Understanding the social and economic impact of Parkinson's disease is essential for resource planning and raising social awareness.
DevelopmentResearchers reviewed the data published to date on epidemiology, morbidity and mortality, dependency, and economic impact of Parkinson's disease in Spain. In addition, a study has been carried out in order to define the public and private health care resources of Spanish patients affected by Parkinson's disease by means of an e-mail survey of all neurologists specialising in this disease and belonging to the Spanish Society of Neurology's study group for movement disorders.
ConclusionsThe incidence and prevalence rates of Parkinson's disease in Spain are similar to those in the rest of Europe. According to current population estimates, there are at least 300 000 patients with Parkinson's disease and one new case per 10 000 habitants per year in Spain. This has a major impact on the patient's quality of life and nearly doubles patient mortality. In addition, the disease generates sizeable costs for the country that may exceed 17 000€ per year per patient; costs will rise due to the ageing of the population and the new therapies employed. Healthcare professionals and administrators dedicate their efforts to providing quality care to patients. Despite the above, we still have a long way to go in order to provide quality, efficient, multidisciplinary, and universal healthcare.
Conocer el alcance socieoeconómico de la enfermedad de Parkinson es esencial para la planificación de recursos y concienciación social.
DesarrolloSe ha realizado una revisión de los datos publicados hasta el momento sobre epidemiología, morbimortalidad, dependencia e impacto económico de la enfermedad de Parkinson en España. Además se ha llevado a cabo un estudio cuyo objetivo principal ha sido definir los recursos asistenciales públicos y privados que tienen los afectados por la enfermedad de Parkinson en nuestro país mediante una encuesta por mail a todos los neurólogos con dedicación especial a esta enfermedad, pertenecientes al grupo de Trastornos del Movimiento de la Sociedad Española de Neurología.
ConclusionesLa enfermedad de Parkinson en España tiene una incidencia y prevalencia similar al resto de Europa. Con la estimación de población actual se obtiene que debe haber en España al menos 300.000 pacientes con enfermedad de Parkinson y a al menos un nuevo caso por 10.000 habitantes año. Esta produce gran impacto en la calidad de vida del paciente y aumenta a casi el doble la mortalidad de los pacientes. Además supone un coste económico muy importante para el país, que puede llegar hasta más de 17.000 € anuales por paciente y que con el envejecimiento de la población y las nuevas terapias va a ir incrementándose. Los profesionales y administraciones realizan un gran esfuerzo para proporcionar una asistencia de calidad a los pacientes. A pesar de ello es mucho el camino que nos queda por recorrer para que una asistencia de calidad, eficaz y multidisciplinar sea universal para todos los pacientes con esta enfermedad.
The Spanish Foundation for the Brain was created to raise social awareness of neurological diseases and present the most accurate information to patients, family members, and non-neurological healthcare workers. It also aims to educate these groups, the media, social agents, and society at large not only about scientific topics, but also about health-related, social, professional, and family issues. In compliance with some of these objectives, the Foundation of the Brain prepares reports on the societal impact of different neurological diseases in Spain.
In our setting, Parkinson's disease is the second most frequent neurodegenerative disease after Alzheimer disease.1 It has a sizeable impact on the quality of life of both patients and carers from time of diagnosis. Society and health authorities alike are becoming increasingly aware of the need for quality specialist care for these patients, since patient management in such cases is complex and the treatment provided is crucial and may even change the prognosis over the long term.
With this in mind, the Foundation of the Brain has completed a report on the social implications of PD in which it presents what we know about the disease's epidemiology, morbidity and mortality, and economic impact, as well as care for these patients in Spain.
DevelopmentEpidemiology of Parkinson's disease in SpainEstimates of the incidence and prevalence of degenerative diseases, such as PD, are important not only as epidemiological information, but also in resource planning. Prevalence and incidence estimates for PD worldwide vary from study to study. This is mainly due to methodological differences, but genetic and environmental factors are also present in the different populations that have been studied. From a methodological viewpoint, the main differences are due to differing diagnostic criteria, the age of the study population, and the case selection methods.
PD is diagnosed clinically since it has no biomarkers.2 The most commonly used clinical criteria were drawn up by the United Kingdom Brain Bank.3 While clinicopathological validation studies are scarce, one series of 100 patients clinically diagnosed with PD4 found that only 75 met pathology criteria for that disease. Another more recent study5 found a better ratio at 72:79, indicating improved awareness among doctors performing the diagnosis. Out of all clinical data, asymmetry and response to levodopa are the most discriminatory in a differential diagnosis of PD and other types of parkinsonism.6‘Door-to-door’ studies provide the best methodology for identifying cases; in these studies, all individuals pertaining to a given population, or more commonly a representative sample, are examined one by one to determine if they have the disease. The most important epidemiological data are generated by door-to-door studies, which include such large longitudinal epidemiological studies as the Framingham study, the East Boston study, and EURODEM.
Incidence studies, in addition to being helpful for resource planning, teach us about the natural history of diseases. Sex-based or age-based variations in disease incidence in a certain place also let us study the environmental or other factors that may affect that disease.
Compared to prevalence studies (Table 1), few incidence studies have been carried out. The worldwide incidence of PD is thought to vary between 1.5 and 22 patients/100000 population/year.1 Twelves et al.,7 in their systematic review of all incidence studies performed around the world as of December 2001, concluded that the annual incidence rate extracted from the best-designed studies with comparable methodology came to 17/100000 population/year, although this was probably underestimated. Peak incidence was observed in individuals aged 70 to 79 years (probably biased by how difficult it is to diagnose patients with very late-onset PD); data were contradictory with regard to incidence by sex, although some studies showed a slight male predominance. The main conclusion was that studies with a better design are necessary for calculating PD incidence. An earlier systematic review by De Pedro-Cuesta and Stawiarz8 presents similar conclusions. These researchers feel that methods vary considerably between incidence studies, which limits the validity of comparisons between them. They also believe these studies underestimate the real numbers since many are based on diagnostic records and overlook patients who do not see a doctor.
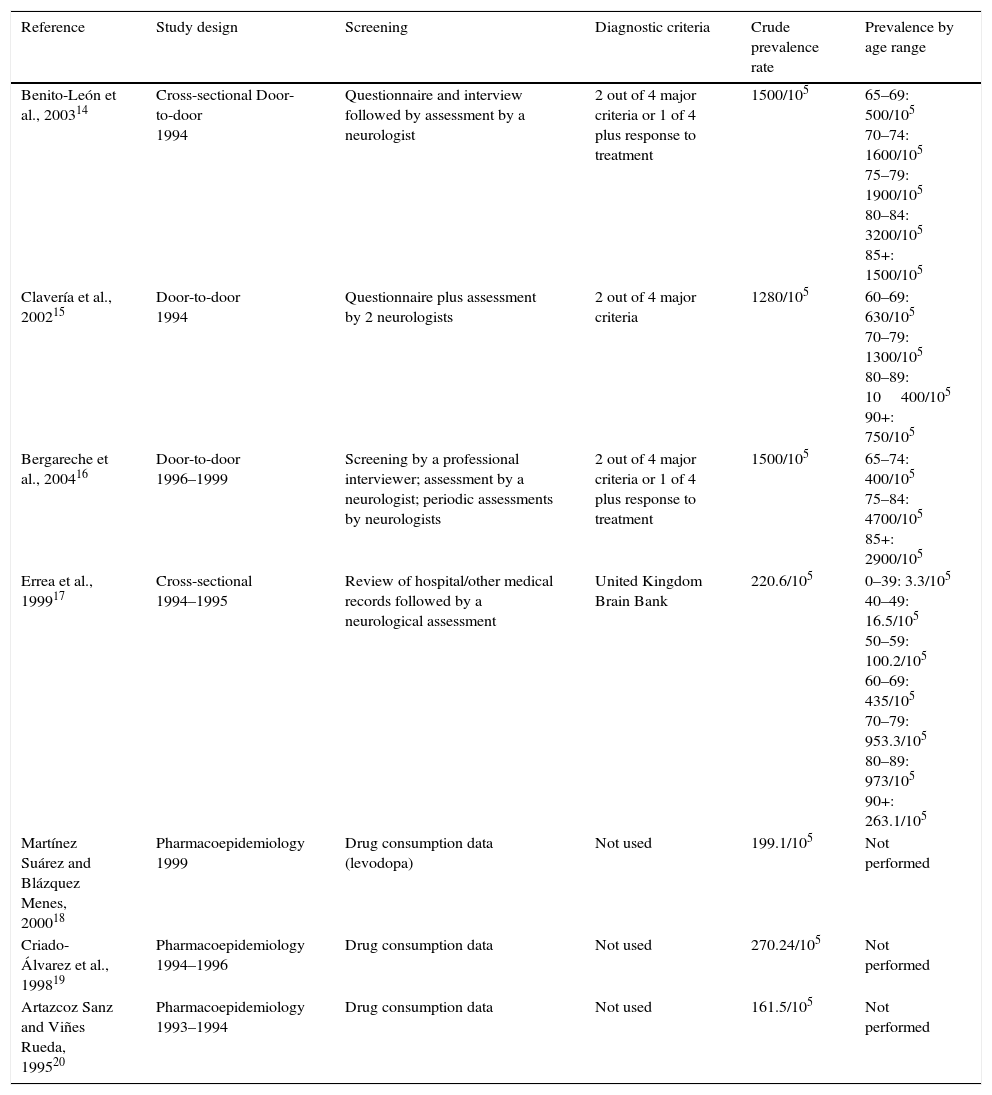
Prevalence studies of Parkinson's disease in Spain.
| Reference | Study design | Screening | Diagnostic criteria | Crude prevalence rate | Prevalence by age range |
|---|---|---|---|---|---|
| Benito-León et al., 200314 | Cross-sectional Door-to-door 1994 | Questionnaire and interview followed by assessment by a neurologist | 2 out of 4 major criteria or 1 of 4 plus response to treatment | 1500/105 | 65–69: 500/105 70–74: 1600/105 75–79: 1900/105 80–84: 3200/105 85+: 1500/105 |
| Clavería et al., 200215 | Door-to-door 1994 | Questionnaire plus assessment by 2 neurologists | 2 out of 4 major criteria | 1280/105 | 60–69: 630/105 70–79: 1300/105 80–89: 10400/105 90+: 750/105 |
| Bergareche et al., 200416 | Door-to-door 1996–1999 | Screening by a professional interviewer; assessment by a neurologist; periodic assessments by neurologists | 2 out of 4 major criteria or 1 of 4 plus response to treatment | 1500/105 | 65–74: 400/105 75–84: 4700/105 85+: 2900/105 |
| Errea et al., 199917 | Cross-sectional 1994–1995 | Review of hospital/other medical records followed by a neurological assessment | United Kingdom Brain Bank | 220.6/105 | 0–39: 3.3/105 40–49: 16.5/105 50–59: 100.2/105 60–69: 435/105 70–79: 953.3/105 80–89: 973/105 90+: 263.1/105 |
| Martínez Suárez and Blázquez Menes, 200018 | Pharmacoepidemiology 1999 | Drug consumption data (levodopa) | Not used | 199.1/105 | Not performed |
| Criado-Álvarez et al., 199819 | Pharmacoepidemiology 1994–1996 | Drug consumption data | Not used | 270.24/105 | Not performed |
| Artazcoz Sanz and Viñes Rueda, 199520 | Pharmacoepidemiology 1993–1994 | Drug consumption data | Not used | 161.5/105 | Not performed |
In Europe, incidence figures range from 9 to 22 cases per 100000 population/year.9 Two incidence studies have been performed in Spain. The first was completed by Viñes et al.10 in Navarre using a population register of cases diagnosed in 1994 and 1995. This information had been obtained by neurologists, general practitioners, and doctors at residential care centres. Patients identified in this register were later assessed by a participating neurologist who confirmed the diagnosis according to the Ward and Gibb criteria. The study determined a PD incidence of 8.2/100000 population/year. A higher incidence rate was observed for men (10.2/105 population/year) than for women (4.02/105 population/year). The highest incidence among men is seen between the ages of 70 and 74; in contrast, incidence in women increases with age until the 85-year mark.
The second incidence study was performed in the NEDICES cohort.11 The method used in this study was as follows: after a PD prevalence study, the cohort declared without parkinsonism was monitored for 3 years. These individuals then underwent preliminary screening to detect PD, followed by a neurological examination to confirm the disease. Researchers followed the PD criteria put forth by the United Kingdom Brain Bank. The resulting adjusted incidence of PD for patients aged 65 to 85 was 186.8/105 population/year. While incidence increases with age in men, it decreases in women older than 79. For this study population, the calculated risk of PD was 2.55 (CI 1.21–5.37). Incidence rates determined by this epidemiology study design are higher than those found using other methods. Furthermore, many diagnosed patients confirmed that they had never received neurological care. The many limitations of this study include having used only patients older than 65 and not having performed the neuroimaging studies that would in some cases have ruled out vascular parkinsonism.
Epidemiology studies of PD prevalence are much more common than incidence studies. They have been completed in many countries around the world, but their results differ greatly. Likewise, the methodology also shows considerable variation. There are 3 main types: community or clinical register-based studies; pharmacoepidemiology studies (those based on drug consumption); and door-to-door studies, which have the advantage of being able to identify patients who do not visit medical centres.12
Door-to-door studies followed by clinical examination of suspected cases probably offer the most reliable results, and they depict worldwide prevalence as ranging between 167 and 5703 per 100000 population. Studies based on clinical registers, hospital records, etc. are less strict, and their numbers point to a worldwide prevalence of 100 to 300 cases per 100000 population. We find many differences in the data from studies performed in different countries, especially between studies from Europe and the United States and those from Africa, Asia, and South America. These discrepancies are probably due to variations in life expectancy and in the methods used, but genetic or environmental factors cannot be ruled out.2
Based on prevalence studies published as of 2007, Dorsey et al.13 estimated the number of PD patients in Western Europe and the 10 most populous countries in the world in 2005 as between 4.1 and 4.6 million. Their estimate for the year 2030 is more than double at 8.7 to 9.3 million.
Mean PD prevalence is estimated at 108 to 207/105 in Europe.9 The prevalence of sporadic PD increases in older cohorts, although it tends to decrease in those over 80 due to comorbidities and lack of response to levodopa for other reasons. There is no consensus regarding whether prevalence is higher in men than in women.
Numerous prevalence studies have been carried out in Spain. Results obtained range from 150 to 1500/105 population. Mean prevalence of PD in Spain is 682.2/105 (CI: 127.4/105-1491.7/105). Table 1 presents the methodological characteristics and results of each of these studies. In addition to the studies listed in Table 1, we also find some door-to-door studies. In 2 cases, only the abstract from an oral presentation is available (studies by López et al. and Acosta et al.). The EUROPARKINSON study21 included data from 2 locations in Spain (Gerona and Pamplona). Here, patients were initially selected using a clinical interview followed by an examination in which a neurologist diagnosed the disease based on finding 2 of 4 key criteria, or 1 of the 4 plus a response to treatment. This yielded a prevalence of 700 to 1100/105 population/year.
Many of the prevalence studies completed in Spain have clear methodological limitations, but they provide the only information available on PD in our setting. Furthermore, we should be mindful that these studies are very costly in terms of both time and money, and they are often underrated.
Spanish studies show a marked increase in prevalence after age 70, with decreasing prevalence in older cohorts that is probably due to patient mortality. Only the study by Errea et al.17 examines patients younger than 60; these researchers observed a PD prevalence of 3.3/105 in subjects under 40, and a prevalence of 16.5/105 in those under 50. In addition to those described above, we find a cross-sectional retrospective multi-centre study showing that PD onset before the age of 40 was predominant in men in urban settings.22
Regarding prevalence by sex, the study by Benito-León et al.14 found prevalence to be higher in men in all age ranges studied except for the age group between 80 and 85 years. Bergareche et al.16 calculated a PD prevalence of 1.3% in men vs 1.6% in women. Clavería et al.15 also pointed to higher prevalence rates in men than in women, with a ratio of 1.7:1. According to Errea et al.,17 age-adjusted prevalence in men is also higher than that in women, with a ratio of 1.2:1. While this tendency seems to arise in Spanish studies, data from other European countries do not support it.
In the study by Benito-León et al.,14 more than 60% of the patients were in Hoehn and Yahr stages I and II; some 25% were in stages IV and V. In the study by Clavería et al.,15 55% of the patients were in Hoehn and Yahr stages I and II, 15% were in stages IV, and none were in stage V.
The aetiology of sporadic PD remains unknown. Environmental and genetic factors contribute to its physiopathogenesis. PD cases that are genetic in origin account for 10% of all patients with PD. A mutation in LRRK2 is the most frequent cause of PD known to date; it may account for up to 40% of the genetic forms of PD (in Asia and northern Africa), and up to 2% of the sporadic forms.23 The most frequent pathogenic mutation is LRRK2 G2019S, and researchers have determined that it varies greatly depending on the region of study. It accounts for 0.5% to 2% of sporadic cases of PD, and 5% of the familial cases, in Europe and the United States.24 This mutation is present in 3.82% of PD patients in the Basque population, which is somewhat lower than the European mean.25,26 In Cantabria,27 this mutation accounts for 8.7% of all PD cases and it has reduced penetrance (47% at 80 years). A case series found the same mutation in just a few patients in Extremadura28 (22% of familial cases, no sporadic cases). The frequency of the mutation in sporadic PD cases in Seville29 was determined to be 1.7%. The LRRK2 R1441G mutation was first identified in Basque families, and it is responsible for 46% of familial PD cases and 2.5% of sporadic PD cases in the Basque population; penetrance is 12.5% at the age of 65 and 83.4% at the age of 80.26 In a study carried out in Catalonia,30 the G2019S mutation has been found in 6.4% of patients with familial PD, and 3.4% of those with sporadic PD. These data are similar to those from other regions of Europe. The R1441G mutation that is so common in the Basque population figures only as a rare cause of PD in the Catalan study; it was present in 0.7% of the total PD patients.
Morbidity, mortality, and dependency in Parkinson's disease in SpainMorbidityMorbidity is defined as the percentage of individuals who contract an illness in a specific time and place, but from an epidemiological standpoint, the concept can be applied to the study and measurement of the presence of a specific disease and its effects on a population. In the case of PD, we can define 2 perspectives on quantifying these effects.
- -
Firstly, overall effects on the population. The concept of ‘burden of disease’ has acquired vital importance as part of recent analyses of this perspective.
- -
Secondly, specific effects on a given patient. The concepts best describing all types of impact of this disease on the patient are quality of life, defined as an individual's perceived position in life within the context of his/her culture and value system, and health-related quality of life, including physical and mental health and their consequences. Since PD is studied within the biopsychosocial model, analysing how all medical and social interventions directed at patients affects their quality of life has become increasingly important.
Spanish publications began referring to these concepts in the 1990s,31 beginning with the article by Martínez-Martín32 on quality of life in PD and the development of such tools as the Parkinson's Disease Questionnaire Spanish version (PDQ-39).33–35 We stress that quality of life is a concept applying to the patient's self-evaluation of the impact of his/her disease. There are many reasons for diminished quality of life in patients with PD, including reduced mobility, falls, motor complications, affective disorders, and sleep disorders. Since many of these aspects go unnoticed in routine clinical assessments, doctors require instruments specifically intended to measure quality of life that can be applied systematically. With this end in mind, a pilot study and a multi-centre study concluded that the Spanish version of the PDQ-39 was valid and consistent as an instrument for the assessment of physical, emotional, and psychosocial aspects of quality of life in patients with PD.
Numerous publications have also studied the impact of surgical treatment for PD on health-related quality of life. A study of 11 patients undergoing pallidotomy36 and in which quality of life was measured with the PDQ-39 found a statistically significant improvement in the global index as well as in 4 of its aspects of well-being: mobility, activities of daily living, emotional well-being, and bodily discomfort. Another study in 17 patients who underwent bilateral stimulation of the subthalamic nucleus37 analysed the impact of this treatment on quality of life using the Spanish-language version of the PDQ-39. It found statistically significant improvement on its summary index and on the dimensions for mobility and activities of daily living. Benefits were less marked for other dimensions, such as bodily discomfort, emotional well-being, and stigma; none were detected for the rest. A third study38 examined the changes in the PDQ-39 score in 14 patients a year after they underwent subthalamic stimulation, as well as 2 years after the procedure in 11 of those patients. The significant improvement in the questionnaire's summary score remained stable during this time, and carers’ quality of life also improved. In light of these results, these authors highlight the importance of evaluating quality of life as part of the pre- and post-surgical assessment for patients with PD.
Using a sample of 158 patients with PD referred to a specialised unit, Cubo et al.39 determined that educational and psychological factors affect quality of life more than physical factors. Specifically, low educational level, memory complaints, and psychotic symptoms are associated with poorer quality of life; along with depression, parts I and II of the Unified Parkinson's Disease Rating Scale (UPDRS) and educational level are the most important predictors of variation in scores on quality of life questionnaires.
In a 2006 review article,40 Martínez-Martín mentions the paucity of data on the influence of cognitive impairment on health-related quality of life in PD,41,42 pointing out that the typical measures for health-related quality of life may not be valid for patients with cognitive impairment or dementia.43–45
Regarding symptoms of the disease and their impact on quality of life, one study in 110 patients46 analysed the correlation between years living with the disease and PDQ-39 with the UPDRS score. These researchers identified 3 variables that account for 51% of the variability in the PDQ-39: cognitive state, gait alterations, and complications of treatment with dopaminergic drugs. In another study,47 an isokinetic dynamometer was used to assess axial rigidity in 36 patients and correlated findings with PD severity, number of years since diagnosis, functioning, and health-related quality of life. The authors concluded that axial rigidity affected quality of life because increased rigidity was associated with greater disability for trunk movements, an increase in perceived stigma, and poorer cognitive function.
A presentation published in 2010 addressed non-motor symptoms,48 focusing on their prevalence, rate of underdiagnosis, and impact on quality of life. These symptoms are the leading cause of morbidity and the main reason for institutionalisation and hospitalisation of patients with PD. The authors also stressed that numerous scales had been developed to detect and measure these symptoms, including quality of life scales. A prospective study focusing on non-motor symptoms such as pain, and including 159 PD patients assessed in a movement disorders unit,49 found a very high prevalence of pain in the sample (72.3%). They also determined that this symptom behaved as an independent predictor of poorer quality of life measured with the PDQ-39, and of reduced autonomy on the Schwab and England scale; it was also associated with depression and increased stress and burden on the part of the carer.
We should also highlight the 2006 launch of ELEP (in Spanish, longitudinal study of patients with Parkinson's disease).50 This study is Spain's contribution to the international project SCOPA-Propark, which is also supported by Instituto Carlos III and its intramural research programme. ELEP is a national multi-centre longitudinal and observational follow-up study over the long term (6 years). It includes 320 patients who undergo cross-sectional evaluations every year during the study period. ELEP is also part of the Spanish Consortium on PD, together with the VIP project,51 which is a multi-purpose patient cohort focusing on the collection of biological samples and the development of genetic and neuroimaging research. This project has 2 angles:
- -
Applying the assessment methods developed in the first phase of this project — Scales for Outcomes in Parkinson's Disease (SCOPA) — which together constitute a full PD evaluation system.
- -
Using these assessment systems to obtain data that increase knowledge about PD evolution in the long term. Until now, this knowledge has been limited by a lack of protocolised, systematic studies using the right instruments.
The ELEP group has already presented numerous publications on the validation of PD scales and how they relate to quality of life,52–56 and others on the diverse clinical features of PD.57–60 It has also found that severity of the disease, motor disability, and motor complications have a larger impact on the direct costs of PD than do non-motor symptoms; also, age and limits on activities of daily living are the main predictors of giving up driving among patients with parkinsonism.58
MortalityIn 1967, Hoehn and Yahr published the first study to examine mortality in a population with parkinsonism.60 This retrospective study found a standardised mortality rate of 2.9, which is more than double that occurring in an age-matched control population. Since then, the international literature has featured multiple studies generating a wealth of statistics on mortality in PD; in general, these articles point to higher mortality.
Obtaining mortality data for study purposes is possible in Spain by accessing the National Statistics Institute data61 on deaths broken down by cause of death and coded according to the ICD-10 classification system. In any case, finding this information is only possible if the death certificate states the diagnosis of PD, and this is not the case for a variable percentage of certificates which may be quite high according to different international studies.62–64
Especially noteworthy are the publications on PD mortality in Spain; an example would be the study by Burguera et al.65 from 1992 that analysed PD mortality in Spain between 1980 and 1985. Data on annual deaths and their distribution by sex, age group, and Spanish province were provided by the National Statistics Institute. The overall mortality rate was 2.14/100000 population; specific mortality was somewhat higher in men. The most interesting finding here is the presence of a geographical ‘mortality gradient’, meaning that mortality is higher in northern Spanish provinces than in southern ones; it is also higher in rural than in urban areas. These observations call for further studies to clarify whether place of residence may have an effect on the development of PD.
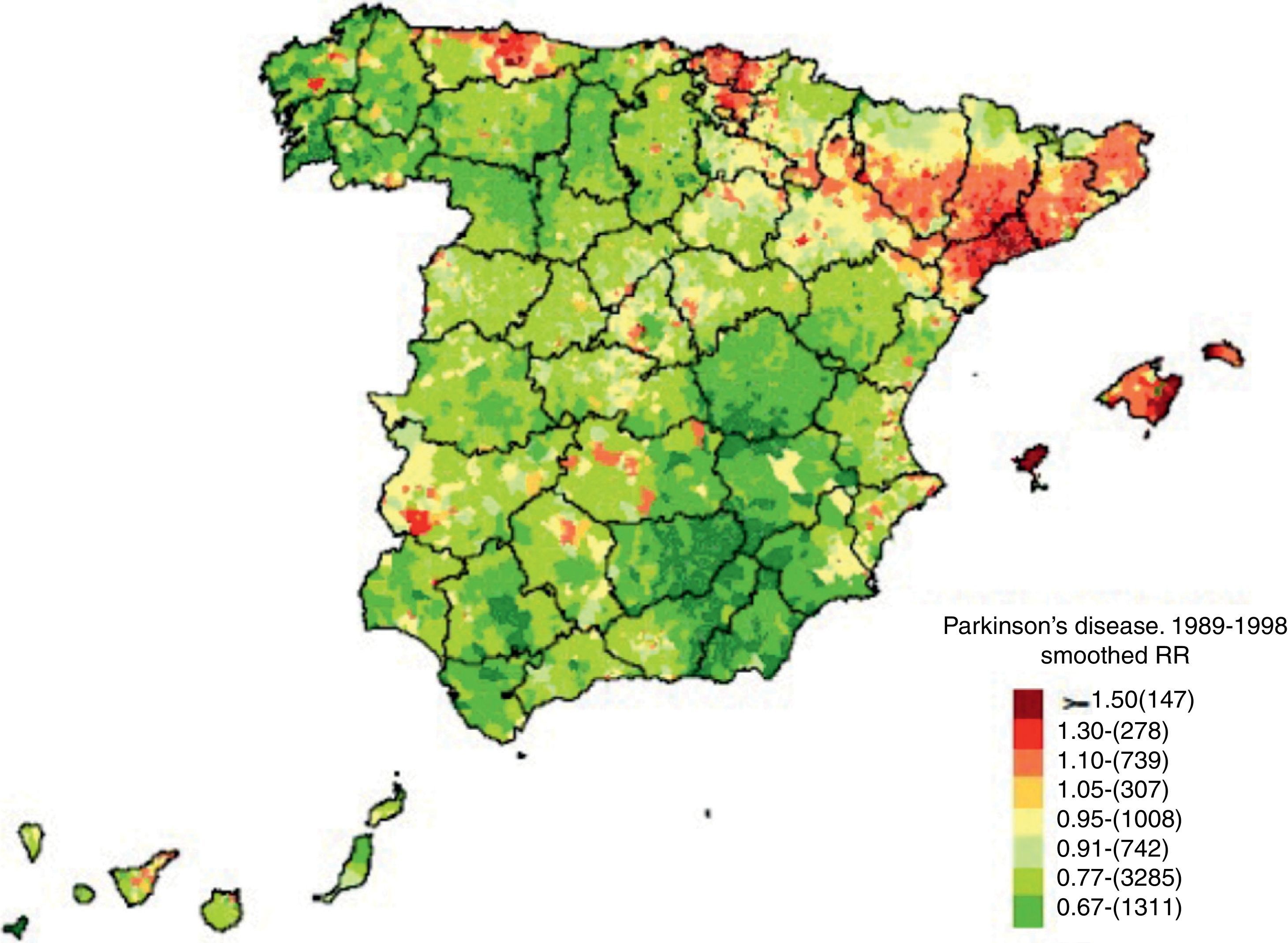
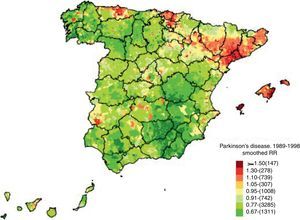
The mortality gradient we describe was analysed in great detail by De Pedro-Cuesta et al.66 in 2009 (Fig. 1). The study addressed the geographical distribution of PD mortality in Spain by city between 1989 and 1998 in order to detect any non-coincidental distribution tendencies and examine their causes. These data were also obtained from the National Statistics Institute. Pedro-Cuesta et al. obtained several key findings in a register of 12531 deaths due to PD during that period, with no significant differences between the sexes. High-mortality areas were discovered in the northeastern Lower Ebro river valley and other parts of Catalonia, especially Tarragona, with low-mortality areas in the southeastern provinces of Jaén, Granada, Almería, Albacete, and Murcia. This distribution can be superimposed on that reported by the earlier study65; furthermore, a previous study67 of levodopa consumption in Spain between 1990 and 1995 reported high sales in the north and low consumption in the south. As such, the pattern seems to correspond to selective areas in which PD is underdiagnosed, rather than to aetiological factors having to do with the presence of the disease itself. The situation calls for measures to improve diagnosis (the authors mention a lack of neurologists in smaller Andalusian hospitals). Nevertheless, the authors did identify isolated towns or town clusters with high mortality located near industrial facilities, and we cannot rule out the possibility that these ‘hotspots’ could point to genetic and environmental risk factors for parkinsonism. The authors suggest conducting specific aetiological studies in these populations.
Mortality in Parkinson's disease. Source: De Pedro-Cuesta et al.66 (used with the authors’ permission).
The methodology and main neurological findings from the NEDICES elderly cohort were published in 2008.68 An analysis of PD mortality within this cohort was published in 2011.69 The full cohort includes 5262 elderly patients with a follow-up period of 13 years (1994-2007). Of this total, 81 patients had PD at baseline, and there were 66 deaths during follow-up. Risk of mortality for patients with PD (2.29) was higher than for controls without PD, and remained high (1.67) after various adjustments for age, dementia, heart disease, and number of medications, as well as when adjusted for variables associated with PD or with death (1.75). Risk of mortality was higher among patients with dementia. For that reason, this study concluded that PD is an independent predictor of mortality in elderly patients, and that risk is especially high among those with dementia. It should be said that PD is mentioned on death certificates in only 18.2% of all cases, and the most frequent cause of death is cardiovascular disease; in this sense, PD patients do not differ from controls without PD.
Another longitudinal study70 with 20 years of follow-up (1978-1998) included 273 patients in the province of Segovia. It found a standardised mortality rate of 1.39, and the following factors were independent predictors of mortality: age at onset, akinetic-rigid clinical manifestations, and initial treatment without dopamine agonists.
DependencyIn recent years, disability and dependency have become 2 extremely relevant concepts in politics and social health. Spain's National Statistics Institute has completed 3 major surveys on disability and dependency (1986, 1999, 2008)71–73 which present their importance in quantitative terms. Similarly, a featured article in Gaceta Sanitaria74 analyses the evolution of our understanding of these concepts. The initial concept of disability was the array of deficiencies and illnesses suffered by an individual and which were addressed with medical treatment, rehabilitation and care; at present, we stress the primordial importance of the resulting disability, understood as a need for personal care.
Data from the last 2 surveys can be accessed from the National Statistics Institute's webpage72,73 and they include data related to PD. For example, in the group of patients of both sexes aged 65 to 79, we find 38235 patients with disability and a diagnosis of PD at this time; in the group of patients of both sexes aged 65 to 69 years, the proportion of patients with disability and diagnosed with PD was 6.6 per 1000 individuals older than 6.
Spanish authors have also referred to the concept of ‘burden of disease’, which includes patient-related factors, such as disability, early death, the need for care and treatment; and environmental factors such as social, economic, and family support. These authors have shown keen interest in the relationship of these social concepts with health-related quality of life in both patients and carers. One study on the burden of PD-related disease in Spain in the year 2000 presents comparisons with data from similar countries in Europe and around the world.75 The study evaluated burden of disease using disability-adjusted life years (DALY) as a measure of premature mortality and disability, since it equates to years of life lost due to a given condition. PD-related DALY values in Spain were higher than worldwide and European figures (84/100000 population), and disability was the main factor contributing to the difference. Although the authors interpret this data cautiously, they stress the need for a better understanding of the burden of PD in Spain. They feel that disability in this context may arise due to multiple factors; although PD is a motor disorder, depression, dementia, and psychosis are manifestations of advanced stages of the disease that increase burden.
Additional publications76–79 have also addressed carer burden in PD and its impact on quality of life. A 2005 study76 analysed the impact of PD on those caring for patients and aimed to identify the main factors associated with carer stress. Its conclusions were that patients’ functional status was significantly associated with the psychosocial burden on the carer. Factors having to do with patients’ health was shown to be another factor linked to the carers’ quality of life; therefore, lessening the patient's disability and improving health-related quality of life may ameliorate the burden on the carer. Another cross-sectional multicentre study77 evaluated 80 patients and their carers and delivered the following findings: the time devoted to care and the stress caused by the patient's state affect carer burden; there is an association between carer burden and health-related quality of life; disability and disease severity have an impact on carer burden and on the mental aspects of health-related quality of life; carer depression is associated with increased burden and poorer health-related quality of life; and depression in the patient also indirectly affects these factors. In summary, the psychological well-being of carers, clinical aspects of the disease, the patient's mental state, and the quality of life related to patient and carer health are predictors of the burden of the disease.
The ELEP group78 has also presented an article on these topics in which it studied 289 patients and their carers to reach the following conclusions: main carers are married female homemakers providing permanent assistance to the patient; as a group, carers for PD patients are more likely to develop anxiety, depression, and poor health than the general population; and carer burden and depression increase alongside disease severity. Lastly, the carer's emotional state is the factor with the greatest influence on the carer's burden and self-perceived state of health; as such, ameliorating this aspect may lessen the burden and prevent a decrease in health-related quality of life. This will have positive repercussions on both care for the patient and use of healthcare resources.
In a recent article, Martínez-Martín et al.79 refer once again to the concept of carer burden, its relationship to quality of life and health-related quality of life, and its dependence on sociodemographic, psychological, and disease-related factors. They also highlight the importance of applying effective interventions to promote carer well-being, which will result in the patient being able to remain at home and still receive appropriate care.
Economic impact of Parkinson's disease in SpainDifferent studies have illustrated the social health repercussions of PD, both in terms of the medical expenses it generates and in terms of decreased productivity and lower quality of life related to the disease. Considering the increase in the population's life expectancy, and that the highest prevalence and incidence of PD is found in older individuals (>60 years, 1280–1500×105 population and 346×105 population-year, respectively), it is believed that the costs generated by the disease will rise in the coming years.9
The costs generated by PD were estimated by including both direct and indirect costs. Direct costs were those generated directly by primary care, other levels of medical care, and treatments. Indirect costs were those generated by the decrease in productivity due to the patient's early retirement or decreased participation in the workforce on the part of the carer. We also find intangible costs that gauge the patient's degree of suffering due to the decrease in quality of life.
In addition to evaluating the motor symptoms that characterise PD, it is important to assess non-motor symptoms, especially psychiatric symptoms, which contribute greatly to the decision to institutionalise patients. Multiple studies evaluate the direct and indirect costs of PD associated with both motor and non-motor symptoms.80–83 A population study in southern Europe found that the general costs generated by PD amounted to a half-yearly expense of €8640 (95% CI: €6700-11240); direct costs made up the largest percentage of this amount, at €6030 (95% CI: €4700-7970). Treatment with dopaminergic drugs accounted for the largest part of the direct costs at €1456 (95% CI: €100-310), with dopamine agonists generating the most expense in this category. Furthermore, monetary expenses rise as the disease progresses, such that a higher score on the UPDRS signifies higher direct costs generated by the disease.84
In the Spanish population, the economic impact of PD was measured in a cross-sectional multicentre study in a cohort of 82 patients in 2004.5 The study measured motor and non-motor symptoms, quality-of-life values and disease severity, treatments, and epidemiological data from the 3 months prior to launching the study. Using this approach, it analysed the association between clinical variants and their direct and indirect costs. Direct costs, mainly represented by drug treatments, incurred a mean per capita cost of €669±405.7 (34% direct costs). Dopaminergic drugs made up the largest part of the drug costs at 83%. Regarding surgery in PD, the mean cost per patient was €475±2951.7. The indirect costs, whether medical or non-medical, included specialist and primary care visits, diagnostic tests, prescribed orthotics, transport, homecare services, home adaptations, etc. Indirect costs generated by the disease were linked to a decrease in workplace productivity as well as to early retirement. This item was listed as amounting to €6691±16283 per capita in the 3 months prior to beginning the study. Lastly, an examination of clinical and epidemiological factors found that direct costs were significantly higher (P<.05) in patients who were younger, with more advanced Hoehn and Yahr stages, longer disease duration and greater severity, greater disability, and more severe motor signs and complications.
As has been shown on multiple occasions, drug costs place the highest burden on the healthcare system. Data from a recent Spanish study85 comparing the costs of such advanced PD treatments as surgery with deep brain stimulation (DBS), continuous intraduodenal levodopa infusion (CILI) and continuous subcutaneous apomorphine infusion (CSAI). Considering the costs of each of the above procedures, we calculated 5-year costs and found that DBS was the least costly treatment (€88104), followed by CSAI (€141393) and CILI (€233986) (P<.0001). The annual costs of each treatment amounted to €17603 for DBS, €28279 for CSAI, and €46796 for CILI. The lower costs linked to surgical treatment was the result of the reduced drug consumption in patients treated with DBS rather than the other techniques requiring continuous administration of dopaminergic drugs. Data support using advanced PD therapies rather than conventional drug treatment in patients referred to more specialised hospitals. Generally speaking, evidence shows that costs incurred by patients undergoing DBS may be as much as 54.7% higher than those for patients treated conventionally; including indirect costs, however, expenses on patients treated with DBS are 34.7% lower than in those receiving conventional drug treatment.86 Indirect and intangible costs as well as direct costs are affected by PD treatments, since treatment changes patients’ level of disability and quality of life. The appearance of motor complications worsens quality of life in addition to increasing costs. Using advanced treatments in these cases has been shown to improve patients’ motor and non-motor function, as well as increasing quality of life.87,88 These treatments therefore decrease indirect and intangible costs in patients with complications.
As the second most common neurodegenerative disease, PD incurs major healthcare expenses in our population. The severity of this disease, and the extent of the disability caused by motor and non-motor symptoms, contribute greatly to increases in both direct and indirect costs. Evaluating use of advanced treatments for these cases of PD is therefore of the utmost importance. Projections for the future, taking into account the tendency of life expectancy to increase, indicate that there will be rising demand for social and healthcare resources having to do with PD. Developing and optimising not only treatments but also health protocols able to reduce the social and economic impact of PD on the population is therefore a crucial undertaking.
The state of neurological care for patients with Parkinson's disease in SpainSince no data on this type of neurological care are available in Spain, the present report includes a study whose main objective is to define the public and private resources offered to patients with PD in our country.
Public care resourcesAn e-mail survey was sent to all neurologists specialising in PD and members of the Movement Disorder Study Group of the Spanish Society of Neurology. We received answers from a total of 40 neurologists from 40 different hospitals throughout Spain. Respondents included neurologists from all of Spain's autonomous communities. Although data do not reflect the entire panorama of PD care in Spain, since we do not have data from every health district, it does map out an approximate idea of how neurological care is provided to PD patients at this time.
Secondly, we consulted data from the Imserso publication89 on the situation, needs, and priorities of patients with PD.
According to the analysis of data provided by the surveys, all of Spain's autonomous communities have at least one specialised PD unit. Health districts with fewer than 200000 inhabitants also have specialist clinics. The Region of Madrid has specialised units in all of its major hospitals, with specialist consults in smaller hospitals. Catalonia is another example of an autonomous community with specialised units in all major hospitals, most of which are in Barcelona. The types of patients cared for by these units will vary from place to place. Half of the units provide care to all patients diagnosed with PD who are referred to the neurology department. The patient referred by a general practitioner as a suspected PD case will be seen by a general neurologist. This doctor in turn will assign the case to the specialised unit, and all follow-up work will be performed by that unit. This is the dominant model in Madrid and Barcelona. Other units, such as those in Seville and in most hospitals in the Valencian Community and Castile-Leon, only follow up on complicated patients, young patients, or those undergoing advanced therapy with perfusion or deep stimulation techniques.
PD units include one to 5 neurologists, who are not solely dedicated to that unit in most cases. They will also be active in other areas of neurology. Patients in the early stages of PD are examined in the specialised unit once or twice yearly, whereas patients in later stages are seen every 3 months on average. However, respondents from most hospitals stated that the patients had a direct line of contact in case they needed to move up their appointment.
We note that only 10 of the 40 units on which we have data are supported by a specialised nursing consult. This consult is usually offered once a week. Another 10 hospitals have general nursing staff assisting neurologists specialised in PD in their consults. Telephone consults are not a common practice.
What we have discovered is that although PD units should be multidisciplinary, the vast majority of them consist solely of neurologists. Some 30% include neuropsychologists, and most of these units receive private funding. Except for the units at Hospital Clínic (Barcelona) and Virgen del Rocío (Seville), these teams do not include speech therapists and physiotherapists, psychologists, and psychiatrists. Neurologists work with neurosurgeons and neurophysiologists in those units performing DBS.
There are no specific rehabilitation programmes in any of these units, or in any rehabilitation departments in public hospitals. This coincides with the Spanish National Health System's list of common services approved by Royal Decree in 2006; according to this document, rehabilitation, including physical, occupational, and speech therapy, is currently considered only for those patients with a reversible functional loss. This being the case, most patients with PD do not have continued access to these therapies in hospitals forming part of the Spanish National Health System. Rather, this role is filled by patient associations, which will be described in a later section.
According to the data obtained, advanced therapies are covered sufficiently in most of Spain's autonomous communities. All 40 hospitals from which we received a response indicated that they can perform apomorphine and duodopa pump therapy. While DBS is performed in 15 of the 40 hospitals, it is available for patients in all autonomous communities. Nevertheless, waiting periods are long at 6 months to a year at most hospitals.
Our data are limited, but they do show that once patients have been referred to extremely specialised units, which is an option in most provinces, their neurological care is well covered. Units with neurologists able to dedicate at least part of their time to specialised care are available in all provinces in Spain. Intervals between follow-up visits are reasonable, and patients experiencing emergencies can contact their doctors directly. However, there are drawbacks including a marked lack of the other treatments that are fundamental to the complex task of managing PD: rehabilitation, psychological treatment, and nursing care. The waiting lists for surgical treatment are also very long.
According to data from Imserso,90 the main problem seems to stem from the preceding level, primary care. Long periods of time may pass before some patients are suspected of having Parkinson's disease and referred to a neurologist. One possible explanation is that this disease has numerous manifestations and may therefore be difficult for a general practitioner to recognise. Furthermore, PD has a low prevalence in the primary care setting compared to other diseases, so general practitioners may be less familiar with its signs.
Private healthcare resources: the role played by associationsAs mentioned before, the Spanish public health system does not contemplate part of the integral treatment that patients with PD need, and which includes physical, speech, and occupational therapy, and psychological support. This gap is currently filled by patient associations.91,92 Nevertheless, many patients are not aware that these associations exist, and neither are they told about the benefits of these treatments. Part of the problem arises from their neurologists not providing this information. Patients are more commonly informed of these options in specialised units, where neurologists have a deeper understanding of integral treatment of the disease and tend to work with associations in their area.
However, these treatments are essential at all stages of the disease. As we have seen, patients in the early stages of Parkinson's disease are not seen in specialised units. Rather, they are assessed by general neurologists who do not provide information about patient associations and other treatment options available because they are not aware of them. Doctors at the primary care level are also unlikely to know of any patient associations.
Associations also provide material to patients and their family members that healthcare professionals may not explain fully. Among other activities, associations organise training courses, sessions, and workshops on the disease which are aimed at both patients and their carers. This being the case, good care will require raising awareness of the role played by associations and better coordination between them and healthcare professionals at all levels.
Effect of Parkinson's disease on the familyIn addition to its effects on patients, PD has an impact on those living with them. Patients with PD present an array of motor symptoms that will give rise to various difficulties and disabilities over the course of the disease. As the disease progresses, the patient will experience decreased autonomy, obliging the family to gradually take responsibility for his or her daily activities. This will involve a loss of free time, working time, or leisure activities on the part of the patient's main carer. However, patients will present other types of symptoms that are equally important and which limit quality of life and social relationships: psychological symptoms include depression, apathy, anxiety, cognitive impairment, sexual disorders (whether hypersexuality or impotence), and impulse control disorders that may give rise to gambling addictions, compulsive buying, jealousy, and other behaviour disorders. All of these manifestations mean that family relationships will be affected at all stages of the disease.
The extent to which all of these considerations will affect the family is fundamentally determined by the patient's age. Older patients tend to be more accepting of their condition, whereas the workplace and economic problems that PD poses for younger patients make their experience more traumatic. Behaviour disorders in younger patients, especially compulsive behaviours, are common and they can result in serious difficulties in the home, including divorce and family rupture.
The consequences of these disruptions are extremely negative for families in some cases. Carers may experience depression, apathy, exhaustion, and social isolation. They will require substantial psychological support, in addition to social assistance and information. These aspects of the disease are normally covered by associations.
ConclusionsThis report allows us to conclude that PD incidence and prevalence in Spain are similar to rates in the rest of Europe. Given the current population estimates, we calculate that there are at least 300000 patients with PD in Spain. The disease has a major impact on the patient's quality of life and mortality rates are nearly twice that in non-patients. With yearly costs of more than €17000 per patient, it also places a heavy economic burden on the country; as the population ages and new treatments become more widespread, these costs will rise. Healthcare professionals and government bodies are making a concerted effort to provide patients with quality care. Nevertheless, much remains to be done to ensure high-quality, effective multidisciplinary care for all patients with PD in Spain.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García-Ramos R, López Valdés E, Ballesteros L, Jesús S, Mir P. Informe de la Fundación del Cerebro sobre el impacto social de la enfermedad de Parkinson en España. Neurología. 2016;31:401–413.