass data acquisition software, version 3.1 (Astro Med., Warwick, USA, 2003). The EEG was continuously obtained in basal conditions (a baseline of 20min before pilocarpine injection) and 4h after the first injection of pilocarpine. Latency and duration to the first generalized seizure and spike frequency during generalized seizures were analyzed in both PC and PFC. To evaluate the frequency of epileptic spikes, only those with an amplitude at least three times above the baseline were considered. At the end of the EEG experiments, rats were killed by an overdose of sodium pentobarbital and the implant site was verified histologically.
Tissue processing for histologyTwenty-four hours after SE, rats were anesthetized with sodium pentobarbital (100mg/kg i.p.) and transcardially perfused with 0.9% NaCl, followed by 4% paraformaldehyde (in 0.1M phosphate solution, pH 7.4). Brains were post-fixed in 4% paraformaldehyde for 2h and processed for paraffin embedding. Sequential coronal sections (10μm thickness) were obtained at the level of the dorsal hippocampus using a microtome (Leica) and mounted on gelatin-coated slides.
Fluoro-jade B stainingDegenerative cells were identified by Fluoro-Jade B (F-JB) staining as described previously.23 Sections were analyzed with a fluorescence microscope (Olympus AX70) at an excitation wavelength of 450–490nm. A semi-quantitative analysis to count F-JB positive (F-JB+) cells per mm2 was performed in 4 consecutive coronal sections for each animal in both hemispheres. Cell counting in the dorsal hippocampus was performed in the CA1 pyramidal layer and the hilus. Regions of interest were photographed, and cell counting was performed using the open-source platform for biological-image analysis Fiji.
Statistical analysisSeizure and histology data were analyzed using a one-way ANOVA to identify differences between the experimental groups and subsequently, when necessary, a Tukey post hoc test was performed. Data from EEG recordings were analyzed with a Student's t-test to identify differences between groups. To determine the difference between two proportions, it was used a proportion test. Graph Pad Prism 6.0 or Statistica 7.0 software were used for statistical analysis. A p-value<0.05 was considered statistically significant.
ResultsSeizure severityOur results unexpectedly showed that any of the rats pre-treated with 120ng GH had SE after 30mg/kg of pilocarpine. Thus, all rats in the group of 120ng GH required 2 or 3 injections of pilocarpine to develop SE, a proportion significantly greater than the rest of the experimental groups (0–16%) (p<0.05) (Fig. 1). Consequently, the latency to the first generalized seizure was larger in the 120ng GH group (79.8±3.3min) compared to the rest of the experimental groups (ACSF=44.4±6.1min); 70ng GH=50.5±2.4min; 220ng GH=46.2±6.3min) (F(3, 25)=14, p=0.0001). Similarly, the latency to SE was greater in the group of 120ng GH (89.2±2.9min) compared to the other groups (ACSF=50.8±5.4min; 70ng GH=55.6±2.7; 220ng GH=52.5±6.3min) (F(3, 25)=19.7, p=0.0001). No differences were observed among experimental groups in the duration of the first generalized seizure (ACSF=29.3±3.1s; 70ng GH=38.4±6.8s, 120ng GH=31.9±4.1s; 220ng GH=44.0±9.7s) (F(3, 25)=1.3, p=0.3) or of the SE (ACSF=5.6±0.3h; 70ng GH=5.6±0.2h; 120ng GH=4.6±0.3h; 220ng GH=5.5±0.4h) (F(3, 24)=2.6, p=0.1); all experimental groups had a similar number of generalized seizures until the application of diazepam (Stage IV seizures: ACSF=2.6±0. 5; 70ng GH=2.4±0.4; 120ng GH=1.6±0.3; 220ng GH=2.8±0.5 (F(3, 25)=1.9, p=0.2); Stage V seizures: ACSF=2.8±0.3; 70ng GH=2.6±0.4; 120ng GH=2.8±0.2; 220ng GH=2.3±0.2 (F(3, 25)=0.7, p=0.6) and no differences in mortality were observed.
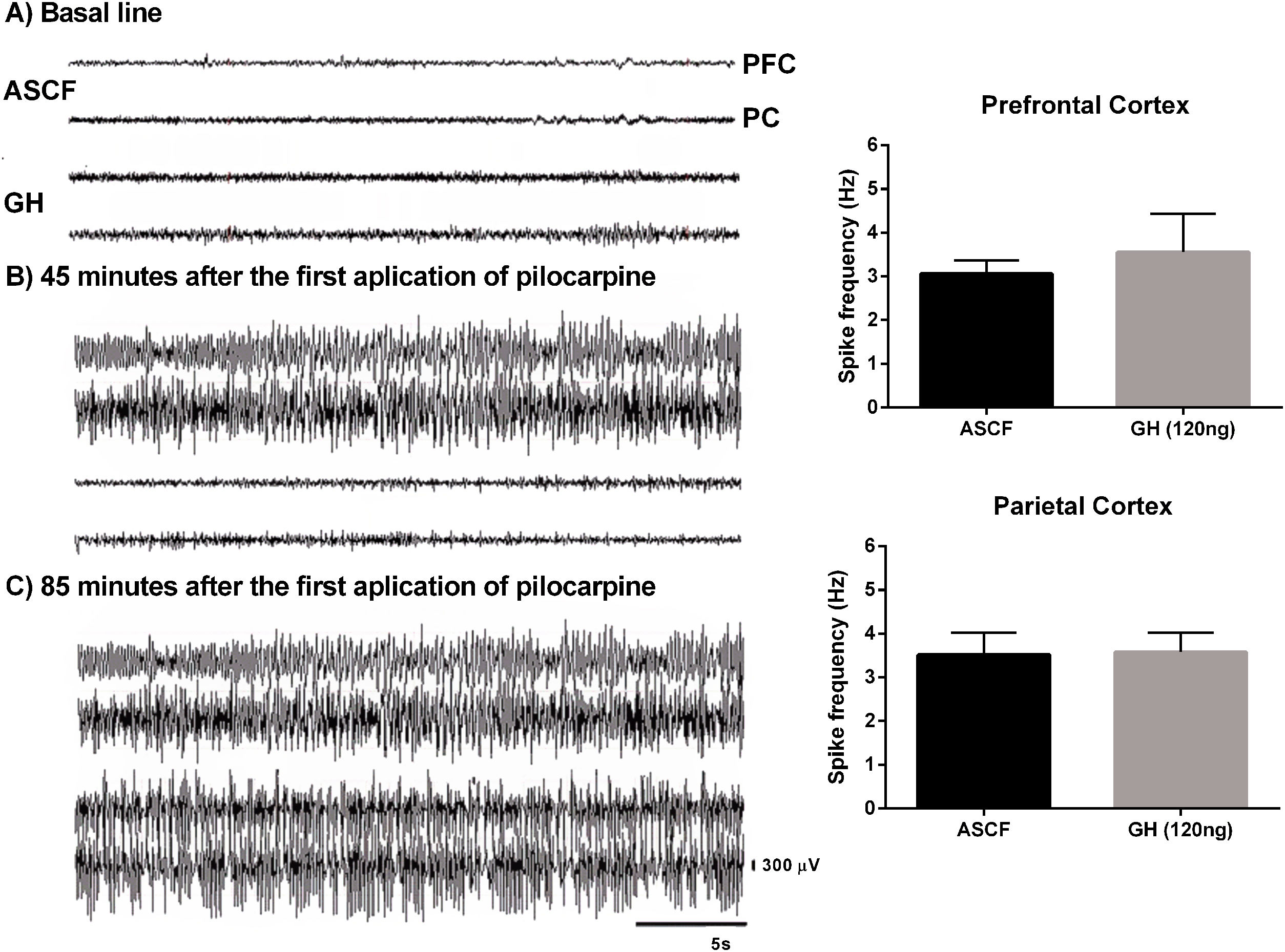
EEG recordings confirmed that rats pre-treated with 120ng GH required 2 or 3 injections of pilocarpine to develop SE, and that latency to the first electrographic seizure and SE was significantly higher in the GH group (84.7±6.7 and 102.2±4.5min, respectively) than in the ACSF group (31.8±3.9 and 43.4±3.9min, respectively) (t=6.4, 9, p<0.05, and t=9.5, 9, p<0.05, respectively). No difference in the duration of the first EEG generalized seizure was observed between GH group (34.8±7.5s) and vehicle group (31.3±6.8s) (t=0.34, 7, p>0.05). Finally, 120ng GH treatment did not affect the spike frequency during generalized seizures either in the PC (3.6±0.4Hz) or in the PFC (3.6±0.9Hz) when compared with the vehicle (3.5±0.5Hz and 3.1±0.3Hz, respectively) (PC, t=0.1, 8, p=0.8; PFC, t=0.5, 8, p=0.1) (Fig. 2).
Effect of growth hormone (GH, 120ng) on electrographic activity recorded from Prefrontal (PFC) and Parietal (PC) cortex during the status epilepticus (SE) induced by lithium–pilocarpine. (A) Basal line, (B) Electrographic activity 45min after the first application of pilocarpine (the rat injected with artificial cerebrospinal fluid [ACSF] is already in SE), (C) Electrographic activity 85min after the first application of pilocarpine (at this time, both rats have electrographic and behavioral SE). Graphs on the right panel show the spike frequency during the first generalized electrographic seizure recorded in the PFC and PC after the injection of ACSF or GH. Data are expressed as the mean±S.E.M. and were analyzed by a Student's t-test. No differences were identified between experimental groups. Scale bars: 5s (horizontal) and 300μV (vertical).
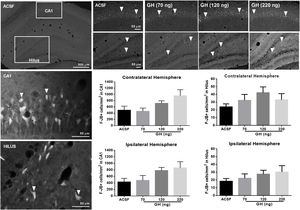
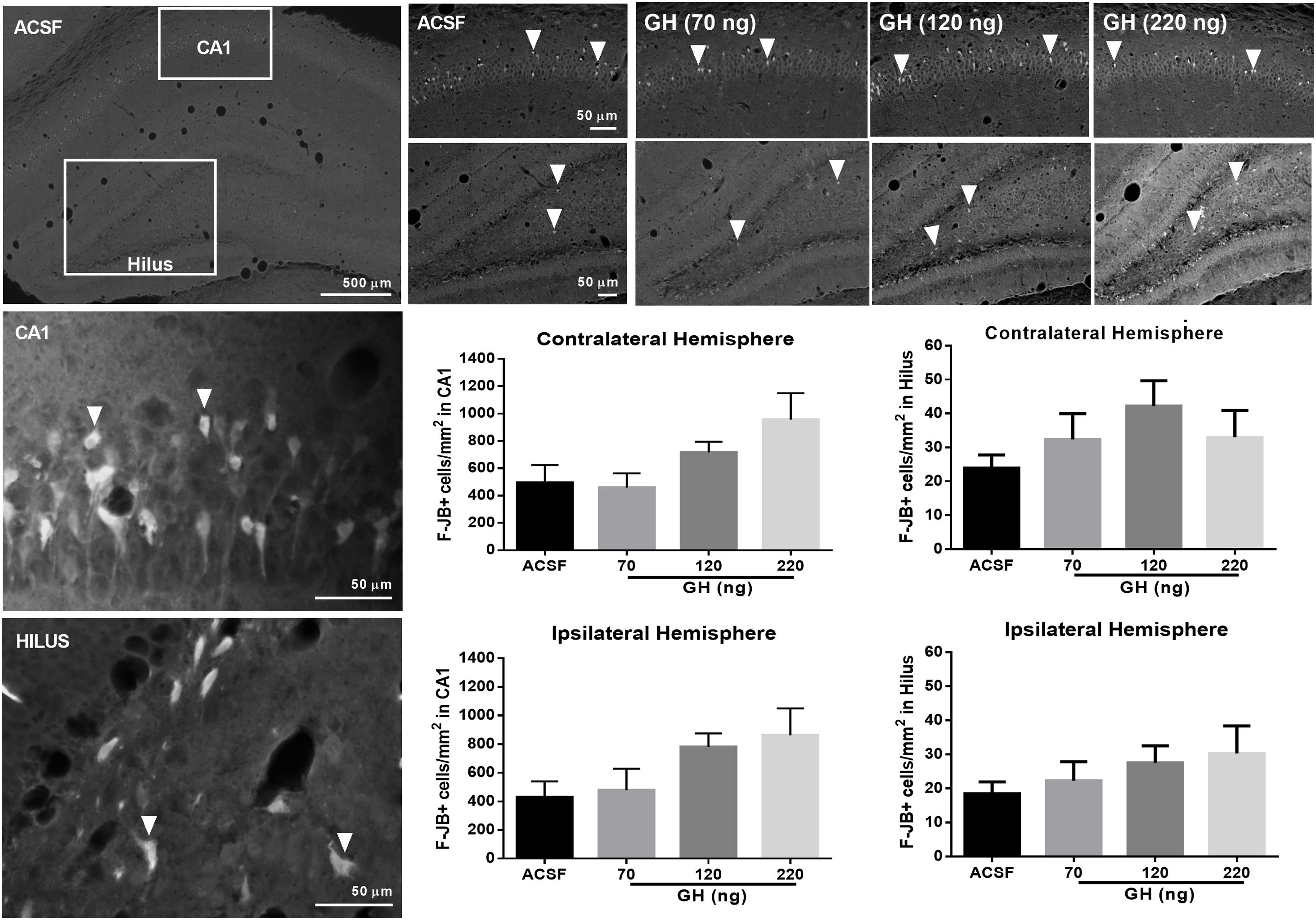
FJ-B+ cells were detected in the hippocampus in all rats in the CA1 area and the hilus, but not in CA2, CA3 or dentate gyrus. No significant differences in the number of F-JB+ cells/mm2 in CA1 were observed between the groups, either in the ipsilateral (ASCF=428.5±101.5; 70ng GH=478±150.1; 120ng GH=779.5±95.1; 220ng GH=862.1±187.1) (F(3, 25)=2.9, p=0.05) or in the contralateral hemisphere (ASCF=493.9±129.9; 70ng GH=457.8±104.7; 120ng GH=715.8±78.3; 220ng GH=955.6±194.0) (F(3, 25)=2.7, p=0.07) (Fig. 3). The number of F-JB+ cells/mm2 in the hilus was similar after all the treatments and for both hemispheres, ipsilateral (ASCF=13.8±4; 70ng GH=22.3±5.6; 120ng GH=27.5±5, 220ng GH=20.2±8.2) (F(3, 25)=1.2, p=0.3) and contralateral to the site of injection (ASCF=24±3.4; 70ng GH=32.4±7.6, 120ng GH=42.2±7.5, 220ng GH=22±8.62) (F(3, 25)=2, p=0.1) (Fig. 3).
Effect of growth hormone (GH) on hippocampal neurodegeneration after status epilepticus (SE) induced by lithium–pilocarpine. Microphotographs show Fluoro-Jade B positive (F-JB+) cells (in white) in the dorsal hippocampus of rats treated with artificial cerebrospinal fluid (ACSF) (upper left side panels) or GH (70, 120, or 220ng) (upper right side panels). Arrowheads in high and low magnification microphotographs point to F-JB+ cells in CA1 and the hilus. Original pictures were converted to 8-bit greyscale images with Fiji. The graphs represent the number of FJ-B+ cells/mm2 in CA1 and the hilus for each experimental group. Data are expressed as the mean±S.E.M. and were analyzed by a one-way ANOVA. No difference between experimental groups was identified.
Our study shows that sub-chronic i.c.v. administration of 120ng GH has an anticonvulsant effect against SE induced by pilocarpine; however, once SE is triggered, the pattern of behavioral and electrographic seizures and the subsequent hippocampal neurodegeneration are not affected.
A growing body of evidence suggest a central role of the GH in the hippocampal function. Interestingly, the expression of this hormone and its receptor are locally up regulated in this brain region by insults of different nature, while GH exogenous administration counteracts their negative effects.3,11,12 The present study aimed to analyze the effect of the GH on SE and the subsequent neurodegeneration in the hippocampus. As experimental strategy, we decided to deliver the GH through i.c.v. microinjection to avoid pharmacokinetic issues associated to the systemic administration, and as a potential therapeutic route.24 Moreover, since it has been reported that daily injection of GH increases overall transcript abundance in the hippocampus more than continuous infusion,25 GH was administrated five days before the SE insult to evaluate the potential anticonvulsant and hippocampal neuroprotective effect of GH, as previously described.11
The role of GH on epilepsy and seizures has not been deeply investigated. The work by Kato and colleagues19 showed a pro-convulsive effect of GH in the electrical kindling model of epilepsy. In that study, chronic administration of GH (12mg/ml) in the mouse hippocampus during the induction of kindling, increased the number of spikes during the afterdischarge, which suggests that GH decreases the threshold for seizure generation.19 In contrast, our study showed an anticonvulsive effect of GH pretreatment. We tested three different amounts of GH, but unexpectedly, only 120ng had an anticonvulsant effect. Previous studies have reported that daily i.c.v. administration of 120ng of GH for seven days significantly increased the expansion rate in long-term neurosphere cultures derived from wild-type mice26 and induces changes in the length of the dendritic trees and the density of dendritic spines in the CA1 region of the dorsal hippocampus and in the PFC (detected 21 days after GH treatment).5 However, considering that GH can exert its multiple biological activities at different concentrations and that those effects are characterized by a bell-shaped dose-response curve, where the optimal concentration could vary,27–30 we decided to probe two additional amounts of GH. Our results confirmed that 120ng per day is the optimal amount for exert an anticonvulsant effect in the SE model and that inferior or superior amounts are ineffective. Differences in the effect produced by GH in both epilepsy models may also be explained by the unlike mechanisms of seizure generation for each one; whereas the electrical kindling is a model of epileptogenesis (chronic), pilocarpine-induced SE is an acute event. Additionally, the effect of GH pretreatment versus its co-administration with the pro-convulsant stimulus may affect differently the mechanism of seizure generation.
Our results suggest that the subchronic administration of GH decreases the susceptibility of the brain to the neuronal excitability initiated by pilocarpine, but once seizures generate, there is no difference in SE severity (seizure latency, frequency, and duration). Several studies have reported the participation of GH on structural5 and synaptic plasticity in the hippocampus.31–34 Specifically, GH enhance excitatory synaptic transmission in the CA1 area of hippocampus, increasing both AMPA and NMDA receptor-mediated excitatory post-synaptic potentials without alteration of GABA-receptor mediated inhibitory synaptic transmission.31–34 GH also regulates NMDA receptor subunit mRNA expression levels in the rat hippocampus.35,36 We do not know if five days of GH treatment is enough to promote changes in structural plasticity that may explain its anticonvulsant effect. It is possible that under our experimental conditions GH may have modified excitatory synaptic plasticity through changes in glutamate receptor expression. GH can activate the PI 3 kinase-Akt pathway,37 which is involved in the expression of glial and neuronal excitatory amino acid transporter.38,39 Thus, GH could also increase the expression of glutamate transporters and regulate the primary mechanism for the removal de glutamate from the extracellular space to promote its anticonvulsant effect. Further experiments are needed to understand the anticonvulsant mechanism of GH.
As mentioned above, once the SE was induced, there was no difference between GH or control groups in seizure latency, frequency or duration, suggesting an equivalent extension of neural damage in the hippocampus. Contrary to what was expected, the number of degenerative cells as long as their histological pattern in the hippocampus were unaffected after GH treatments, but similar to formerly reported by others in the lithium–pilocarpine model.40,41 Hippocampal neuronal cell death induced by lithium–pilocarpine SE can be necrotic and apoptotic, as a result of excessive activation of glutamatergic receptors.42–44 Previous studies have demonstrated the neuroprotective effect of GH against different insults to the brain, associated with its capability to regulate apoptosis (i.e., an anti-apoptotic effect).3,11,45–49 In those experiments, GH was administered either before or after the damage inducer and the neuroprotective effect was evaluated at different times, suggesting that this effect is an intrinsic property of this hormone. We initially expected that the i.c.v. administration of GH would stimulate an anti-apoptotic response in the hippocampus that will protect the neurons against the SE insult as we have reported previously.11 Our results showed the absence of neuroprotection under our experimental conditions, but it is worth noting that the current report is the first study designed to assess the anticonvulsant and neuroprotective effect of GH against neuronal damage caused by SE. Further studies are needed in this field to characterize the exact role of GH in epilepsy and its consequences.
In conclusion, the i.c.v. administration of GH has an anticonvulsant effect since it increases the amount of pilocarpine necessary to induce SE. However, once SE is triggered, the pattern of hippocampal neurodegeneration is not modified, i.e., GH has not a neuroprotective effect. Our findings are relevant in understanding the role of GH in epilepsy.
Authors’ contributionsMLLM, EJA, and IZB conceived and designed the study. IZB and AM performed the research. IZB, MLLM, and LBP analyzed the experimental data. MLLM, IZB, EJA and ISR wrote the paper.
Sources of supportThis work was supported by CONACYT Mexico (fellowship awarded to IZB, 309011).
Conflict of interestsThe authors declare no conflict of interests.





![Effect of growth hormone (GH, 120ng) on electrographic activity recorded from Prefrontal (PFC) and Parietal (PC) cortex during the status epilepticus (SE) induced by lithium–pilocarpine. (A) Basal line, (B) Electrographic activity 45min after the first application of pilocarpine (the rat injected with artificial cerebrospinal fluid [ACSF] is already in SE), (C) Electrographic activity 85min after the first application of pilocarpine (at this time, both rats have electrographic and behavioral SE). Graphs on the right panel show the spike frequency during the first generalized electrographic seizure recorded in the PFC and PC after the injection of ACSF or GH. Data are expressed as the mean±S.E.M. and were analyzed by a Student Effect of growth hormone (GH, 120ng) on electrographic activity recorded from Prefrontal (PFC) and Parietal (PC) cortex during the status epilepticus (SE) induced by lithium–pilocarpine. (A) Basal line, (B) Electrographic activity 45min after the first application of pilocarpine (the rat injected with artificial cerebrospinal fluid [ACSF] is already in SE), (C) Electrographic activity 85min after the first application of pilocarpine (at this time, both rats have electrographic and behavioral SE). Graphs on the right panel show the spike frequency during the first generalized electrographic seizure recorded in the PFC and PC after the injection of ACSF or GH. Data are expressed as the mean±S.E.M. and were analyzed by a Student](https://static.elsevier.es/multimedia/21735808/0000003900000001/v1_202401010508/S2173580823000664/v1_202401010508/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)