There is sufficient evidence on the usefulness of surgery as a therapeutic alternative for patients with drug-resistant epilepsy; however this treatment is underutilised, especially in developing countries.
MethodsWe describe the outcomes of epilepsy surgery in 27 paediatric patients at Hospital Baca Ortiz in Quito, Ecuador. Our analysis considered the following variables: reduction in seizure frequency, surgery outcome according to the Engel classification, improvement in quality of life, and serious complications due to surgery.
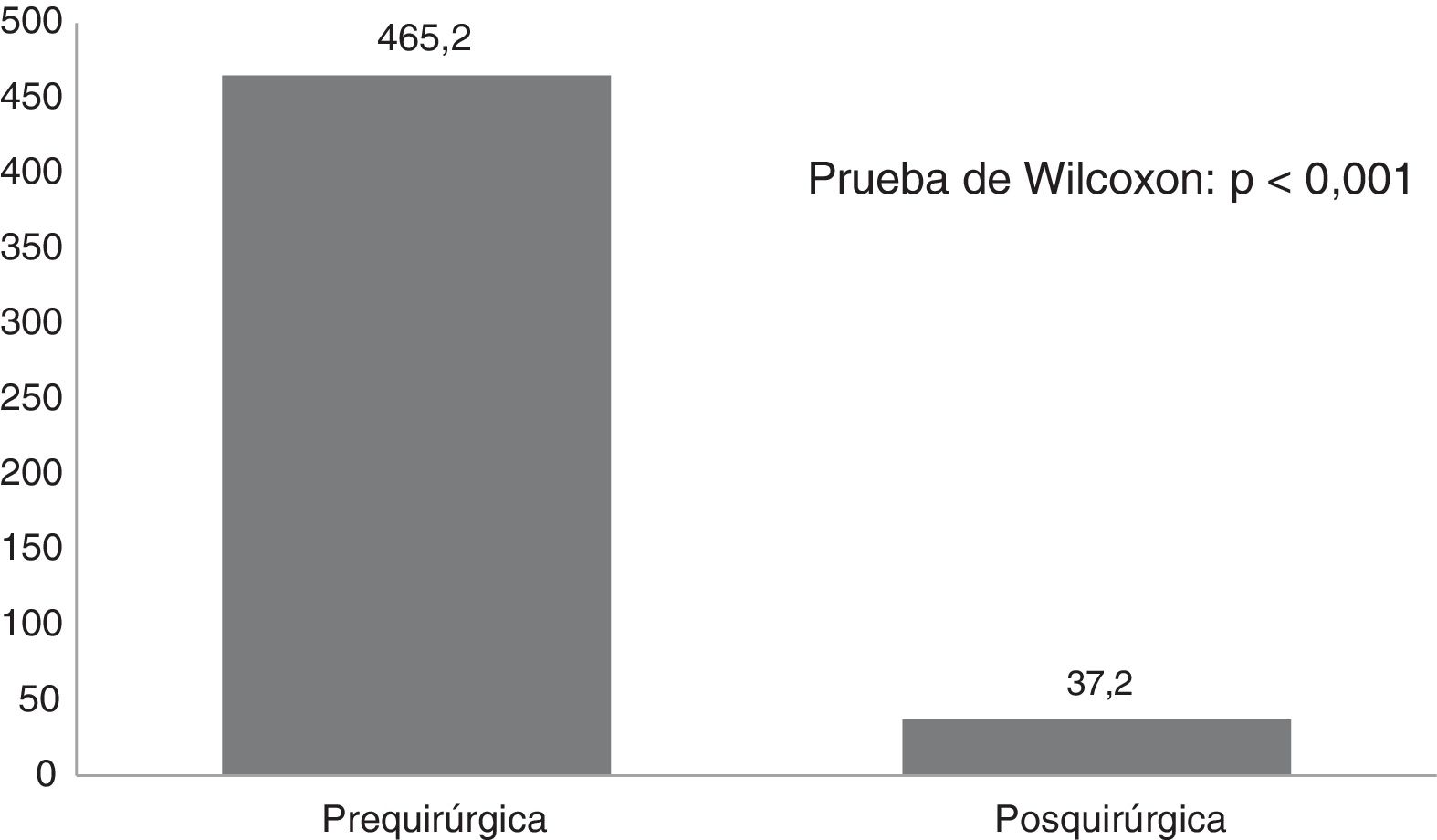
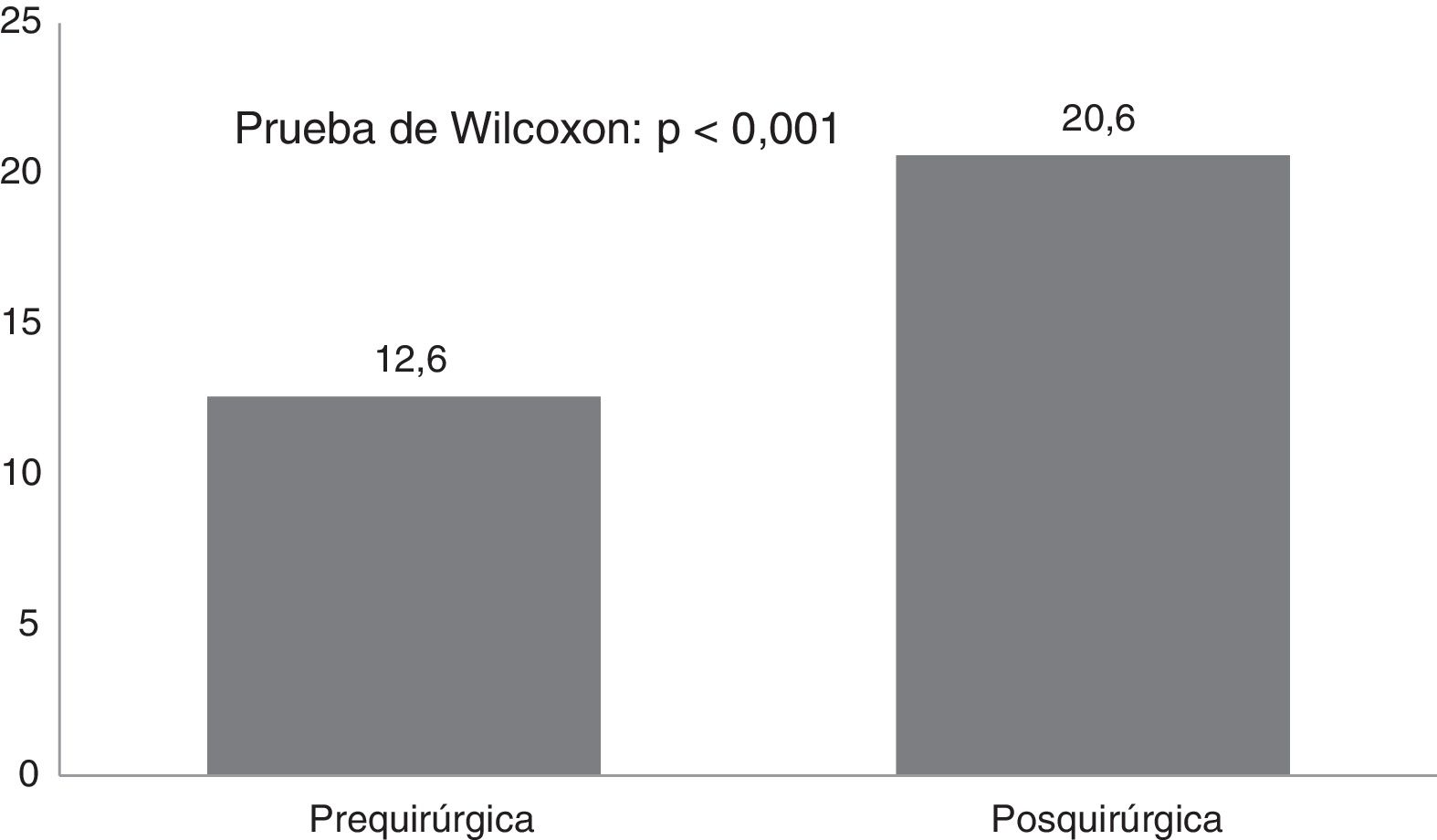
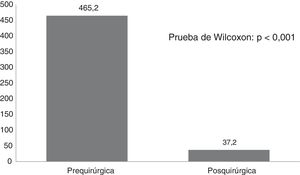
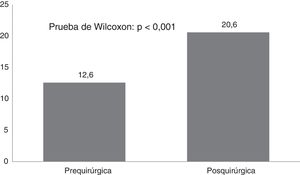
ResultsTwenty-one Corpus callosotomies and 6 resective surgeries were performed. The mean seizure frequency decreased from 465 per month before surgery to 37.2 per month thereafter (P<.001); quality of life scale scores increased from 12.6 to 37.2 (P<.001), and quality of life improved in 72.7% of patients. Regarding resective surgery, 2 patients with temporal lobe epilepsy and one with posterior quadrant epilepsy achieved Engel class IA, and one patient undergoing hemispherotomy due to Rasmussen encephalitis achieved Engel class IIA. Two patients underwent surgery for hypothalamic hamartoma: one achieved Engel III and the other, Engel IA; however, the latter patient died in the medium term due to a postoperative complication. The other major complication was a case of hydrocephalus, which led to the death of a patient with refractory infantile spasms who underwent corpus callosotomy.
ConclusionsFavourable outcomes were observed in 92.5% of patients.
Existe suficiente evidencia sobre la utilidad de la cirugía como alternativa terapéutica para pacientes con epilepsia farmacorresistente, sin embargo este tratamiento es subutilizado especialmente en países en desarrollo. El objetivo de este trabajo fue determinar la efectividad y seguridad de la cirugía de epilepsia en un hospital terciario de Ecuador.
MétodosSe describe el resultado de la cirugía de epilepsia en 27 niños y adolescentes en el Hospital Baca Ortiz, de Quito, Ecuador, teniendo en cuenta las siguientes variables antes y después de la cirugía: reducción de la frecuencia de crisis, Engel posquirúrgico, mejoría en la calidad de vida y presencia de complicaciones graves por la cirugía.
ResultadosSe realizaron 21 callosotomías y seis cirugías resectivas. La frecuencia de crisis media se redujo de 465 mensual antes de la cirugía a 37,2 mensual después de la misma (p<0,001), mientras que la puntuación en la escala de calidad de vida aumentó de 12,6 a 37,2 puntos (p<0,001), el 72,7% de los pacientes mejoró la calidad de vida. Entre las cirugía resectivas, en dos epilepsias del lóbulo temporal y una del cuadrante posterior se logró Engel Ia, una hemisferotomía por encefalitis de Rasmusen quedó en Engel IIa y dos hamartomas hipotalámicos, uno logró Engel III y otro Engel Ia pero falleció a mediano plazo por complicación posquirúrgica. La otra complicación grave fue un hidrocéfalo que llevó a la muerte a un lactante con espasmos infantiles refractarios sometido a callosotomía.
ConclusiónEl resultado favorable se observó en el 92,5% de los pacientes.
Epilepsy is the most common neurological disorder in children, with a prevalence of 3.2-5.5 cases per 1000 children in developed countries and 3.6-44 cases per 1000 children in developing countries.1 Latin America is the region with the highest prevalence of epilepsy in the general population, with a rate of 17.8 cases per 1000 population.2,3 Children with epilepsy present higher rates of behavioural, cognitive, emotional, social, and academic problems than healthy children or those with other chronic diseases.4 This has consequences for healthcare systems, with costs for these patients being higher than those associated with epilepsy in adults.5
Drug-resistant epilepsy (DRE) is defined by the International League Against Epilepsy as the “failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom.”6 DRE accounts for 20%-30% of cases of epilepsy in paediatric patients,7 although this figure can rise to 65% in patients younger than one year of age.5 DRE is associated with poorer quality of life4,8 and a mortality rate of approximately 20%9; this rate is greater than that of healthy children or those with controlled epilepsy.10 Furthermore, polytherapy is common in these patients, with the associated increases in the rate of adverse reactions to antiepileptic drugs.11 Total costs associated with DRE are 3 times higher than the mean for all patients with epilepsy.12
Surgery is demonstrated to be an effective13–15 and cost-effective16,17 treatment alternative for patients with DRE. According to international guidelines on childhood epilepsy, assessing eligibility for surgery should be standard practice in epilepsy management, even in primary and secondary healthcare centres.18 Data from various studies in developed countries suggest an increase in the number19 and complexity20 of surgical procedures for paediatric epilepsy; nonetheless, the treatment continues to be underused.15,17,18 Developing countries show a much greater therapeutic gap due to economic factors and a lack of education on the subject among healthcare professionals and the general public.21
While seizure freedom is the fundamental objective of epilepsy surgery, associated benefits include cognitive and behavioural improvements and a reduction in the number and dose of antiepileptic drugs administered.13,22 Furthermore, generalised or multifocal epilepsy can be treated with palliative surgery, which significantly reduces seizure frequency.13
Most studies on epilepsy surgery were conducted in developed countries. Data from Latin American countries are very scarce: to our knowledge, no study on the subject has been published to date in Ecuador. This study aims to determine the safety and effectiveness of epilepsy surgery at a tertiary hospital in Ecuador.
Patients and methodsWe performed a descriptive study of a series of 27 paediatric patients treated surgically for DRE at Hospital Baca Ortiz in Quito, Ecuador. We included all patients treated with palliative or resective surgery between 2014 (when these techniques were introduced at our centre) and 2016. The only exclusion criterion was a postoperative follow-up time of less than 6 months.
Hospital Baca Ortiz is a tertiary paediatric hospital pertaining to the Ecuadorian Ministry of Public Health. It has paediatric neurology and neurosurgery departments and, being a national reference centre for paediatric neurology, treats a great number of patients with DRE. In 2014, epilepsy surgery was introduced as a treatment alternative for these patients.
We gathered data on patient and disease characteristics: age, sex, age of disease onset, age at the time of surgery, diagnosis (according to the 2001 International League Against Epilepsy criteria23), aetiology, presence of cerebral palsy, presence and degree of intellectual disability, presence of autism spectrum disorders, preoperative monthly seizure frequency, and quality of life prior to surgery. We also collected data on surgery type (resective or palliative) and the specific procedure performed.
Surgical outcomes were measured using variables assessing seizure control: postoperative seizure frequency, percentage reduction in seizure frequency, Engel Epilepsy Surgery Outcome Scale classification, postoperative quality of life, and improvement in quality of life. We also recorded surgical complications and deaths related to the procedure. These data were recorded a minimum of 6 months after surgery.
Surgical outcomes were considered favourable if complete seizure freedom was achieved after resective surgery (Engel class IA) or if seizure frequency was reduced by over 50% after palliative surgery, and in the absence of severe complications or patient mortality related to the surgery. Quality of life outcomes were considered successful if the patient's quality of life rating increased according to the CAVE questionnaire for assessing the quality of life of children with epilepsy.
The CAVE questionnaire was designed by Spanish researchers and determines the negative impact of epilepsy on children's behaviour, school attendance, learning, personal independence, and social relationships, as well as seizure frequency and intensity and the parents’ opinion.24 The questionnaire is scored from 8 to 40, with patients’ quality of life rated poor to very poor (≤ 15 points), poor to normal (16-23), normal to good (24-31), or good to very good (32-40).
We followed the protocols established by our centre's management and research ethics committee for accessing medical records for research purposes.
Our results are analysed with descriptive statistics; quantitative variables are expressed as means (standard deviation [SD]) and categorical variables are expressed as frequencies (percentage). Non-parametric statistics were used to analyse relationships between variables: the Wilcoxon test was used to compare the means of dependent samples for quantitative variables, and categorical variables were compared using the chi-square test. Data analysis was conducted with the SPSS package, version 20.0 (SPSS, Inc., Chicago, IL, USA).
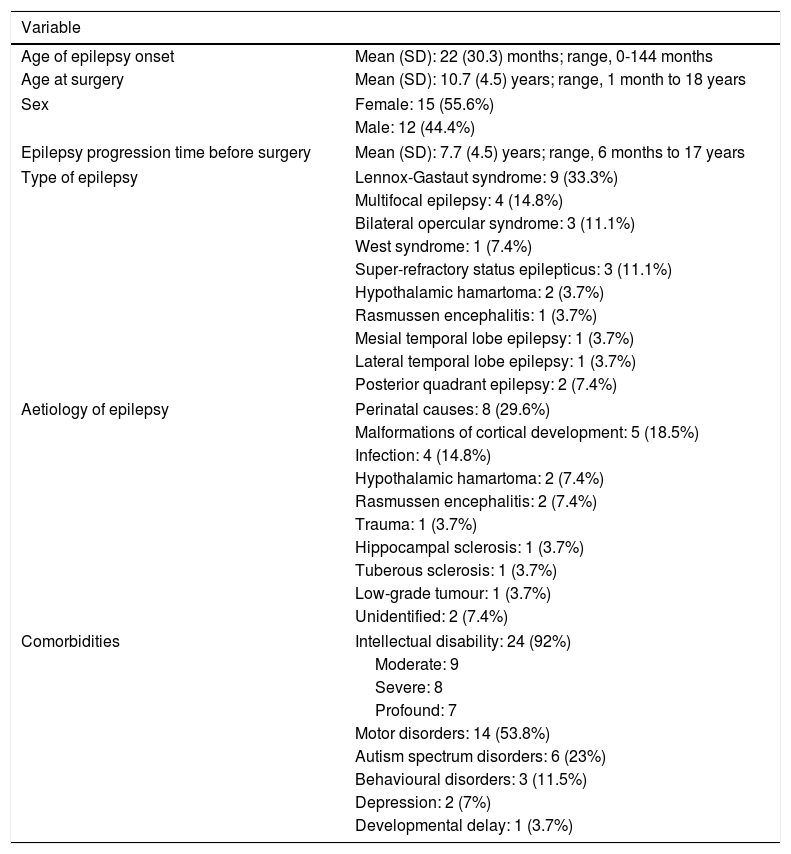
ResultsTable 1 summarises patient and disease characteristics. Mean age of epilepsy onset was younger than 2 years, while mean age at epilepsy surgery was approximately 10 years. Symptomatic generalised epilepsy and multifocal epilepsy were the most common subtypes. The most frequent aetiologies were perinatal causes, malformations of cortical development, and central nervous system infections. We also observed a high rate of comorbidities, with the most frequent being intellectual disability and motor disorders; depression was diagnosed in 2 adolescents diagnosed with temporal lobe epilepsy and not presenting intellectual disability.
Patient and disease characteristics. Hospital Baca Ortiz, 2014-2016.
| Variable | |
|---|---|
| Age of epilepsy onset | Mean (SD): 22 (30.3) months; range, 0-144 months |
| Age at surgery | Mean (SD): 10.7 (4.5) years; range, 1 month to 18 years |
| Sex | Female: 15 (55.6%) |
| Male: 12 (44.4%) | |
| Epilepsy progression time before surgery | Mean (SD): 7.7 (4.5) years; range, 6 months to 17 years |
| Type of epilepsy | Lennox-Gastaut syndrome: 9 (33.3%) |
| Multifocal epilepsy: 4 (14.8%) | |
| Bilateral opercular syndrome: 3 (11.1%) | |
| West syndrome: 1 (7.4%) | |
| Super-refractory status epilepticus: 3 (11.1%) | |
| Hypothalamic hamartoma: 2 (3.7%) | |
| Rasmussen encephalitis: 1 (3.7%) | |
| Mesial temporal lobe epilepsy: 1 (3.7%) | |
| Lateral temporal lobe epilepsy: 1 (3.7%) | |
| Posterior quadrant epilepsy: 2 (7.4%) | |
| Aetiology of epilepsy | Perinatal causes: 8 (29.6%) |
| Malformations of cortical development: 5 (18.5%) | |
| Infection: 4 (14.8%) | |
| Hypothalamic hamartoma: 2 (7.4%) | |
| Rasmussen encephalitis: 2 (7.4%) | |
| Trauma: 1 (3.7%) | |
| Hippocampal sclerosis: 1 (3.7%) | |
| Tuberous sclerosis: 1 (3.7%) | |
| Low-grade tumour: 1 (3.7%) | |
| Unidentified: 2 (7.4%) | |
| Comorbidities | Intellectual disability: 24 (92%) |
| Moderate: 9 | |
| Severe: 8 | |
| Profound: 7 | |
| Motor disorders: 14 (53.8%) | |
| Autism spectrum disorders: 6 (23%) | |
| Behavioural disorders: 3 (11.5%) | |
| Depression: 2 (7%) | |
| Developmental delay: 1 (3.7%) | |
SD: standard deviation.
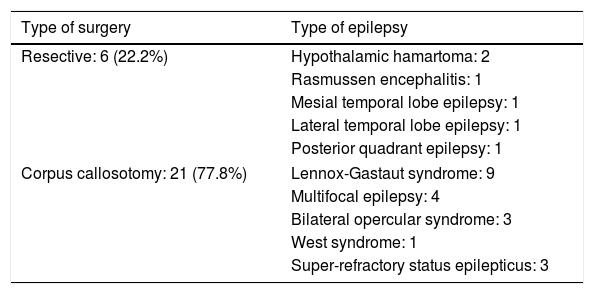
Table 2 lists the surgical procedures performed for each type of epilepsy. In 17 cases, complete corpus callosotomy was performed in a single procedure; the remaining 4 patients initially underwent partial callosotomy and subsequently complete callosotomy in a second procedure. Regarding the resective surgeries, the patient with mesial temporal lobe epilepsy with hippocampal sclerosis underwent right amygdalohippocampectomy; the patient with low-grade tumour–related lateral temporal lobe epilepsy underwent left anterior temporal lobectomy, and the patient with posterior quadrant epilepsy of perinatal aetiology underwent right posterior lobectomy. One patient with Rasmussen encephalitis was treated with left hemispherotomy, and both patients with hypothalamic hamartoma underwent open surgery, via a transcallosal approach in one case and a subtemporal approach in the other.
Types of surgeries performed. Hospital Baca Ortiz, 2014-2016.
| Type of surgery | Type of epilepsy |
|---|---|
| Resective: 6 (22.2%) | Hypothalamic hamartoma: 2 |
| Rasmussen encephalitis: 1 | |
| Mesial temporal lobe epilepsy: 1 | |
| Lateral temporal lobe epilepsy: 1 | |
| Posterior quadrant epilepsy: 1 | |
| Corpus callosotomy: 21 (77.8%) | Lennox-Gastaut syndrome: 9 |
| Multifocal epilepsy: 4 | |
| Bilateral opercular syndrome: 3 | |
| West syndrome: 1 | |
| Super-refractory status epilepticus: 3 | |
Fig. 1 shows seizure frequencies before and after surgery. Generally, seizure frequency decreased significantly (P<.001) following surgery. Our patients showed a mean (SD) reduction in seizure frequency of 90.9% (11.8%). The mean reduction in seizure frequency was 331 per patient per month, ranging from 10 in the patient with mesial temporal lobe epilepsy with hippocampal sclerosis to 1890 in a patient with Lennox-Gastaut syndrome.
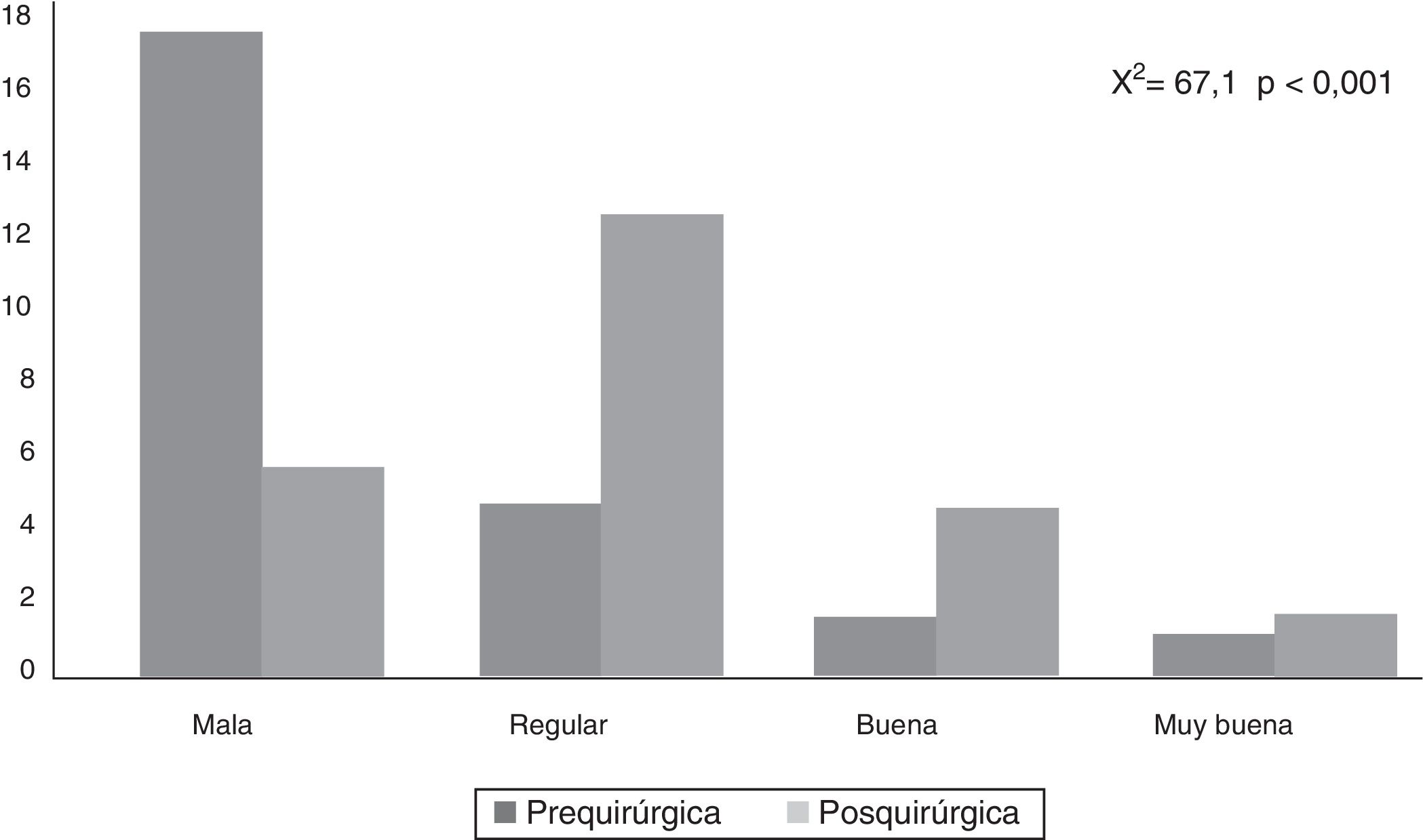
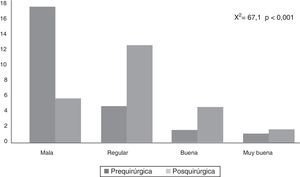
CAVE scores increased by 7.8 (3.9) points after surgery (P<.001; Fig. 2). Fig. 3 shows CAVE quality of life ratings before and after surgery; ratings increased by at least one category in 72.7% of patients.
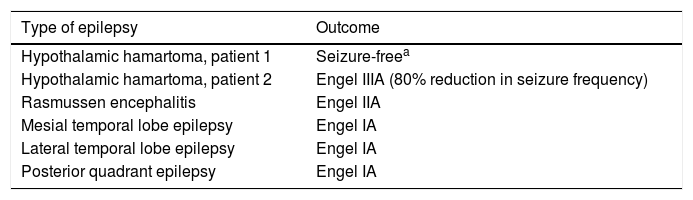
Table 3 shows the Engel classifications of the 6 patients undergoing resective surgery. The patients undergoing right amygdalohippocampectomy, left anterior temporal lobectomy, and right posterior lobectomy achieved seizure freedom. Of the patients with hypothalamic hamartoma, one presented considerable improvements in seizure frequency and behaviour, and the other remained seizure-free, but died 3 months later. The patient undergoing hemispherotomy for Rasmussen encephalitis presented sporadic focal motor seizures.
Outcomes of resective procedures in terms of seizure control. Hospital Baca Ortiz, 2014-2016.
| Type of epilepsy | Outcome |
|---|---|
| Hypothalamic hamartoma, patient 1 | Seizure-freea |
| Hypothalamic hamartoma, patient 2 | Engel IIIA (80% reduction in seizure frequency) |
| Rasmussen encephalitis | Engel IIA |
| Mesial temporal lobe epilepsy | Engel IA |
| Lateral temporal lobe epilepsy | Engel IA |
| Posterior quadrant epilepsy | Engel IA |
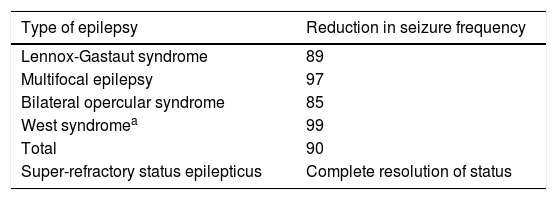
As shown in Table 4, all patients undergoing complete callosotomy presented a > 50% reduction in seizure frequency; none achieved seizure freedom, but frequency decreased by over 80% in all patients.
Outcomes of palliative corpus callosotomies. Hospital Baca Ortiz, 2014-2016.
| Type of epilepsy | Reduction in seizure frequency |
|---|---|
| Lennox-Gastaut syndrome | 89 |
| Multifocal epilepsy | 97 |
| Bilateral opercular syndrome | 85 |
| West syndromea | 99 |
| Total | 90 |
| Super-refractory status epilepticus | Complete resolution of status |
Three patients underwent surgery for super-refractory convulsive status epilepticus, which resolved in all cases. One patient with multifocal epilepsy continued to present isolated focal motor seizures, and died 15 days later due to a complication unrelated to the procedure; a patient with Rasmussen encephalitis continued to present isolated hemibody seizures of differing behaviour (hemispheric surgery was not performed, in accordance with the parents’ wishes); and a patient with focal epilepsy due to right frontal cortical dysplasia (treated with corpus callosotomy plus resection of the dysplastic area) continued to present frequent hemibody motor seizures due to inappropriate pharmacological management; she remained seizure-free following adjustment of the treatment.
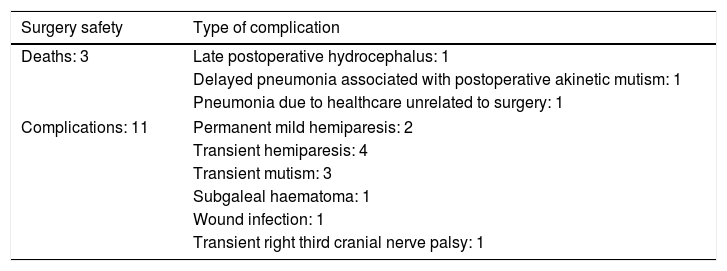
Table 5 shows the complications observed, which were mainly mild and/or transient. Three patients died: the patient treated for refractory West syndrome died 2 months after surgery due to hydrocephalus with severe intracranial hypertension, which was not promptly detected and treated; a 13-year-old boy treated for hypothalamic hamartoma presented akinetic mutism, leading to aspiration pneumonia 3 months later; and a girl with super-refractory status epilepticus, which resolved with complete corpus callosotomy, developed ventilator-associated pneumonia. Therefore, severe surgery-related complications accounted for 2 patient deaths (7.4% of patients).
Complications in patients undergoing epilepsy surgery. Hospital Baca Ortiz, 2014-2016.
| Surgery safety | Type of complication |
|---|---|
| Deaths: 3 | Late postoperative hydrocephalus: 1 |
| Delayed pneumonia associated with postoperative akinetic mutism: 1 | |
| Pneumonia due to healthcare unrelated to surgery: 1 | |
| Complications: 11 | Permanent mild hemiparesis: 2 |
| Transient hemiparesis: 4 | |
| Transient mutism: 3 | |
| Subgaleal haematoma: 1 | |
| Wound infection: 1 | |
| Transient right third cranial nerve palsy: 1 | |
Our hospital's epilepsy surgery programme is safe and effective for the majority of patients, as shown by the successful outcomes recorded: 92.6% of patients presented improvements in seizure frequency and quality of life, with no severe complications.
Most patients underwent palliative surgery, specifically corpus callosotomy, which is widely used as a palliative treatment for generalised and multifocal epilepsy, and is particularly indicated for tonic and atonic seizures.20,21 Luat et al.25 report a > 50% reduction in seizure frequency in all patients undergoing the procedure, while Graham et al.26 report an average reduction of 58.6% in 88.2% of patients. Although seizure freedom is not the objective, some patients do achieve this: Iwasaki et al.27 report seizure freedom in 19% of patients, and Luat et al.25 report a rate of 35%.
The preference for complete or partial corpus callosotomy also merits discussion. According to various authors, partial corpus callosotomy prevents transient disconnection syndrome, with similar results for seizure control,26 whereas others report more favourable results with complete callosotomy. In a series of 113 patients, Shimuzu28 reports that atonic seizures resolved in 67% of patients undergoing anterior corpus callosotomy and 91% of those undergoing complete callosotomy.
In our series, patients undergoing callosotomy showed a mean 90% reduction in seizure frequency. In the 4 patients who initially underwent partial callosotomy, the expected outcome was not achieved, hence the decision to complete the callosotomy in a second procedure. Transient disconnection syndrome was observed in 11% of patients. According to these results, complete corpus callosotomy is associated with better outcomes, with a lower frequency of transient complications, at least in the paediatric population.
Surgery for super-refractory convulsive status epilepticus achieved the desired result (seizure freedom) in all 3 cases. Seizure freedom persisted in one of these patients, whereas in the other 2, subsequent progression was not favourable. Surgery is considered a treatment option for super-refractory status epilepticus after other potential treatments have been exhausted, and may be the only alternative for a condition associated with high morbidity and mortality rates.29–31
The available evidence on surgery for super-refractory status epilepticus is from small series and case reports; most cases are treated with resective surgery. Alexopoulos et al.32 present a series of 10 patients treated with hemispherectomy and lobectomy; status epilepticus resolved in all cases and 70% remained seizure-free. Schrader et al.33 report a series of 3 infants undergoing lobectomy, with status epilepticus resolving in all 3 (one remained seizure-free in the long term); Ng et al.34 report status epilepticus resolution with resective surgery in 5 patients. The literature includes reports of status epilepticus resolving with corpus callosotomy.35,36
Unlike adult patients undergoing epilepsy surgery, paediatric patients most frequently present extratemporal epilepsy, which typically has less satisfactory results.11 In a review of extratemporal resective procedures, including a total of 1259 patients from 36 studies, 56% of patients achieved seizure freedom, whereas surgery on the temporal lobe achieves an Engel class I outcome in 60%-100% of cases.11 Hirfanoglu et al.37 studied a series of 61 children and adolescents undergoing resective surgery, with 89% of those with temporal epilepsy and 50% of those with extratemporal epilepsy achieving Engel class I outcomes. Englot et al.38 report Engel class I outcomes in 76% of 110 patients treated with resective surgery, with better outcomes in patients with tumours and mesial temporal sclerosis and worse ones in patients with cortical dysplasia. Frontal localisation is also associated with less favourable outcomes.37 In patients with posterior quadrant epilepsy, various authors report that parieto-occipital or temporo-parieto-occipital disconnection achieve Engel class I outcomes in nearly all patients.39–41
Our series includes 2 cases of temporal resective surgery: one mesial temporal lobectomy and one lateral temporal lobectomy; outcome was rated Engel class I in both cases. Extratemporal surgery was performed on a patient with posterior quadrant epilepsy, who also achieved seizure freedom. These successful surgical outcomes are consistent with those expected for these procedures. No patient underwent resective surgery to the frontal lobe.
One patient, a 13-year-old girl with Rasmussen encephalitis, underwent hemispherotomy. She remained seizure-free for one year, subsequently developing sporadic focal motor seizures. In a recent series, Hoffman et al.42 report the cases of 13 patients treated surgically for Rasmussen encephalitis, with 63% remaining seizure-free and 100% showing a >50% reduction in seizure frequency. In an Italian series of 16 patients, Granata et al.43 achieved seizure freedom in 9 patients, with all the remaining patients presenting a considerable reduction in seizure frequency; the authors also report motor and cognitive improvements.43 In a series of 20 patients undergoing various forms of hemispheric surgery, Guan et al.44 report Engel class I outcomes in 16 patients; in a Brazilian study, 11 of 25 patients remained seizure-free.40 Hemispheric surgery continues to be the treatment of choice for these patients; the outcome observed in our patient is consistent with those reported in the literature.
The other 2 patients undergoing resective surgery had hypothalamic hamartomas. An anterior transcallosal interforniceal approach was used in one patient, who presented seizure freedom for the first 3 months, but subsequently died due to a disability associated with the surgery. In the other, we used a subtemporal approach, achieving an 80% reduction in seizure frequency and significant improvements in the patient's behaviour disorder and hypersexuality. The patient presented third cranial nerve palsy, which fully resolved within weeks. Seizures fully resolve in around 50% of patients undergoing open or endoscopic surgery45,46 but only in 40% of those undergoing radiosurgery.47 The treatment of choice for hypothalamic hamartoma is surgical extraction or disconnection, with several techniques available.48
Another therapeutic objective is to improve the quality of life of the patient and his/her parents, which often constitutes a key factor in the decision to opt for surgery. Our patients’ quality of life improved both in terms of CAVE score and CAVE classification. Various authors report significantly greater improvements in quality of life among patients with DRE who receive surgical treatment than among those continuing with pharmacological treatment alone40–52; some researchers attribute this improvement to seizure freedom,48,49 although it has been shown also to exist among patients with Engel class III outcomes.53 Quality of life among patients with epilepsy also depends on whether they present depression or adverse drug reactions.49,52,54 In our series, the 2 adolescent girls with temporal lobe epilepsy who underwent resective surgery both presented depression in the preoperative assessment, which resolved after the procedure.
One limitation of our study is the small number of patients undergoing resective surgery; however, the favourable outcomes support the use of this treatment.
Despite the fact that epilepsy surgery is a well established treatment, its relatively recent introduction in Ecuador has meant that many patients and physicians refuse this alternative due to a lack of knowledge or trust. Our findings may encourage healthcare professionals and patients’ families to opt for surgery.
This is the first study to be published on epilepsy surgery in Ecuador; our results show that our hospital's epilepsy surgery programme offers safe, satisfactory outcomes for the majority of patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fernández-Concepción O, López Jiménez M, Valencia-Calderón C, Calderón-Valdivieso A, Recasén-Linares A, Reyes-Haro L, et al. Efectividad y seguridad de la cirugía para la epilepsia en niños. Experiencia de un hospital terciario en Ecuador. Neurología. 2021;36:271–278.