Approximately 30% of patients with mesial temporal lobe epilepsy (MTLE) will develop drug resistance, which requires early surgical treatment. The success of the surgical procedure largely depends on the correct lateralisation of the epileptogenic zone, which can only be determined in 70% of patients with such conventional diagnostic tools as video electroencephalography and volumetric structural magnetic resonance imaging. We evaluated the performance of a memory functional magnetic resonance imaging (fMRI) paradigm in lateralising the epileptogenic zone in patients with drug-resistant MTLE.
MethodsWe included 18 patients with MTLE attended at the Instituto Neurológico Colombiano in Medellin (Colombia) between 2018 and 2019. The volume of functional activation in both temporal lobes was determined with a memory fMRI paradigm. A concordance analysis was performed to compare the performance of fMRI against that of conventional tests.
ResultsIn patients with left MTLE, lower total activation was found in the hemisphere ipsilateral to the epileptogenic zone as compared to the contralateral hemisphere (121.15 ± 16.48 voxels vs 170.23 ± 17.8 voxels [P < .001]), showing substantial concordance with conventional tests. Patients with right MTLE displayed lower hippocampal activation ipsilateral to the epileptogenic zone (18.5 ± 3.38 voxels vs 27.8 ± 3.77 voxels in the contralateral hippocampus [P = .048]), showing moderate concordance with conventional tests.
ConclusionsThese findings suggest that lower functional activation as determined by a memory fMRI paradigm has a high level of concordance with conventional tests for lateralising the epileptogenic zone in patients with drug-resistant MTLE.
Un 30% de los pacientes con epilepsia del lóbulo temporal mesial (ELTM) desarrollan farmacorresistencia, implicando la necesidad de intervención quirúrgica temprana. El éxito de esta depende de una adecuada lateralización de la zona epileptogénica (ZE), que con pruebas convencionales (video monitoreo electroencefalográfico y resonancia estructural volumétrica) solo se puede determinar en un 70% de los pacientes. Consecuentemente, evaluamos el desempeño de la resonancia magnética nuclear funcional (RMNf) bajo paradigma de memoria como herramienta para la lateralización de la ZE en pacientes con ELTM farmacorresistente.
MétodosSe incluyeron 18 pacientes con ELTM atendidos en el Instituto Neurológico Colombiano (INDEC) de Medellín en los años 2018–2019, a los cuales se les determinó el volumen de activación en estructuras de ambos lóbulos temporales con RMNf bajo paradigma de memoria, y se realizó un análisis de concordancia con pruebas convencionales.
ResultadosEn pacientes con ELTM izquierda se encontró una diferencia de activación total de 121.15 ± 16.48 ipsilateral a la ZE frente a 170.23 ± 17.8 vóxeles contralateral (p < 0.001), con una concordancia sustancial con pruebas convencionales. En pacientes con ELTM derecha se encontró una menor activación hipocampal ipsilateral a la ZE (18.5 ± 3.38 vóxeles vs 27.8 ± 3.77 vóxeles [p = 0.048]) con una concordancia moderada con pruebas convencionales.
ConclusionesEstos hallazgos sugieren que la disminución de la activación funcional en RMNf secundaria a un paradigma de memoria, tiene una concordancia alta con pruebas convencionales para la lateralización de la ZE en pacientes con ELTM farmacorresistente.
The International League Against Epilepsy (ILAE) defines epilepsy as a disorder of the brain characterised by an enduring predisposition to generate abnormal electrical activity, called epileptic seizures, with considerable neurobiological, cognitive, psychological, and social consequences.1
Mesial temporal lobe epilepsy (MTLE) is the most common clinical form of focal epilepsy, becoming drug-resistant in the long term in up to 30%–40% of patients.2–4 For this reason, epilepsy surgery is a highly important clinical treatment option; patients undergoing this procedure not only present a 58% increase in seizure-free time (vs 8% in patients receiving medical treatment only), but also have greater quality of life.5 Therefore, surgical treatment can largely prevent the cognitive impairment and the progressive loss of memory and language observed in patients with drug-resistant MTLE.5,6
While current evidence suggests better prognosis in patients undergoing surgery for MTLE,5,6 successful outcomes are dependent on correct lateralisation of the epileptogenic zone (EZ): success rates (defined by such outcome measures as 3-year seizure freedom) may be as high as 87% when the EZ is clearly identified,7 falling as low as 45% if lateralisation of the lesion zone is not possible.8 Due to this considerable reduction in the effectiveness of epilepsy surgery when EZ lateralisation is not determined, it is extremely important to establish which cortical hemisphere is responsible for seizure onset.
Functional MRI (fMRI) is a non-invasive imaging technique capable of identifying different neural networks by detecting differences in electromagnetic fields generated by changes in the oxyhaemoglobin/deoxyhaemoglobin ratio as a result of increases in neuronal metabolism in different brain regions in response to different paradigms.7,9,10 In this study, we used a memory paradigm to assess patients with drug-resistant MTLE, aiming to obtain complementary information on alterations in neuronal activation11; if these data are consistent with the findings of classical neuropsychological and neurophysiological testing, they may constitute useful complementary information for lateralisation of the EZ in presurgical evaluation.
MethodsStudy populationThis study was conducted in compliance with international ethical guidelines for biomedical research in human subjects. The institutional ethics committee for human research of Universidad CES approved the project (resolution 148 of 2016); the study was also approved by the ethics committee of the Neurological Institute of Colombia (INDEC, for its Spanish initials). All patients and volunteers gave written informed consent to participate in the study. Patients and volunteers were recruited at the Neurological Institute of Colombia in Medellín in the period 2018–2019.
We aimed to recruit a sample of 15 consecutive patients; sample size was calculated based on previous studies and the estimated number of patients with drug-resistant epilepsy undergoing epilepsy surgery at INDEC in an 18-month period.12 We analysed INDEC’s patient databases and prospectively included all adults aged under 65 years with a diagnosis of drug-resistant left or right MTLE established by the medical-surgical committee for epilepsy; with an intelligence quotient greater than 40 as determined with a standard neuropsychological test of 2 years’ validity; and for whom data were available from a volumetric T1-weighted MRI study (vMRI) study and 120-h video EEG study conducted in the last 2 years. Exclusion criteria were: inability to classify the patient in any of the presurgical studies; previous epilepsy surgery or central nervous system surgery; history of alcohol abuse, trigeminal neuralgia, claustrophobia, metallic implants, Parkinson’s disease, Alzheimer disease, migraine, schizophrenia, or dementia; infection or decompensation of an underlying disease at the time of the study; seizures in the 24 hours prior to the study; poor sleep the night prior to the study; use of sedatives other than antiepileptic treatment; and inconclusive fMRI findings. Overall, we recruited a total of 18 patients: 13 diagnosed with left MTLE and 5 with right MTLE. Table 1 presents sociodemographic data on the sample.
To control for information bias, we only included in the study data on patients for whom we had a high percentage of information from structural MRI and/or resting-state or language paradigm fMRI. Missing data were excluded from the analysis, and data were not imputed in any case. All tests were performed in the same place with the same equipment, protocols, and technicians. Finally, inclusion and exclusion criteria were rigorously applied to prevent selection bias.
Anatomical magnetic resonance imaging and determination of the memory network associated with the paradigmImaging methodsStudies were conducted in a 1.5 T Siemens MRI scanner. We obtained volumetric T1-weighted sequences with slices of 1 × 1 × 1 mm3. We initially delimited structural lesions compatible with MTLE, when present; subsequently, to establish neuronal activation, we acquired 240 volumes of 3 × 3 × 6 mm voxels, in dimensions of 64 × 64 × 15, 15 slices, repetition time (TR) of 2 seconds, using the memory paradigm described by Detre et al.12
Cognitive taskThe paradigm described by Detre et al.12 consists in 40-second blocks of complex visual scenes for activation; images were back-projected onto a mirror inside the scanner. Each scene was shown for 2 seconds, followed by 0.5 seconds of a black-and-white screen. For control images, we showed one of the target images, but with degraded light and colour content. Patients were shown a total of 128 images. Before the task, patients were instructed to memorise the images shown, with the exception of the control image, a photograph degraded using an algorithm applied in 10 000 iterations. After application of the paradigm, patients were evaluated to assess the efficiency of visual information encoding. Patients were shown a sequence of 32 images, including 8 targets and 24 distractors, and asked which images they had seen previously. All patients were able to complete the task. It should be noted that the study by Detre et al.12 found symmetrical activation in both temporal lobes.
Pre-processing of fMRI dataFunctional MRI data were analysed in MATLab version R2017a, using the Statistical Parametric Mapping (SPM) toolbox, version 8, with time correction,13 TR of 3, acquisition time (TA) of 2.8846, realignment (full width at half maximum [FWHM] of 5) discarding translations or rotations greater than 2 mm on 6 axes; smoothing was performed with a FWHM of 4 × 4 × 4. A linear regression model was used to correct for movement; we performed co-registration with a 7 × 7 Gaussian smoothing kernel, functional and structural registration check aligned to the anterior commissure (Montreal National Institute template), followed by segmentation in C1 (grey matter) and C2 (white matter), and subsequently smoothing at a FWHM of 7 × 7 × 7.
Post-processing of fMRI dataIn first-level specifications, the unit of analysis for the design was seconds, with an interval of 3 seconds, microtime of 16, and microtime onset of 1; the paradigm began at 30:60:300 (activation interval of 30 s up to 100 acquisitions); duration of 30 seconds; high-pass filtering of 120. The model was estimated using restricted maximum likelihood (ReML) with contrast variates of 1 and 0 for the activation and deactivation periods of the paradigm, with a threshold for significance of P = .05, using the Bonferroni correction for family-wise error (FWE).
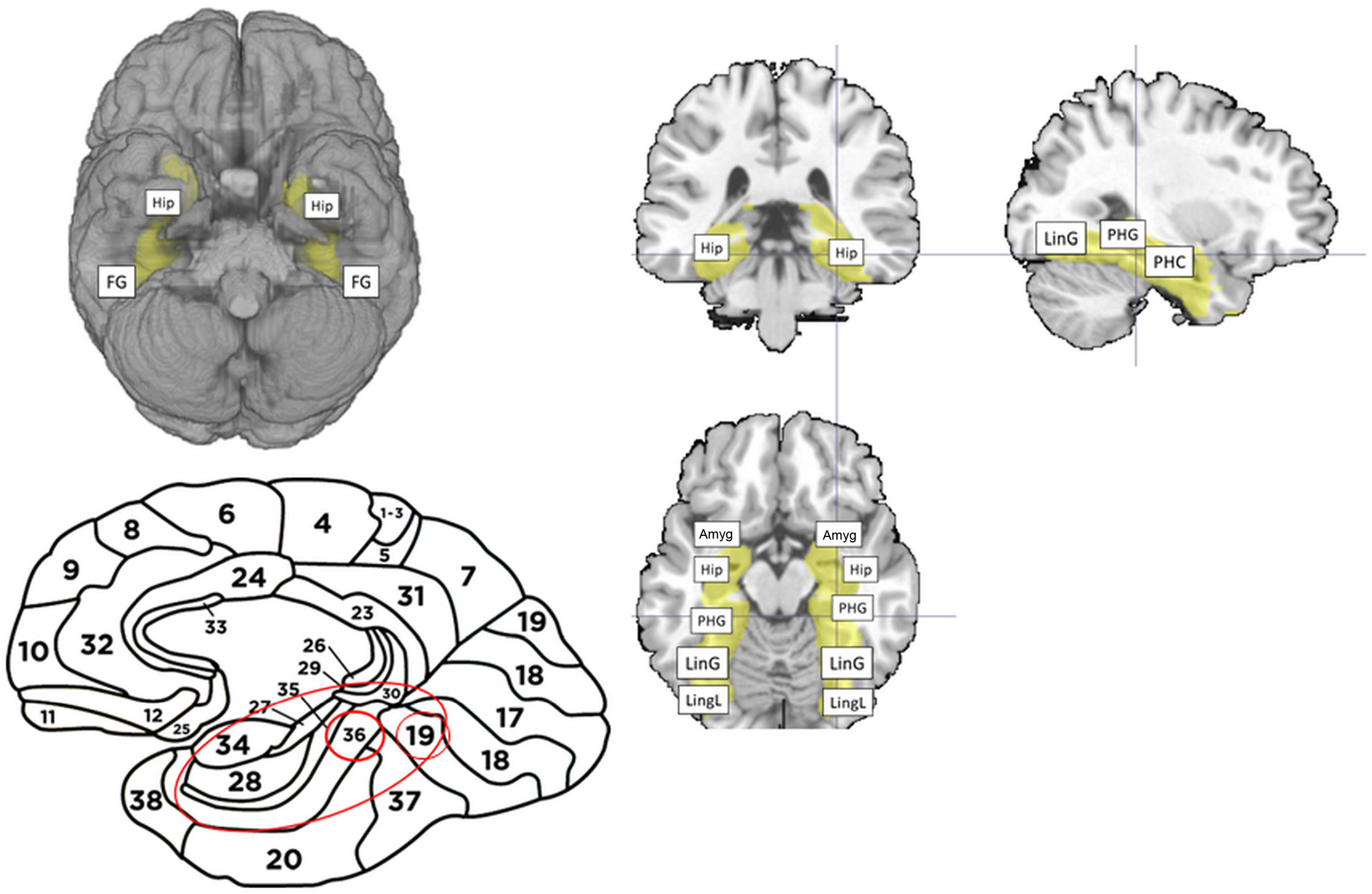
We applied inclusive masking of the regions of interest for this paradigm. The mask was normalised with the inverse matrix generated in the segmentation of each patient’s structural MRI study, and included the functional regions responsible for memory, according to the data reported by Detre et al.12 These regions of interest analysed with the paradigm are shown in Figure 1. Having obtained the activation map, we assumed clusters larger than 20 voxels and with FWE rates < 0.05 to be representative; this selection, based on the recommendations of Roiser et al.,14 is intended to reduce the presence of false negative or false positive results. We obtained mean total activation volumes ipsilateral (damaged) and contralateral (healthy) to the lesion and compared volumes using the t test (Epidat, version 3.0.1).
Brain areas used for inclusive masking in the memory paradigm. (A) Caudal projection of the brain, with activation of both hippocampi and fusiform gyri (FG). (B) Lateral projection of the brain, showing the Brodmann areas included in the analysis (B16, 16, 36, 37). (C) Top left: coronal slice showing the bilateral hippocampi included in the paradigm. Top right: sagittal slice showing the parahippocampal gyrus (PHG), parahippocampus (PHC), and lingual gyrus (LinG). Bottom: axial slice showing anatomical areas included in the memory mask. In anteroposterior order: amygdala (Amyg), hippocampus (Hip), parahippocampal gyrus (PHG), lingual gyrus (LinG), and lingual lobe (LingL).
Both groups (left and right MTLE) underwent a 120-h video EEG monitoring study at INDEC. EEG was performed according to the international standard protocol recommended in the guidelines of the American Clinical Neurophysiology Society. Surface electrodes were placed according to the 10–20 system. Anterior temporal electrodes were recorded using a 32-channel Cadwell EEG system; simultaneously, the entire duration of the study was filmed, with the patient both awake and sleeping. We also added channels for electrocardiography, electro-oculography, and electromyography (surface electrodes on chin and both deltoid muscles). We applied activation techniques including hyperventilation, photostimulation, and sleep deprivation. To lateralise the irritative zone, we suspended antiepileptic treatment prior to monitoring and used ictal/interictal EEG data (only 3 of 13 patients with left MTLE did not present seizures).
Concordance analysisTo analyse concordance between activation volume under the memory paradigm and the findings of neurophysiological and neuroimaging studies, we used the formulae for concordance testing and the kappa index described by Abraira.15 This analysis includes determination of absolute agreement and expected agreement for use in calculating the kappa index; this approach is widely used and broadly accepted in the field of diagnostic testing, as it excludes the influence of random chance in the results of these tests. Concordance and kappa index values calculated for agreement between presence of lesions on the structural MRI and the irritative zone identified in video EEG were used as a reference, as these are the tests routinely used in clinical practice. Subsequently, we determined concordance and Kappa index values between alterations in activation volume overall and in the regions of interest, and the lesions observed in structural MRI findings and video EEG monitoring.
ResultsInterhemispheric differences in activation in left mesial temporal lobe epilepsyIn patients with left MTLE, the left memory network activated under the memory paradigm presented considerable differences with respect to the contralesional hemisphere in total volume of functional activation, and in specific regions of interest in this network, such as the hippocampus (Fig. 2). Total activation volume was 121.15 ± 16.48 voxels (3 × 3 × 6 mm) in the left hemisphere and 170.23 ± 17.8 voxels in the right (P < .001), a difference of 49.08 voxels. Activation volume in the parahippocampal gyrus was 42.66 ± 7.64 voxels on the left side and 58.07 ± 9.59 on the right (P = .008). Activation volume in the hippocampus was 22.3 ± 3.21 voxels on the left side and 30.41 ± 4.9 on the right (P = .018), with the parahippocampus showing values of 21.7 ± 3.1 voxels on the left side and 28.08 ± 3.51 on the right (P = .014) (Fig. 3).
Brain activation of a patient with left mesial temporal lobe epilepsy under the memory paradigm. (A) Three projections from the activation map obtained using the memory paradigm: sagittal, coronal, and axial planes, respectively. The higher P-values (darker tone) in voxels in the right hemisphere (red arrow) represent greater levels of activation. (B) The same projections, with the voxels activated by the memory paradigm; colour values closer to yellow represent higher levels of activation.
Memory network in patients with left mesial temporal lobe epilepsy. (A) Mean activation (in voxels) of the memory network of each hemisphere in a population of patients with left mesial temporal lobe epilepsy under the memory paradigm. From left to right: total activation (Total act.) and activation in the parahippocampal gyrus (PHCG), hippocampus (HIP), and parahippocampus (PHIP); reduced activation was observed on the left side in all regions. (B) Coronal slice showing a schematic representation summarising hypoactivation in total left hemisphere connectivity (yellow shading), the hippocampus (HIP), and parahippocampus (PHC). (C) Axial slice showing a schematic representation of hypoactivation of the left parahippocampal gyrus (PHG).
The interhemispheric activation asymmetry values obtained under the memory paradigm, resulting from reduced total and hippocampal functional activation ipsilateral to the lesion with respect to the contralateral side, were 71.16% and 73.3%, respectively.
Patients with left MTLE and presenting normal MRI findings (n = 4) displayed lower activation in all cases, with an average 38% decrease in total functional activation under the memory paradigm in the mesial temporal lobe ipsilateral to the irritative zone identified in video EEG, compared to the contralateral side; for the ipsilateral hippocampus and parahippocampus, we observed reductions of 38% and 18%, respectively.
Interhemispheric differences in activation in right mesial temporal lobe epilepsyUnder the memory paradigm, patients with right MTLE presented total functional activation volumes of 103.8 ± 22.59 voxels (3 × 3 × 6 mm) in the left hemisphere and 104.2 ± 22.06 in the right (P = 0.48). Activation volume in the parahippocampal gyrus was 54.6 ± 8.9 voxels on the left side and 46 ± 11.5 on the right (P = .08). The hippocampus was the only region of interest displaying significant differences in activation volume under the memory paradigm: 27.8 ± 3.77 voxels in the left hippocampus and 18.5 ± 3.38 in the right (P = .048) (Fig. 4).
Memory network in patients with right mesial temporal lobe epilepsy. A) Mean activation (in voxels) of the memory network of each hemisphere in a population of patients with right mesial temporal lobe epilepsy under the memory paradigm. From left to right: total activation (Total act.) and activation in the parahippocampal gyrus (PHCG), hippocampus (HIP), and parahippocampus (PHIP); the only region showing hypoactivation was the right hippocampus. B) Coronal slice showing a schematic representation summarising hypoactivation (purple shading) in the left hippocampus (HIP). PHC: parahippocampus.
The interhemispheric activation asymmetry values observed in the memory paradigm, resulting from reduced hippocampal functional activation ipsilateral to the lesion with respect to the contralateral side, was 66.5% (hippocampal volume) in patients with right MTLE.
Only one patient in the right MTLE group presented normal structural MRI findings. In this patient, we observed a 9% reduction in total activation volume in the mesial temporal lobe ipsilateral to the irritative zone identified with video EEG, compared to the contralateral hemisphere, with reductions of 66% and 47% in hippocampal and parahippocampal activation volume, respectively.
Concordance between left structural lesions and functional activation network under the memory paradigm in left mesial temporal lobe epilepsyFor all relevant findings in each network in patients with left MTLE, concordance analysis was used to determine their usefulness in lateralising the epileptogenic focus. The first concordance analysis assessed the agreement between structural MRI lesions (considered the presurgical gold standard) and 72-h video EEG. This analysis revealed strong agreement (0.913), with a kappa index of 0.70, considered substantial or good. In 10 of the 13 patients with left MTLE (76.9%) undergoing fMRI with the memory paradigm, we observed a reduction in left hippocampal functional activation, with a concordance value of 0.916 for the lesion observed with vMRI and a kappa index of 0.75, considered substantial or good. Furthermore, 12 of the 13 patients (92.3%) presented reduced total functional activation, with a concordance value of 0.917 with the vMRI lesion, and a kappa index of 0.62, considered substantial or good.
In this study, 4 patients with left MTLE did not present structural MRI findings of hippocampal sclerosis; as a result, it was not possible to calculate concordance between presence of a lesion on MRI and alterations in activation volume under the memory paradigm. Therefore, in this patient subgroup, we calculated concordance between left hippocampal activation and the irritative zone identified in video EEG, observing reduced left hippocampal activation in 3 (75%); the calculated concordance value was 0.75, with a kappa index of 0.68, considered substantial or good.
Concordance between right structural lesions and functional activation network under the memory paradigm in right mesial temporal lobe epilepsyThe first concordance analysis, between the tests routinely applied in clinical practice, evaluates agreement between right temporal lobe lesions in the structural MRI study and findings from the 72-h video EEG. This analysis revealed strong agreement (0.888), with a kappa index of 0.76, considered substantial or good. For this group, analysis of concordance between right hippocampal activation and MRI lesions in the right temporal lobe, despite not surpassing the values observed for the tests used in routine practice, was satisfactory, with 4 of 5 patients (80%) showing decreased right hippocampal activation under the memory paradigm, with a concordance value of 0.8 and a kappa index of 0.57, considered moderate. All patients with right MTLE presented lesions in the vMRI study.
DiscussionFunctional MRI has traditionally been used to lateralise memory function with a view to determining the consequences of a surgical procedure16; it is also important to establish whether the reorganisation of neural networks during the functional activation involved in memory processes contributes valuable information on the lateralisation of the epileptogenic focus in temporal lobe epilepsy.17 Our study demonstrates the existence of interhemispheric differences in functional activation volume under the fMRI memory paradigm, in both total and hippocampal activation in patients with drug-resistant left MTLE and in hippocampal activation in patients with drug-resistant right MTLE. Both findings showed good concordance with conventional studies.
Studies have shown that memory networks may be redistributed in 2 ways, depending on disease progression time.17 In the first distribution pattern in patients with short progression times (< 7 years), increased activation is observed ipsilateral to the lesion, with reduced interhemispheric activation. In the second form, in patients with drug-resistant epilepsy and longer progression times (> 7 years), activation is reduced in ipsilesional networks, with a compensatory increase in the contralateral hemisphere.17 In our patients, with both groups presenting progression times greater than 7 years, the network distribution we observed was consistent with that described by Morgan et al.17 in patients with longer disease duration: application of the memory paradigm found decreased functional activation ipsilateral to the lesion.
The interhemispheric asymmetry observed in activation volumes, as a result of decreased total or hippocampal functional activation ipsilateral to the lesion under the memory paradigm, are similar to those described in the literature, with reported values up to 81.1%–90%.12,18,19 These asymmetries are consistent both in patients with hippocampal sclerosis and in those with other types of neocortical lesions, but are more marked in the former group.18 Temporomesial hypoactivation ipsilateral to the lesion is also associated with damage to the parahippocampal region, a key area in epileptogenesis, which connects the hippocampus with limbic structures and other cortical regions.18 Furthermore, the persistent decrease in total, hippocampal, and parahippocampal activation ipsilateral to the EZ in patients with normal structural MRI findings suggests that these findings cannot be attributed to loss of volume.
In the light of all this evidence, fMRI memory paradigms offer multiple advantages and are highly valuable in lateralising the EZ for several reasons, for instance in clinical situations in which drug-resistant left MTLE is suspected in patients with no evidence of a structural lesion: reduced total or hippocampal activation on the left side would constitute supporting evidence, or even a warning sign if it is absent. Finally, we acknowledge that the convenience sampling method used in this study resulted in a small sample of patients with right MTLE, although results were promising nonetheless. Therefore, there is a need for a larger study with greater sample sizes and longer follow-up periods; it is especially important also to include patients undergoing surgery in the context of negative vMRI findings.
ConclusionsOur findings show that, in the patients studied, protocols analysing resting-state and memory paradigm functional activation may help to lateralise the epileptogenic focus, with strong concordance with such routine tests as vMRI and video EEG. Therefore, we believe that these alterations in temporal activation contribute potentially important complementary information that may be highly valuable in lateralising the epileptogenic focus in the presurgical assessment of patients with drug-resistant MTLE, both in simpler clinical scenarios in which structural MRI identifies a lesion, and in more complex situations where no such lesion is detected in that study.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThis study was funded by Universidad CES(generational changeover grant) and the Neurological Institute of Colombia (project code 270116).
The authors thank the Universidad CES medical school, the graduates’ school, and the Neurological Institute of Colombia for supporting us with the tools needed to conduct this research.