Muscle MRI and electrophysiological exercise testing have been reported as useful techniques in hypokalemic periodic paralysis (HypoPP). Striking clinical differences between men and women with this disorder are well known; however, little information is available on complementary tests in the asymptomatic population.
MethodsWe recruited 11 individuals with HypoPP from 4 independent families, carrying the frequent p.R528H mutation in the calcium channel gene CACNA1S; the sample included 8 symptomatic men and 3 asymptomatic women, together with 9 controls recruited from the same families. Muscle MRI and electrophysiological long exercise test results were evaluated in this homogeneous cohort.
ResultsMuscle MRI showed a consistent pattern of atrophy and fatty infiltration mainly involving posterior compartment muscles of the thigh, with first involvement of the adductor magnus and semimembranosus, both in symptomatic and all the asymptomatic carriers, associated with age. The long exercise test showed a delayed decrement in compound muscle action potential amplitude and area in all carriers, regardless of the symptoms, with results becoming 100% sensitive after 35min.
ConclusionsOur findings redefine the exercise test and muscle imaging findings in HypoPP due to the p.R528H CACNA1S mutation, with a particular focus on asymptomatic carriers, who displayed the same alterations as those described in symptomatic patients, thus highlighting their value as screening tools.
La RM muscular y el test electrofisiológico de ejercicio son técnicas útiles en la parálisis periódica hipocalémica (PPH). Son bien conocidas las sorprendentes diferencias clínicas entre hombres y mujeres con esta patología; sin embargo, hay poca información sobre pruebas complementarias para la población asintomática.
MétodosIncluimos 11 pacientes con PPH (8 hombres sintomáticos y 3 mujeres asintomáticas), de 4 familias independientes, que eran portadores de la frecuente mutación p.R528H en el gen relacionado con los canales de calcio CACNA1S, y 9 controles procedentes de las mismas familias. Se evaluaron los resultados de la RM muscular y del test electrofisiológico de ejercicio en esta cohorte homogénea.
ResultadosLa RM muscular mostró un patrón consistente de atrofia e infiltración grasa que afectaba principalmente a los músculos del compartimento posterior del muslo, manifestándose primero en el aductor mayor y en el músculo semimembranoso, tanto en los portadores sintomáticos como en los asintomáticos; se observó una relación lineal entre dicho patrón de infiltración grasa y la edad. El test de ejercicio largo mostró un descenso tardío en la amplitud y en el área del potencial de acción muscular compuesto en todos los portadores, independientemente de la presencia de síntomas. Los resultados mostraron una sensibilidad del 100% después de 35 minutos.
ConclusionesNuestros resultados redefinen los hallazgos del test de ejercicio y de RM muscular en PPH por mutación p.R528H en el gen CACNA1S, especialmente en los portadores asintomáticos, que mostraron las mismas alteraciones que las descritas en los pacientes sintomáticos, lo que subraya su valor como herramientas de cribado.
Hypokalemic periodic paralysis (HypoPP) is a channelopathy characterized by abnormal sarcolemmal depolarization leading to acute episodes of flaccid muscle weakness, associated with low serum potassium levels during the attacks.1–3 Arginine mutations in S4 regions of the L-type calcium channel (whose α subunit is encoded by the CACNA1S gene) or of the voltage-gated sodium channel (whose α subunit is encoded by the SCN4A gene) account for 90% of cases of HypoPP.4–6 Studies of phenotype–genotype correlations have shown incomplete penetrance in women carrying the p.R528H or the p.H916Q mutation of the CACNA1S gene, although the underlying mechanism is yet to be clarified.4,7 Furthermore, electrophysiological exercise testing in familial periodic paralysis shows different patterns whose diagnostic sensitivity depends on the mutated gene, although ethnicity and frequency of attacks can influence the results.8–11 Muscle MRI is recognized as a useful technique for the diagnosis and follow-up of hereditary muscle diseases,12,13 but little has been published about MRI in channelopathies, with most addressing non-dystrophic myotonia.14–16 Regarding HypoPP, some reports have shown muscle MRI changes in these patients,17,18 and an unusual but consistent muscle MRI pattern was recently reported in a large cohort of individuals with the p.R528H CACNA1S mutation presenting different phenotypes, including one out of 4 asymptomatic carriers.19
The present study, conducted in a Spanish cohort of patients with HypoPP due to the p.R528H mutation in the CACNA1S gene, demonstrates the value of muscle MRI and the long-exercise test as screening tools in asymptomatic carriers. We also confirmed the recently reported muscle MRI pattern in our patients.
Patients and methodsThis study was approved by the ethics committee of Hospital Universitario Virgen del Rocío; all participants provided informed consent.
We selected patients with HypoPP and heterozygous for the common p.R528H mutation (NM_000069.2:c.1583G>A) in the CACNA1S gene, who were under follow-up by our neuromuscular disorders unit. Regardless of symptoms, we contacted as many family members as possible and asked them to participate in the study. The same protocol was applied to patients and relatives: (1) collection of clinical data through review of medical records, interviews, and physical examinations (the collected clinical data are summarized in Table 2); (2) classification of participants as symptomatic or asymptomatic based on the occurrence of well-characterized transient paralysis attacks (at least one) or of permanent weakness during the inter-critical periods; (3) blood collection for DNA isolation and determination of creatine kinase, ion levels, thyroid hormones, and renal function; (4) standardized electrophysiological test during an inter-attack period, as described by Fournier et al.,8 performed by the same examiner and with the same equipment (TECA™ Sinergy T-EP); and (5) MRI of lower limb muscles.
Blinding was applied to studies of all participants, except for the index patients, with sequencing of the entire coding region and the exon/intron boundaries of the CACNA1S gene being performed last. Non-carriers were considered controls for the exercise test and muscle MRI.
Muscle MRI protocolMuscle MRI was performed at the level of the hips, thighs, and lower legs. We used a 1.5T scanner (MRI Intera, Philips Medical Systems). Axial MRI sections were obtained with a T1-weighted spin-echo sequence (TR 600–700ms, TE 30ms) and a short-time inversion recovery (STIR) sequence (TR 2500–3500ms, TE 60ms, TI 150ms), in 10mm slices. Fatty degeneration of muscles was identified according to increased signal in T1 sequences and quantified according to a subjective scale20 (0=normal, 1=mildly increased signal [<30%], 2=moderately increased signal [30–60%], 3=severely increased signal [>60%]); STIR sequences were used to evaluate the presence of edema.
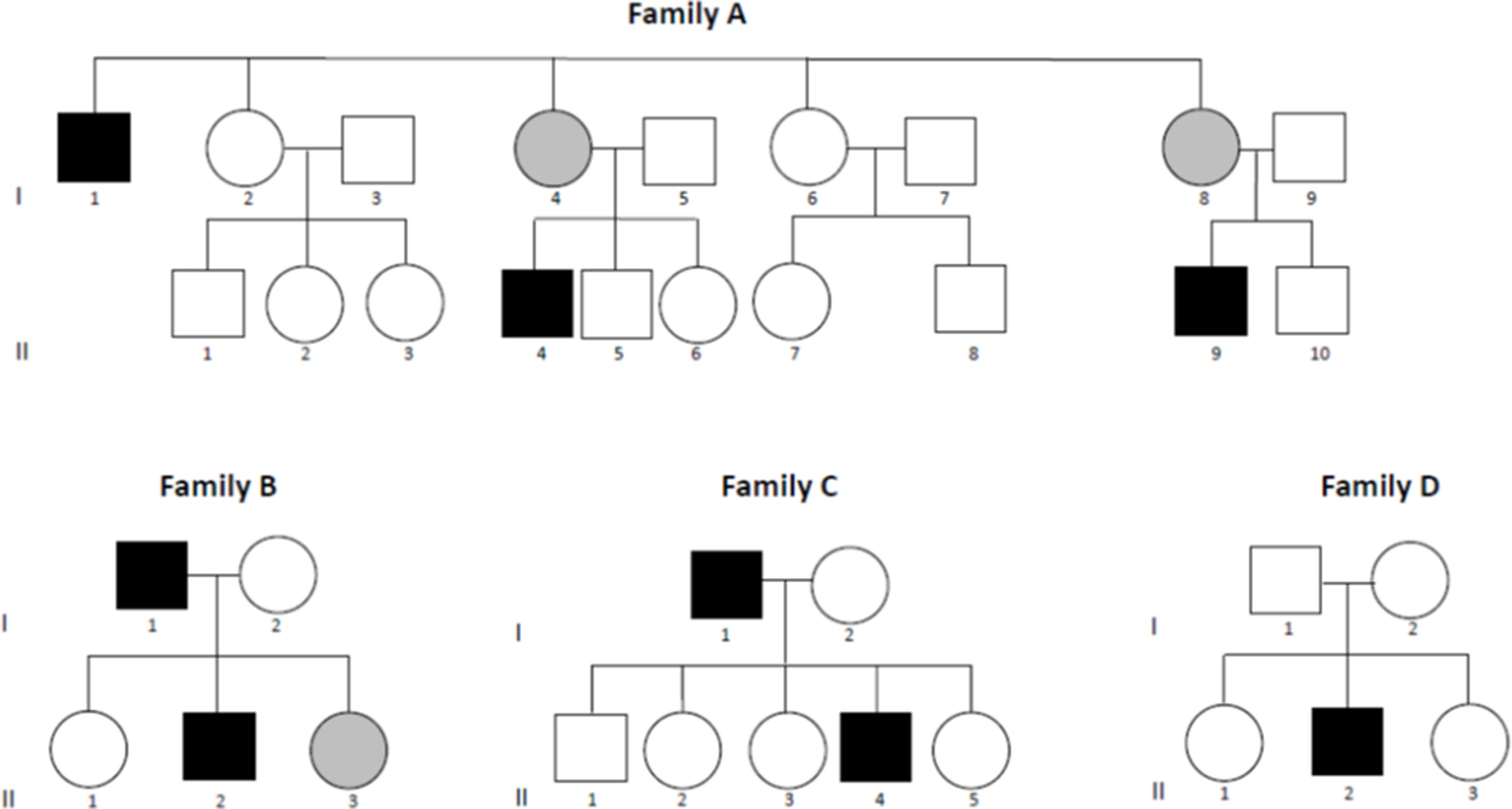
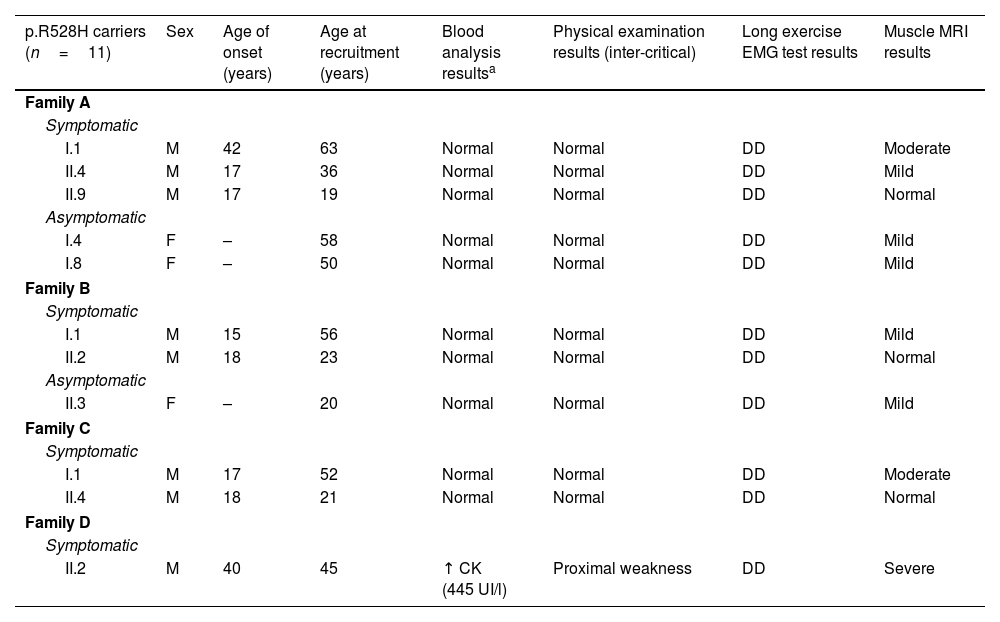
ResultsClinical phenotypeWe recruited 20 participants belonging to 4 unrelated white families from southern Spain (Fig. 1). We detected the p.R528H mutation in 11 individuals, of whom 8 were symptomatic (sHypoPP) and 3 asymptomatic (aHypoPP); interestingly, all sHypoPP were men and all aHypoPP were women. The 9 remaining participants (5 men and 4 women) made up the control group. Mean age (standard deviation [SD]) at recruitment was 42 (16) years (range, 20–58). In the sHypoPP group, the mean age of onset was 23 (11) years (range, 15–42), the age at recruitment was 39.3 (17) (range, 19–63), and disease duration was 16.3 (15.2) years (range, 2–41). All but 2 sHypoPP participants had presented transient paralytic attacks during the previous year, and we were able to identify clear triggers in 6 patients, with exercise being the most frequent. Six out of 8 individuals with sHypoPP were receiving treatment, either acetazolamide or oral potassium, with a full or partial response. All patients had full muscle strength at physical examination, except one, who had presented the first episode of paralysis 5 years prior to recruitment, and showed inter-critical permanent weakness involving proximal muscles of the lower limbs (2–3/5 on the Medical Research Council scale for manual muscle testing; 3/6 on the modified Rankin Scale). The muscle biopsy in this patient displayed striking myopathic features, with abundant subsarcolemmal vacuoles. All patients’ data are summarized in Tables 1 and 2.
Participants’ demographic, electrophysiological, and radiological data.
| p.R528H carriers (n=11) | Sex | Age of onset (years) | Age at recruitment (years) | Blood analysis resultsa | Physical examination results (inter-critical) | Long exercise EMG test results | Muscle MRI results |
|---|---|---|---|---|---|---|---|
| Family A | |||||||
| Symptomatic | |||||||
| I.1 | M | 42 | 63 | Normal | Normal | DD | Moderate |
| II.4 | M | 17 | 36 | Normal | Normal | DD | Mild |
| II.9 | M | 17 | 19 | Normal | Normal | DD | Normal |
| Asymptomatic | |||||||
| I.4 | F | – | 58 | Normal | Normal | DD | Mild |
| I.8 | F | – | 50 | Normal | Normal | DD | Mild |
| Family B | |||||||
| Symptomatic | |||||||
| I.1 | M | 15 | 56 | Normal | Normal | DD | Mild |
| II.2 | M | 18 | 23 | Normal | Normal | DD | Normal |
| Asymptomatic | |||||||
| II.3 | F | – | 20 | Normal | Normal | DD | Mild |
| Family C | |||||||
| Symptomatic | |||||||
| I.1 | M | 17 | 52 | Normal | Normal | DD | Moderate |
| II.4 | M | 18 | 21 | Normal | Normal | DD | Normal |
| Family D | |||||||
| Symptomatic | |||||||
| II.2 | M | 40 | 45 | ↑ CK (445 UI/l) | Proximal weakness | DD | Severe |
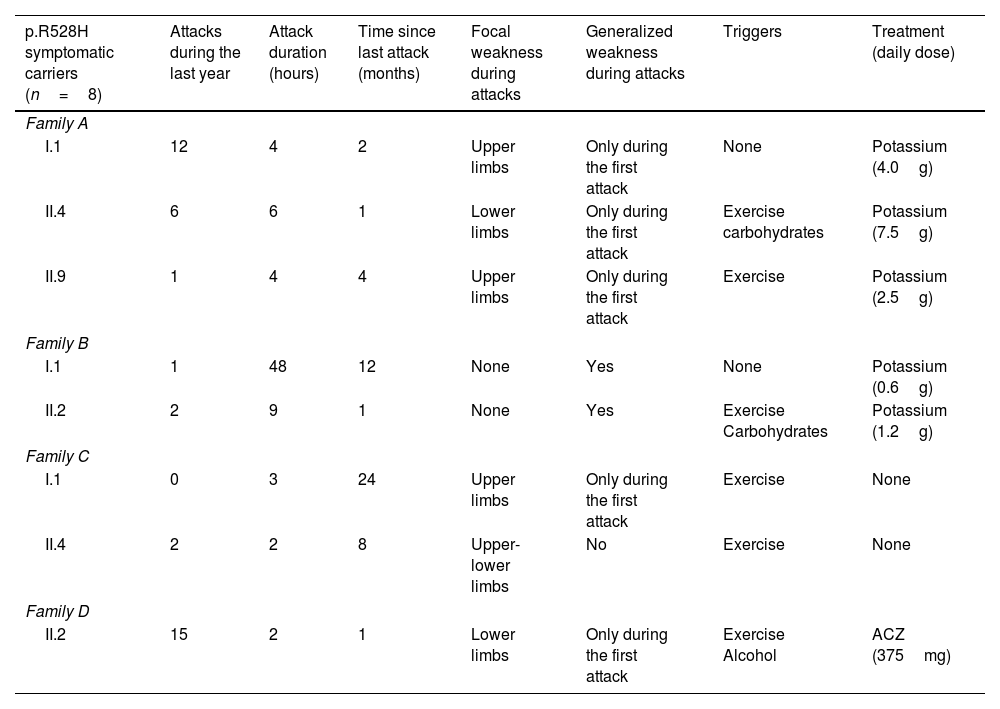
Symptomatic carriers’ clinical data.
| p.R528H symptomatic carriers (n=8) | Attacks during the last year | Attack duration (hours) | Time since last attack (months) | Focal weakness during attacks | Generalized weakness during attacks | Triggers | Treatment (daily dose) |
|---|---|---|---|---|---|---|---|
| Family A | |||||||
| I.1 | 12 | 4 | 2 | Upper limbs | Only during the first attack | None | Potassium (4.0g) |
| II.4 | 6 | 6 | 1 | Lower limbs | Only during the first attack | Exercise carbohydrates | Potassium (7.5g) |
| II.9 | 1 | 4 | 4 | Upper limbs | Only during the first attack | Exercise | Potassium (2.5g) |
| Family B | |||||||
| I.1 | 1 | 48 | 12 | None | Yes | None | Potassium (0.6g) |
| II.2 | 2 | 9 | 1 | None | Yes | Exercise Carbohydrates | Potassium (1.2g) |
| Family C | |||||||
| I.1 | 0 | 3 | 24 | Upper limbs | Only during the first attack | Exercise | None |
| II.4 | 2 | 2 | 8 | Upper-lower limbs | No | Exercise | None |
| Family D | |||||||
| II.2 | 15 | 2 | 1 | Lower limbs | Only during the first attack | Exercise Alcohol | ACZ (375mg) |
ACZ: acetazolamide.
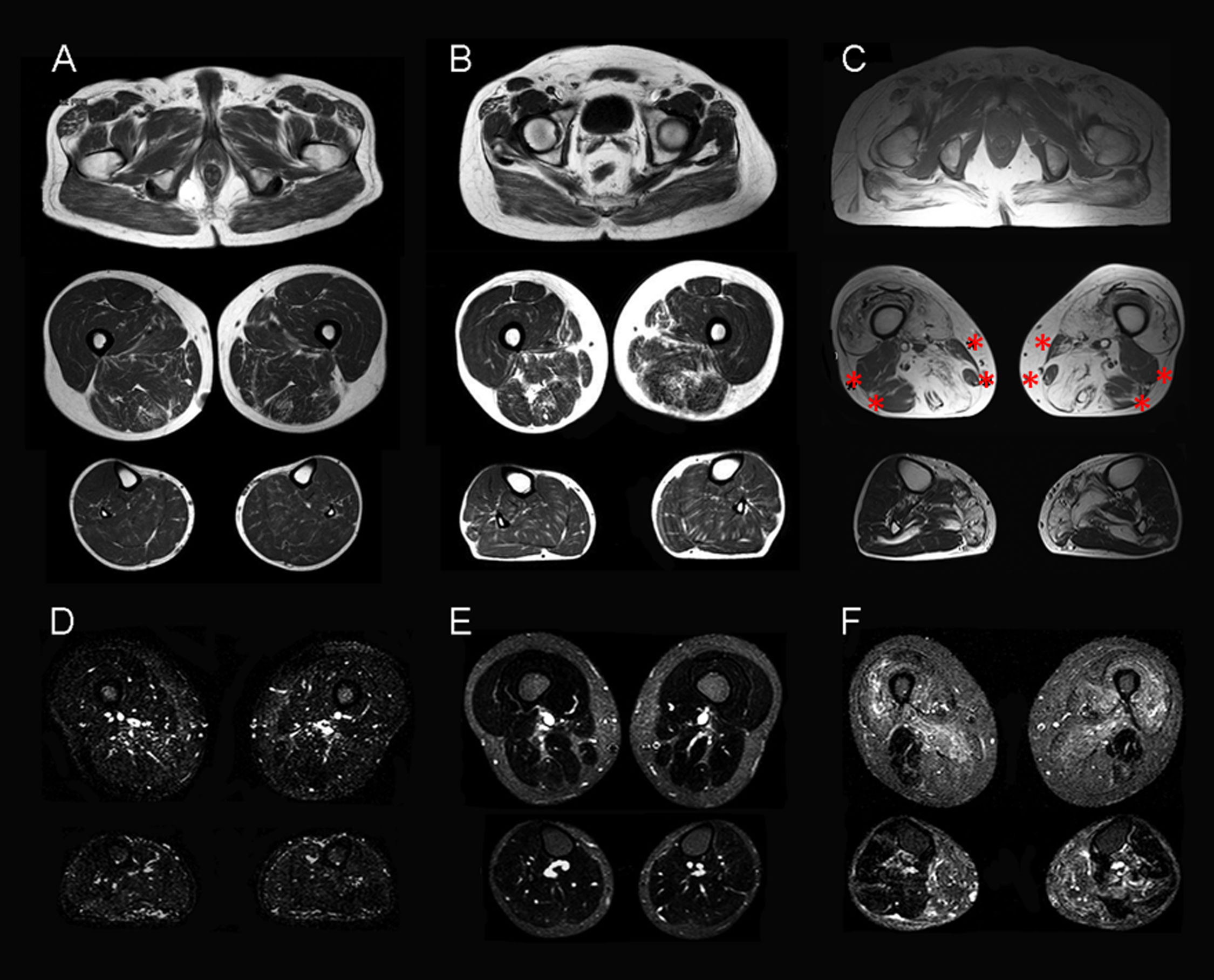
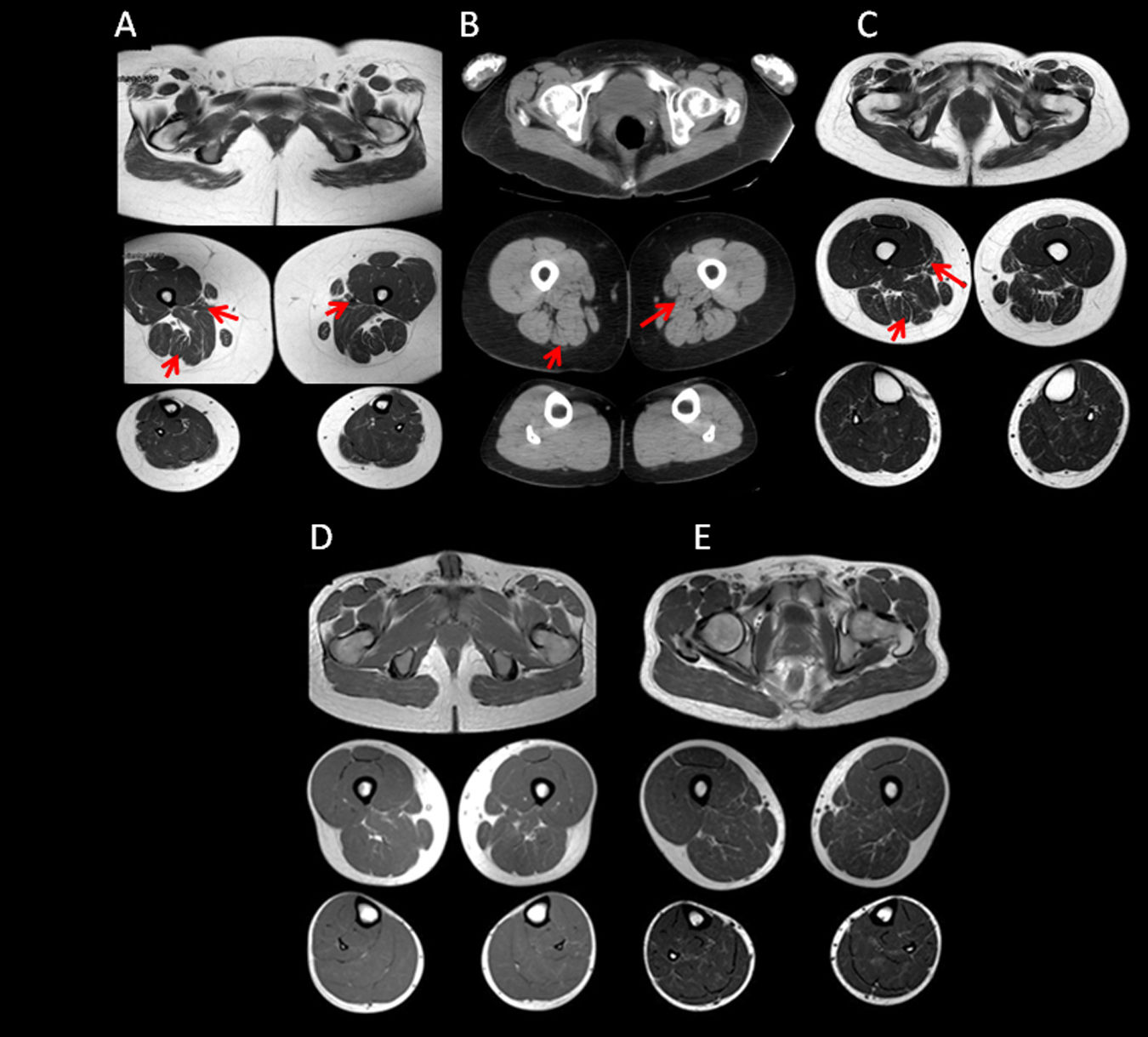
Muscle MRI on T1-weighted sequences showed varying degrees of atrophy and fatty infiltration in all 3 aHypoPP carriers and in 5 of the 8 sHypoPP patients. The distribution pattern was consistent with fatty infiltration and atrophy in the muscles of the posterior compartment of the thigh, with the adductor magnus and semimembranosus being the first muscles affected, and the biceps femori, sartorius, and gracilis muscles being spared. At the hip level, mild fatty degeneration also involved the gluteus major muscle. At the lower leg level, the gastrocnemius medialis and soleus muscles were affected only in the patient with permanent weakness. When MRI findings were ranked according to a subjective scale,20 3 aHypoPP and 2 sHypoPP participants showed mild involvement, and 2 patients with sHypoPP showed moderate involvement, whereas severe fatty replacement was only seen in the sHypoPP patient with permanent weakness, who also showed fatty degeneration of the quadriceps (Figs. 2A–C and 3). There was a direct linear association between severity of fatty infiltration and age (r=0.62; P=.003), but there was no statistically significant association with any other clinical parameter. STIR sequences showed increased signal only in the sHypoPP patient with permanent weakness, revealing edema in some muscles with fatty replacement in the thigh (vastus lateralis, adductor magnus, and semimembranosus) and lower legs (gastrocnemius and soleus) (Fig. 2D, E). Muscle MRI findings in controls were normal.
Muscle MRI studies of the lower limbs in symptomatic patients. Muscle imaging of sHypoPP patients showed different degrees of fatty degeneration in T1-weighted axial images. Patients without permanent weakness (A and B) showed a moth-eaten appearance with scattered small areas of increased signal in the gluteus major muscle and in the muscles of the posterior compartment of the thigh (particularly the adductor magnus and semimembranosus muscles), while an incipient involvement of the soleus muscle was observed only in B. A patient with permanent weakness (C) showed end-stage fatty degeneration in the gluteus major and thigh muscles, sparing the biceps femori, semitendonosus, sartorius, and gracilis muscles (asterisks). In the lower legs, the soleus and gastrocnemius medialis showed pronounced degeneration. STIR sequences showed hyperintensity only in muscles with permanent weakness (F), but were normal in patients with recurrent attacks but not permanent weakness (D, E).
Muscle MRI studies of the lower limbs in asymptomatic carriers. Muscle imaging of the 3 asymptomatic carriers (A–C), showed atrophy and very mild fatty degeneration of the adductor magnus and semitendinosus muscles (arrows), with less marked involvement of the muscles of the posterior compartment of the thighs, and atrophy of the gluteus major muscle. This pattern is similar to that initially observed in sHypoPP. MRI findings in controls from the same families were all normal (D, E).
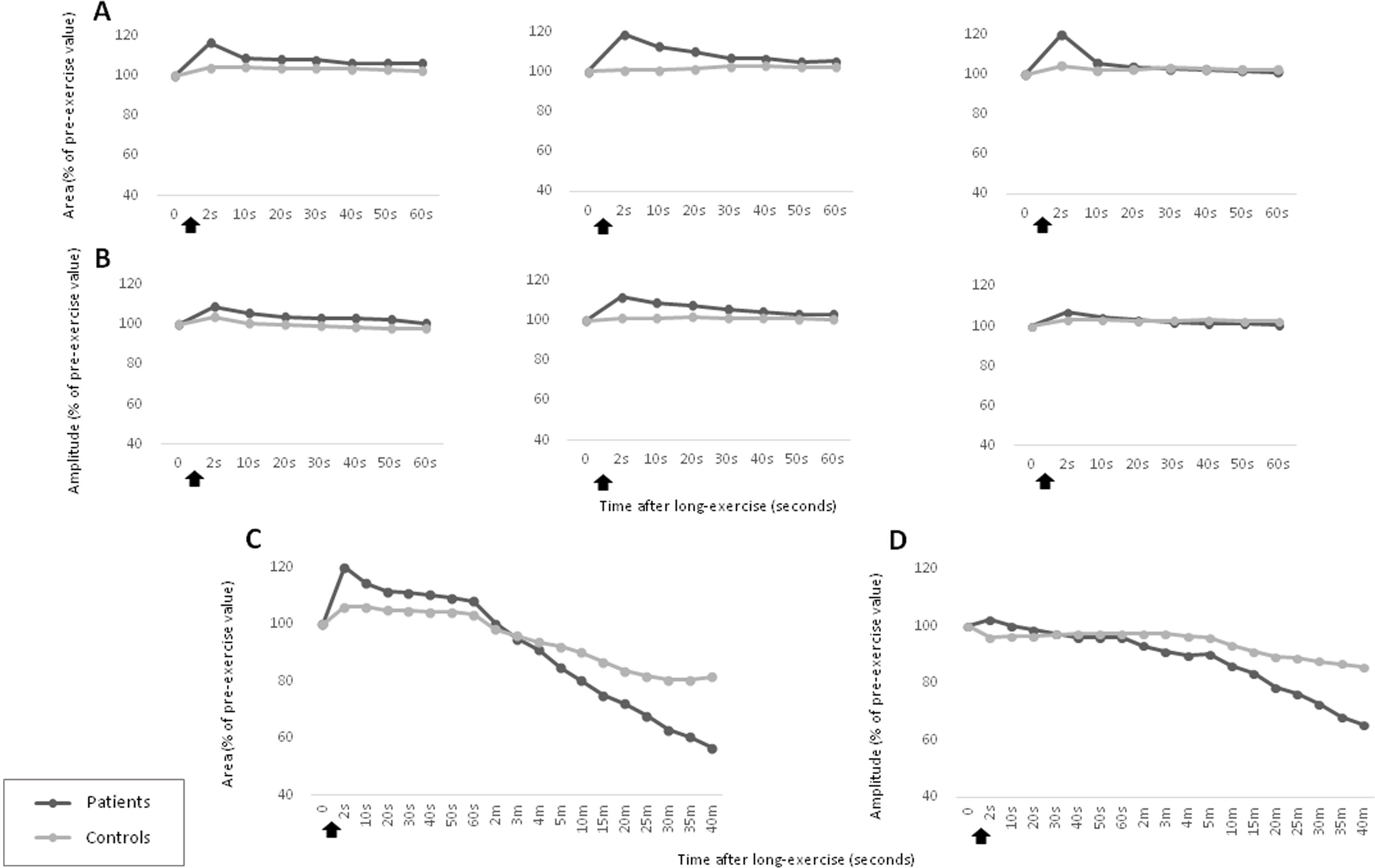
We evaluated the electrophysiological response after short and long exercise tests in the total group of patients (sHypoPP and aHypoPP) and controls. Post-exercise compound muscular action potential (CMAP) amplitude and area (baseline to negative peak) were expressed as percentages of pre-exercise values, as previously described.8 After short exercise (3 trials), we observed a slight, transient increase in CMAP amplitude and area within the first seconds post exercise (11% [7%]), which was not significantly different from that observed in controls (4% [6%]) (Fig. 4A, B, Supplementary Fig. 4).
Short and long exercise tests in the patients with hypokalemic periodic paralysis due to p.R528H mutation and controls. (A, B) Short exercise of the abductor digiti minimi (ADM) muscle was repeated 3 successive times at 1-min intervals. Post-exercise CMAP area (A) and amplitude values (B) were not significantly different from those observed in controls (C, D). Long exercise test in the ADM showed a delayed decrement in CMAP area (C) and amplitude (D), while an early and transient increment was only observed in CMAP area. CMAP amplitude and area of the CMAP, expressed as percentages of baseline values before the trials, were recorded at different times after the exercise (arrows). These data represent the mean value for all individuals in each group (patients and controls).
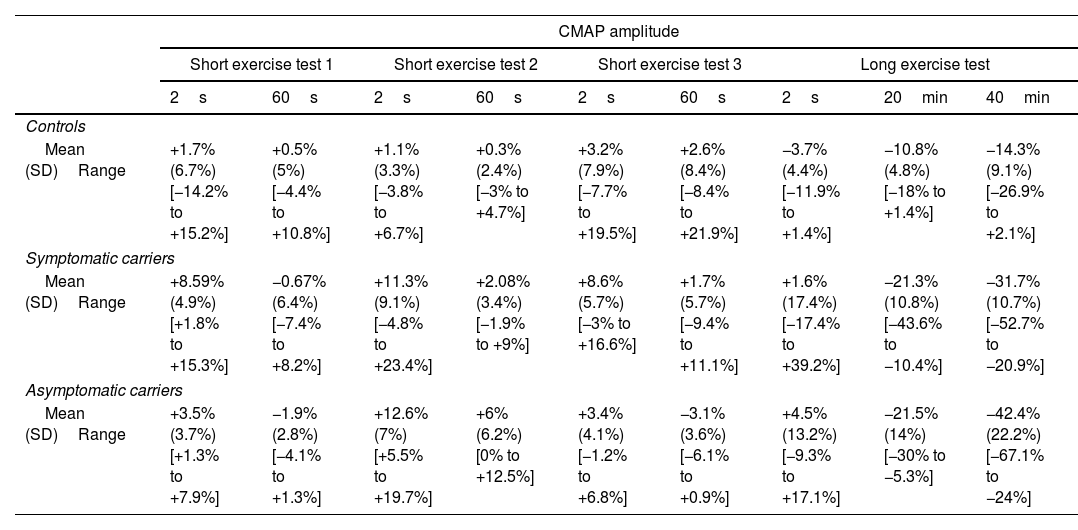
After long exercise, we detected a delayed, progressive decrement in CMAP amplitude, which was statistically significant from 20min post-exercise onwards (−21.45% [11%]; P<.005). The decline reached up to 67% by the end of the recording (Fig. 4D). We consider this an appropriate cut-off point based on amplitudes in our non-carrier control group, which decreased only slightly (−10.8% [4.8%]). The CMAP amplitude was significantly different between patients and controls from 10min (95% confidence interval [95% CI], 2.2–18.4; P<.05) until 40min post-exercise (95% CI, 12.2–36.9; P<.05). A transient increment in CMAP area was slightly but not significantly higher in patients immediately after completion of the long exercise test (Fig. 4C). This electrophysiological pattern was observed in all the mutation carriers, regardless of the symptoms (Fig. 5).
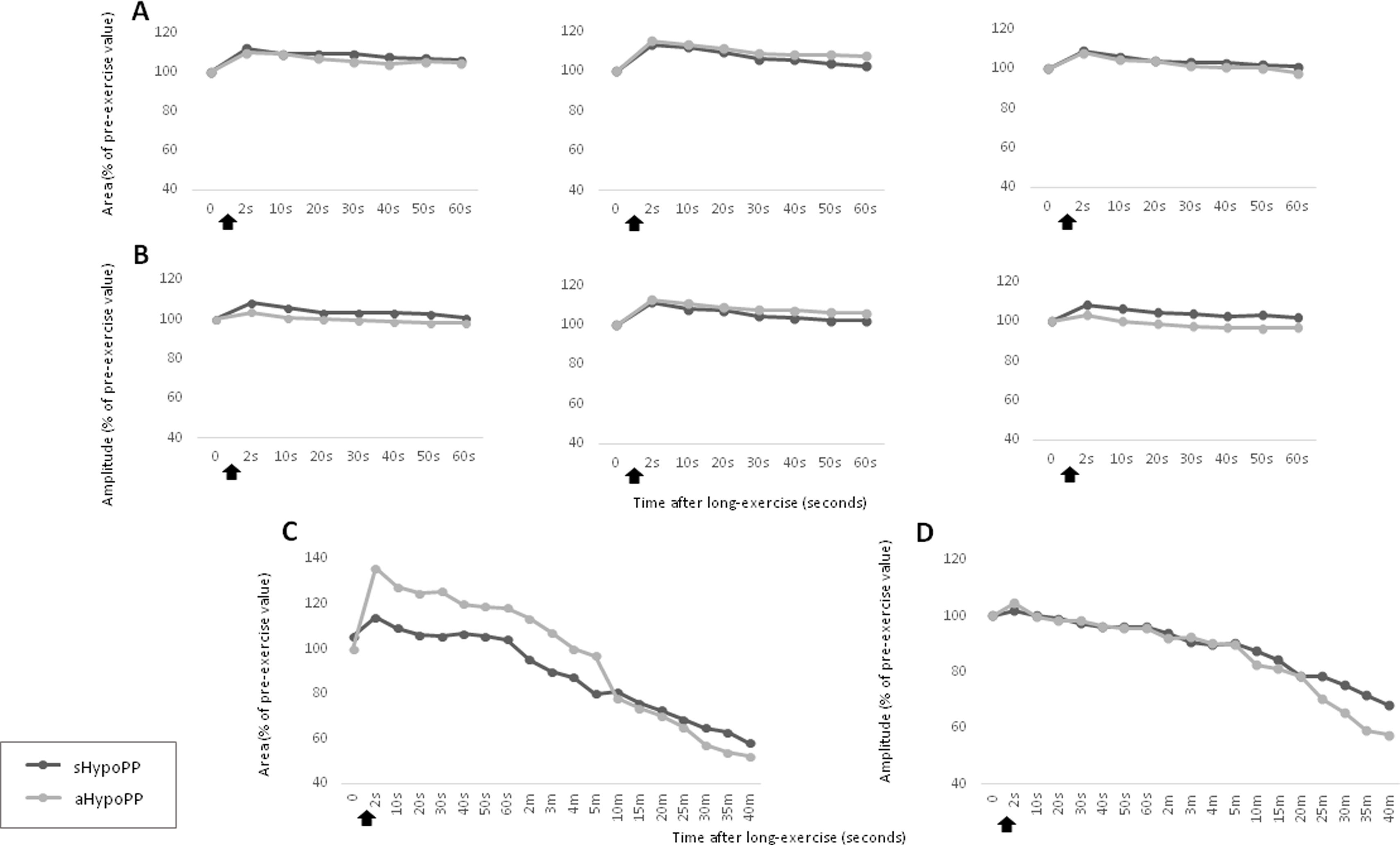
Short and long exercise tests in individuals with asymptomatic and symptomatic hypokalemic periodic paralysis. The response of CMAP area (A, C) and CMAP amplitude (B, D) in the short (A, B) and long exercise tests (C, D) was not significantly different between the aHypoPP and sHypoPP groups. The short exercise test was repeated 3 successive times at 1-min intervals. CMAP amplitude (B, D) and area (A, C), expressed as a percentage of baseline values before the trials, were recorded at different times after the exercise (arrows). These data represent the mean value for all patients in each group (aHypoPP and sHypoPP).
We separately analyzed the results from sHypoPP and aHypoPP. The response of the exercise test in the aHypoPP group (42.6 [16.3] years old; range, 20–58) showed no significant CMAP decline or increase after the short exercise, or after successive trials. In the long exercise, no significant CMAP changes were observed during the first seconds after exercise, but between 10 and 35min after exercise a significant and prolonged decrement in CMAP amplitude appeared at rest in all aHypoPP patients (−42.47% [27.22%]). The maximum decline was −67.14% (range, −24% to −67.14%). In the sHypoPP group (39.37 [17.14] years old; range, 19–63), no significant CMAP change was observed in the short exercise test. However, a significant decrement in CMAP amplitude was observed between 10 and 35min after long exercise (−31.79% [10.76%]), with a maximum decrement of −52.72% (range, −20.93% to −52.72%). Only one sHypoPP patient displayed a progressive decrement in CMAP amplitude from the first minute after long exercise. Six sHypoPP participants, who had presented attacks during the previous year, showed the same pattern of CMAP amplitude decrement in the tests as the 2 sHypoPP participants with no recent attacks.
CMAP amplitude and area results were consistent both in short and long exercise tests (Table 3). The CMAP amplitude decrement was not influenced by age, sex, or disease duration, and no differences were observed between treated and untreated patients. According to our analysis, this pattern for the HypoPP patients with the CACNA1S p.R528H mutation had 63.6% sensitivity and 100% specificity, for values recorded 20min post-exercise. However, at 35min post-exercise, the test showed 100% sensitivity but specificity was reduced to 88%.
CMAP amplitude and area in patients and controls.
| CMAP amplitude | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Short exercise test 1 | Short exercise test 2 | Short exercise test 3 | Long exercise test | ||||||
| 2s | 60s | 2s | 60s | 2s | 60s | 2s | 20min | 40min | |
| Controls | |||||||||
| Mean (SD)Range | +1.7% (6.7%)[−14.2% to +15.2%] | +0.5% (5%)[−4.4% to +10.8%] | +1.1% (3.3%)[−3.8% to +6.7%] | +0.3% (2.4%)[−3% to +4.7%] | +3.2% (7.9%)[−7.7% to +19.5%] | +2.6% (8.4%)[−8.4% to +21.9%] | −3.7% (4.4%)[−11.9% to +1.4%] | −10.8% (4.8%)[−18% to +1.4%] | −14.3% (9.1%)[−26.9% to +2.1%] |
| Symptomatic carriers | |||||||||
| Mean (SD)Range | +8.59% (4.9%)[+1.8% to +15.3%] | −0.67% (6.4%)[−7.4% to +8.2%] | +11.3% (9.1%)[−4.8% to +23.4%] | +2.08% (3.4%)[−1.9% to +9%] | +8.6% (5.7%)[−3% to +16.6%] | +1.7% (5.7%)[−9.4% to +11.1%] | +1.6% (17.4%)[−17.4% to +39.2%] | −21.3% (10.8%)[−43.6% to −10.4%] | −31.7% (10.7%)[−52.7% to −20.9%] |
| Asymptomatic carriers | |||||||||
| Mean (SD)Range | +3.5% (3.7%)[+1.3% to +7.9%] | −1.9% (2.8%)[−4.1% to +1.3%] | +12.6% (7%)[+5.5% to +19.7%] | +6% (6.2%)[0% to +12.5%] | +3.4% (4.1%)[−1.2% to +6.8%] | −3.1% (3.6%)[−6.1% to +0.9%] | +4.5% (13.2%)[−9.3% to +17.1%] | −21.5% (14%)[−30% to −5.3%] | −42.4% (22.2%)[−67.1% to −24%] |
| CMAP area | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Short exercise test 1 | Short exercise test 2 | Short exercise test 3 | Long exercise test | ||||||
| 2s | 60s | 2s | 60s | 2s | 60s | 2s | 20min | 40min | |
| Controls | |||||||||
| Mean (SD)Range | +3.9% (6.6%)[−3.3% to +17.1%] | +2.5% (6.9%)[−8.5% to +15.7%] | +0.6% (3.9%)[−3.8% to +8.6%] | +2.3% (3.5%)[−3.5% to +9.1%] | +4.1% (8.5%)[−7.2% to +20.1%] | +2.1% (6%)[−7.2% to +16%] | +6% (11.3%)[−17% to +22.1%] | −16.4% (6.5%)[−25.4% to −5.9%] | −18.4% (9.7%)[−36.7% to −7.1%] |
| Symptomatic carriers | |||||||||
| Mean (SD)Range | +11.8% (5.6%)[+4.5% to +19.7%] | +5.9% (8.4%)[−14.2% to +10.8%] | +13.6% (10.4%)[−5% to +23.3%] | +2.8% (3.5%)[−0.5% to +8.4%] | +8.8% (7.9%)[−5.9% to +17.2%] | +0.9% (6.1%)[−11.7% to +6.6%] | +14.1% (27.3%)[−32.9% to +61%] | −27.32% (12.2%)[−47.7% to −6.9%] | −41.9% (17.8%)[−82.9% to −23.9%] |
| Asymptomatic carriers | |||||||||
| Mean (S))Range | +9.7% (5.2%)[+4.2% to +14.6%] | +4.4% (4.6%)[−0.7% to +8.4%] | +15.3% (4.4%)[+10.4% to +19.2%] | +7.6% (1%)[+6.6% to +8.7%] | +7.9% (12.4%)[−1.1% to +22.1%] | −2.2% (4.7%)[−5.8% to +3.1%] | +35.8% (29.9%)[+12.2% to +69.5%] | −29.8% (9.6%)[−35.7% to −18.7%] | +52.1% (27.8%)[−71.1% to −16.9%] |
CMAP: compound muscle action potential; SD: standard deviation of the mean.
CMAP amplitude and area are expressed as percentages of pre-exercise baseline values. These data represent the mean value (standard deviation) for all patients in each group (carriers and controls).
We describe the clinical, muscle MRI, and electrophysiological characteristics of a well-defined Spanish cohort of individuals with HypoPP due to the CACNA1S p.R528H mutation, including both symptomatic and asymptomatic individuals. Some radiological changes have been observed in periodic paralysis, and the exercise test has already been described in channelopathies, but this is the first time that muscle MRI and exercise electrophysiology have been performed together in a homogeneous population from a single geographical area, carrying the same mutation. Thus, we have defined a thorough pattern for these tests, with a focus on searching for differences between symptomatic and asymptomatic carriers. Because different clinical or electrophysiological expressions in individuals with HypoPP have been associated with gender and ethnicity,10,21 we considered it important to study a cohort of cases and controls with a homogeneous genetic background and geographical origin.
As previously described, we confirmed the incomplete penetrance of the p.R528H mutation in women, since all the female carriers in our cohort were asymptomatic, whereas we observed 100% penetrance in male carriers.4,7 This is a widely described phenomena, and it is reasonable to assume that hormonal factors may modulate the regulation of channels of the tubular system. This type of regulation is supported by previous data demonstrating that voltage-dependent sodium currents in C2C12 cells were under androgenic influence through a post-transcriptional mechanism.17 However, in the light of the fact that individual studies have reported signs of disease in some asymptomatic carriers, describing these signs is important to detect potential carriers and prevent new cases.
Previous reports of individual cases of HypoPP include information about muscle imaging,17,22,23 but a recent study reported the muscle MRI findings from 54 HypoPP patients with the CACNA1S mutation p.R528H, describing the same pattern of muscle degeneration as that observed in our series.19 This pattern includes fatty degeneration of the gluteus major and posterior compartment of the thigh in most patients with a phenotype of periodic paralysis, associated with degeneration of the gastrocnemius and soleus muscles in the most severe cases with permanent weakness (Fig. 2). Interestingly, compared with the largest reported series,19 in which only one of the 4 aHypoPP participants showed alterations in muscle MRI, we found radiological alterations in all 3 asymptomatic carriers, who mainly displayed atrophy and mild fatty degeneration of the adductor magnus and semimembranosus muscles. Although all our asymptomatic carriers were women and all symptomatic individuals were men, we found no radiological differences between sexes in our control sample, so we can reasonably rule out the possibility that sex affected the pattern of fat distribution in our patients. We found that muscle fatty replacement increased with age; this is consistent with the results of Holm-Yildiz et al.19 The age of the 3 aHypoPP women in our series was 42 (16) years, older than the 4 aHypoPP individuals in the unique report about muscle MRI in aHypoPP (32 [17] years),19 in which the authors found imaging alterations only in one carrier. The older age of aHypoPP participants in our series may be the reason for the muscle MRI alterations found in all 3 of them, although this hypothesis should be tested in larger series of aHypoPP.
In our series, MRI only revealed severe degeneration affecting the pelvic, thigh, and lower leg muscles in the single patient with permanent weakness. Disease duration in this patient was only 5 years, whereas it ranged between 19 and 41 years in the remaining patients with sHypoPP, suggesting that severe fatty degeneration in this disease is unrelated to disease duration. Similarly to our own findings, Holm-Yildiz et al.19 found that muscle fatty replacement was not associated with frequency or total number of attacks. Therefore, further research is needed to identify other factors potentially explaining the severity of muscle degeneration in this disease.
In our study, STIR sequences showed increased signal only in the patient with permanent weakness. While the largest reported series, described by Holm-Yildez et al.,19 did not include data from STIR sequences, Jurkat-Rott et al.24 found edema in the muscles in 25 of the 36 HypoPP patients with permanent weakness; similar results have been published for other muscle channelopathies.18,22,23,25,26 Provocative testing in hyperkalemic periodic paralysis increases edema in weak muscles during attacks,26 and one study showed that edema on muscle MRI was most pronounced in severe phenotypes of periodic paralysis, with up to daily paralytic episodes27; therefore, one would expect to see more severe muscle degeneration and edema in patients with frequent transient episodes of paralysis. In fact, the patient in our series with muscle edema showed the highest attack frequency (one per month), in spite of therapy. In this scenario, muscle MRI could be a potential marker of muscle degeneration to be considered in natural history studies of HypoPP and in further clinical trials.
As this is the first large cohort of Spanish patients with hereditary HypoPP, we performed the exercise test in both symptomatic and asymptomatic patients in order to complete the description of the phenotype and to uncover possible differences from previously published studies. We observed a similar electrophysiological pattern to that reported by other authors in p.R528H mutation carriers, i.e., significantly delayed CMAP amplitude decrement after long exercise with no significant changes following short exercise.8,10 However, we did find some differences: (a) a transient increment of CMAP area, though not significant, was present immediately after long exercise in all our patients; this finding has only previously been described by Kuntzer et al.9; (b) sensitivity was up to 100% in prolonged recording (up to 35min); and (c) the same pattern was observed in symptomatic and asymptomatic individuals; this is contrary to the findings of the only previous study in which 4 asymptomatic carriers underwent exercise testing, showing the same pattern as controls.21
Numerous studies report the usefulness of the long exercise test in PP, but cut-off values to define an abnormal result are not well defined.9,11,21,28 In this regard, Jin et al.28 recommended that different reference values be established for men and women in the long exercise test. They found that the rate of CMAP amplitude decrement was associated only with sex (46.8% in the male group and 26.9% in the female group), and not with age, height, weight, or type of exercise.28 That study included only 45 women, but did not specify whether they were symptomatic or asymptomatic. In contrast, exercise testing results in our study were not influenced by sex, age, or disease duration. Recently, a study using Bayesian principles established the optimal cut-off values for the long exercise test achieving 95% post-test probability of PP in a cohort including different phenotypes (among others, only 12 patients presented genetically defined HypoPP), genes (SCN4A or CACNA1S), and mutations. The authors reported that establishing peak-to-nadir decrement was diagnostically useful, using cut-off values of either 40% amplitude or 50% area decrement for pre-test probabilities ≤50%.29,30 By contrast, our study followed the methodology described by Fournier et al.,8 using baseline-to-peak values and defining amplitude decrement with comparison to the pre-exercise baseline value. The cut-off to define a pathological long exercise test result was defined as either an amplitude or an area decrement>20%±2 standard deviations. Compared to the previous study,29 our group of individuals with HypoPP was more phenotypically and genetically homogeneous; therefore, our values are probably more accurate for the most frequent HypoPP population, carriers of the CACNA1S p.R528H mutation. Interestingly, we detected the same long exercise pattern and values regardless of symptoms, and we cannot rule out an association between this pattern and age, since the only report including exercise test findings in 3 individuals with aHypoPP showed a normal pattern but did not describe the sample.21
While disease burden caused by repeated attacks of paralysis or permanent muscle weakness may explain the fatty muscle degeneration, it would not explain the radiological manifestations in asymptomatic cases. In addition, the pattern of long exercise test results observed in our aHypoPP participants was not different from that seen in sHypoPP. Taken together, these results suggest that, to some extent, the underlying pathophysiological mechanism is active even in asymptomatic carriers.
Authorship contributionsP. Carbonell recruited and examined the patients, acquired the data, and drafted the manuscript. M. Cabrera and E. Rivas handled the patients and biological samples. A. García-Redondo performed the molecular studies. A. Fernández conducted the statistical analysis. C. Paradas recruited the patients, interpreted the results, designed the study, and revised and edited the manuscript.
Study sponsorship or funding (industry, government, or institutional)This research has not received specific support from industry, government, or institutional entities.
Conflicts of interestNothing to report.
We thank the patients and their families for their participation in this study.