Quality of life (QOL) is becoming increasingly important to measure the effect of interventions on the life of patients with Alzheimer's disease (AD), particularly on the most meaningful issues. However, most of the instruments used to measure QOL have not been validated in the Spanish population. The aim of this study was to determine the psychometric properties of a Spanish version of QoL scale in patients with AD, carers and health professionals.
Material and methodsOn hundred and two patients, their carers and 25 health professionals were recruited from day centres. Patients’ QOL was rated by patients, carers and health professionals. The Health Utilities Index, Clinical Insight Rating Scale and Mini Mental State Examination were also administered.
ResultsThe internal reliability and external reliability of QoL-AD were excellent. Criterion validity was indicated by a significant correlation of QoL-AD scores with HUI-3 and QoL-AD global item scores (P<.05). Lack of insight and cognitive impairment did not have an effect on these properties. QoL-AD scores were not significantly different between groups made according sociodemographic characteristics and cognitive impairment (P>.05). The exploratory factor analysis result revealed a three factor solution, which accounted for 61.3% of variance: health factor, functional status factor, and social relationship–environment factor.
ConclusionsQoL-AD scale has proved to be a valid and reliable instrument to measure QoL of Spanish AD patients with mild-to-moderate cognitive impairment and a wide range of anosognosia.
La calidad de vida (CV) está adquiriendo cada vez más relevancia como medida para evaluar los resultados de las distintas intervenciones terapéuticas sobre los pacientes con enfermedad de Alzheimer (EA), dado que contempla aspectos que son especialmente valiosos en su vida diaria. Sin embargo, son escasos los instrumentos para medir la CV que han sido validados en población española. El objetivo de este estudio es explorar las propiedades psicométricas de la escala QoL-AD en pacientes, cuidadores y profesionales sanitarios.
Pacientes y métodosSe seleccionó a 102 pacientes con EA en fase leve-moderada, sus cuidadores y 25 profesionales sanitarios. La CV de los pacientes fue valorada por pacientes, cuidadores y profesionales mediante la escala QoL-AD. Además, se administraron MMSE, escala de valoración de insight clínico (CIR) e índice de utilidades de salud (HUI-3).
ResultadosLa fiabilidad interna y externa de la escala QoL-AD fueron excelentes. La escala presenta validez de criterio dado que sus puntuaciones correlacionaron con las de HUI-3 y la medida global de CV (p<0,05). La falta de insight y el deterioro cognitivo no tuvieron un efecto sobre estas propiedades. Las puntuaciones en la escala QoL-AD no difirieron entre grupos establecidos según MMSE y factores sociodemográficos (p>0,05). En el análisis factorial se obtuvo una solución de tres factores que explica el 61,3% de la varianza: factor salud, factor estado funcional y factor relaciones sociales-ambiente.
ConclusionesLa escala QoL-AD es un instrumento válido y fiable para medir la CV en la pacientes españoles con AD que presenten deterioro cognitivo leve-moderado, sea cual fuese su grado de insight.
The purpose of the therapies and social resources allocated to patients with Alzheimer's disease (AD) is to improve their quality of life (QoL). In fact, it is increasingly common to incorporate assessments of this construct into clinical trials.1,2 There is an agreement that quality of life involves a subjective evaluation by an individual of different aspects of her/his life.3,4 In the case of patients with dementia, the generic QoL questionnaires designed to be applied in a wide range of pathologies are sometimes not sensitive enough.5,6 Therefore, specific scales that match the characteristics of this special population have recently been designed and have shown their validity.4,7–9 Currently, an attempt to validate and adapt these questionnaires to the Spanish population is being made.10,11 The QoL-AD scale has been supported in the literature as one of the best tools to measure QoL.12,13 This study was intended to test the adequate psychometric behaviour of the Spanish version of this scale in outpatients with AD, caregivers and healthcare professionals, as well as to compare the effect that cognitive impairment and lack of insight of patients may exert on it.
Material and methodsSubjectsThe sample consisted of 102 patients, their caregivers and 25 professionals from day care centres in the Region of Murcia. All patients had been diagnosed with probable or possible AD (NINCDS-ADRDA criteria), were able to communicate and lived daily with a caregiver. All participants consented to participate in the study. The project was approved by the bioethics committee of the University of Murcia.
Patients had a mean age±standard deviation of 78.09±7.02 years, with a mean schooling of 4.72±2.70 years and mean disease duration of 4.15±2.42 years. A total of 68.80% of them were female, 56.90% were married and the rest were widowed. In total, 47.10% of the patients were homemakers, 31.30% were skilled workers and 21.60% were non-skilled workers. Mean scores on the MMSE, Clinical Insight Rating Scale (CIR) and HUI-3 were 18.51±5.99 (range 9–27), 4.16±2.66 (range 0–8) and 18.14±4.78 (range 10–28), respectively.
Caregivers had a mean age of 58.86±15.99 years. Of these, 70.60% were female, 78.43% were married and the rest were single. In 41.2% of cases, they were the spouses of patients, in 51%, offspring and in 7.8%, non-related caregivers.
InstrumentsWe applied the following questionnaires:
- –
QoL-AD4 scale. This has 13 items related to the perception of health status, mood, functional capacity, personal relationships and leisure, financial situation and life as a whole. Each item is answered according to a Likert scale from 1 (bad) to 4 (excellent). In this study we used a modified version, such as Edelman et al.,13 in which items relating to “money” and “marriage” had been replaced by “ability to decide” and “people you live with”. Questionnaires with up to 2 missing items were considered eligible.
- –
HUI-314. This is a generic QoL scale including 8 attributes (vision, hearing, speech, gait, dexterity, emotion, cognition and pain) which, in turn, include 5 or 6 levels of severity of involvement. For our study, we calculated the multi-attribute usefulness scores in the manner described by Ruiz et al.14
- –
MMSE. We used a version of this test validated for the Spanish population, correcting scores for age and schooling.15 Two groups were formed for comparison, taking as cut-off value that corresponding to the median of the scores.
- –
CIR. This scale consists of 4 items that measure situational awareness, cognitive impairment, functional dependency and disease progression, and those are scored from 0 (not aware) to 2 (fully aware).16 We established 2 groups, as did Ready et al.17
Questionnaires were applied to patients (MMSE, QoL-AD, CIR), caregivers (QoL-AD, HUI-3) and professionals (QoL-AD), with the interviews being performed separately. Caregivers and professionals were instructed to perform the assessments of patient QoL attempting to think as they would. One month after the baseline interview, the QoL-AD scale was administered again to 25 patients and 25 caregivers selected randomly.
Statistical analysisWe evaluated the acceptability (rate of completed items and distribution of scores), internal consistency (Cronbach's alpha coefficient and item-total correlation) and test–retest reliability (intraclass correlation coefficient). We determined the validity of concurrent criterion and divergent validity by studying: (a) the correlation between total scores on QoL-AD and HUI-3 and item 13 of QoL-AD; (b) the relationship between total scores on QoL-AD and cognitive function (MMSE) and socio-demographic factors (Mann–Whitney U and Kruskal–Wallis tests). Construct validity was verified through factor analysis (principal components method) on the assessments of patients (12 items). The factors obtained were rotated by the Varimax method.
ResultsAcceptabilityThere were no problems in understanding any of the items. The mean time required for completion by patients, caregivers and professionals was 15.4±5.3, 7.2±1.3 and 7.5±1.2min, respectively. The percentage of invalidated questionnaires was less than 1%. There was an accumulation of responses greater than 25% in the lowest quartile of item 5 (memory) and in the top quartile of item 6 (relations with family). The mean and median scores on the QoL-AD scale were: patients 34.92±6.48 and 36; caregivers 29.76±4.92 and 30; professionals 30.27±4.82 and 30.50, respectively. The percentage variation in the medians with respect to the means was less than 3.2%. The asymmetry coefficients of all items were in the range between −1 and +1.
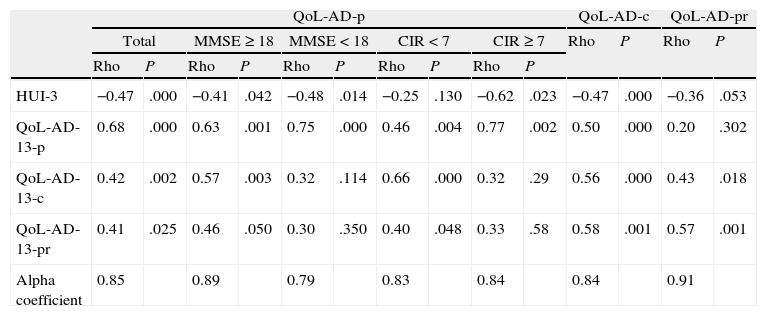
ReliabilityThe internal consistency of the assessments of patients, caregivers and professionals was excellent (alpha coefficient=0.85, 0.84 and 0.91, respectively). All correlations between item scores and overall scores were over 0.3. Neither the degree of insight nor cognitive impairment of patients implied decreases in the internal consistency below acceptable levels (Table 1). The test–retest reliability was excellent in both patients and caregivers (ICC=0.87 and 0.86, respectively).
Validity and reliability criteria of the QoL-AD scale.
| QoL-AD-p | QoL-AD-c | QoL-AD-pr | ||||||||||||
| Total | MMSE≥18 | MMSE<18 | CIR<7 | CIR≥7 | Rho | P | Rho | P | ||||||
| Rho | P | Rho | P | Rho | P | Rho | P | Rho | P | |||||
| HUI-3 | −0.47 | .000 | −0.41 | .042 | −0.48 | .014 | −0.25 | .130 | −0.62 | .023 | −0.47 | .000 | −0.36 | .053 |
| QoL-AD-13-p | 0.68 | .000 | 0.63 | .001 | 0.75 | .000 | 0.46 | .004 | 0.77 | .002 | 0.50 | .000 | 0.20 | .302 |
| QoL-AD-13-c | 0.42 | .002 | 0.57 | .003 | 0.32 | .114 | 0.66 | .000 | 0.32 | .29 | 0.56 | .000 | 0.43 | .018 |
| QoL-AD-13-pr | 0.41 | .025 | 0.46 | .050 | 0.30 | .350 | 0.40 | .048 | 0.33 | .58 | 0.58 | .001 | 0.57 | .001 |
| Alpha coefficient | 0.85 | 0.89 | 0.79 | 0.83 | 0.84 | 0.84 | 0.91 | |||||||
CIR: Clinical Insight Rating Scale; QoL-AD-p: assessments by patients; QoL-AD-c: assessments by caregivers; QoL-AD-pr: assessments by professionals; HUI-3: Health Utilities Index 3; QoL-AD-13-c: item 13 of QoL-AD rated by caregivers; QoL-AD-13-p: item 13 of QoL-AD rated by patients; QoL-AD-13-pr: item 13 of QoL-AD rated by professionals; MMSE: mini-mental state examination; Rho: Spearman correlation coefficient.
There were significant correlations between total scores of patients, caregivers and professionals in QoL-AD scores and HUI-3 and item 13 of QoL-AD. The subgroup analysis found that the scores given by patients with greater cognitive impairment and those given by those with lower levels of insight correlated significantly with two of the measures adopted as a criterion (Table 1).
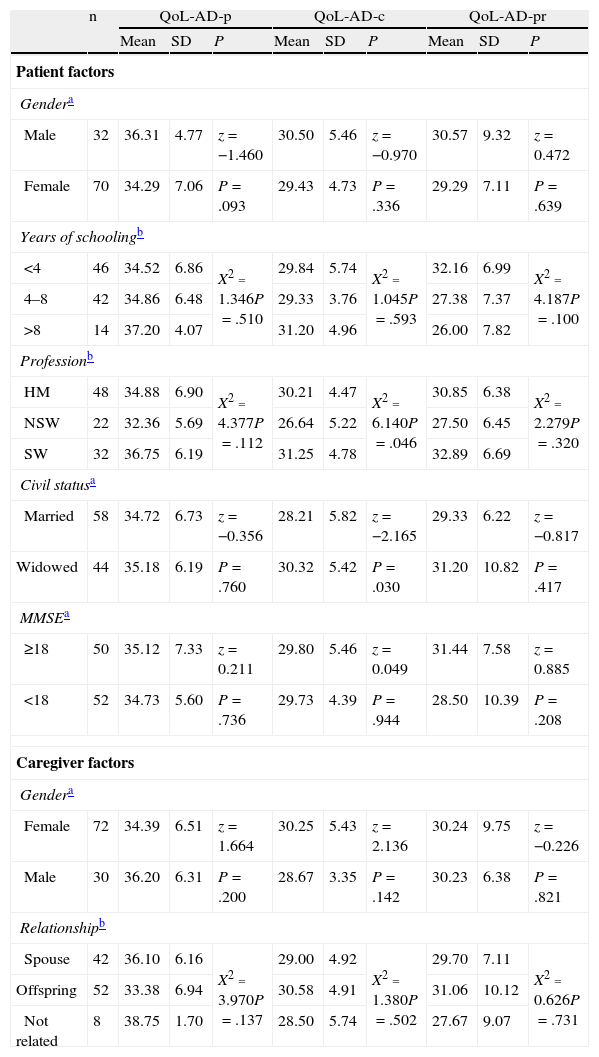
Divergent validityThe scores on the QoL-AD scale given by patients and professionals were not significantly different in the groups established based on age, gender, educational level, profession, marital status and MMSE of the patients (Table 2). The scores given by caregivers were influenced by marital status and occupation of patients (P=.030 and P=.046, respectively) and were weakly correlated with patient age (r=0.204; P=.04). Caregiver socio-demographic factors were not associated with significant differences in QoL (P>.05).
Socio-demographic and clinical factors of patients and caregivers and their relationship with QoL-AD scale scores.
| n | QoL-AD-p | QoL-AD-c | QoL-AD-pr | |||||||
| Mean | SD | P | Mean | SD | P | Mean | SD | P | ||
| Patient factors | ||||||||||
| Gendera | ||||||||||
| Male | 32 | 36.31 | 4.77 | z=−1.460 | 30.50 | 5.46 | z=−0.970 | 30.57 | 9.32 | z=0.472 |
| Female | 70 | 34.29 | 7.06 | P=.093 | 29.43 | 4.73 | P=.336 | 29.29 | 7.11 | P=.639 |
| Years of schoolingb | ||||||||||
| <4 | 46 | 34.52 | 6.86 | X2=1.346P=.510 | 29.84 | 5.74 | X2=1.045P=.593 | 32.16 | 6.99 | X2=4.187P=.100 |
| 4–8 | 42 | 34.86 | 6.48 | 29.33 | 3.76 | 27.38 | 7.37 | |||
| >8 | 14 | 37.20 | 4.07 | 31.20 | 4.96 | 26.00 | 7.82 | |||
| Professionb | ||||||||||
| HM | 48 | 34.88 | 6.90 | X2=4.377P=.112 | 30.21 | 4.47 | X2=6.140P=.046 | 30.85 | 6.38 | X2=2.279P=.320 |
| NSW | 22 | 32.36 | 5.69 | 26.64 | 5.22 | 27.50 | 6.45 | |||
| SW | 32 | 36.75 | 6.19 | 31.25 | 4.78 | 32.89 | 6.69 | |||
| Civil statusa | ||||||||||
| Married | 58 | 34.72 | 6.73 | z=−0.356 | 28.21 | 5.82 | z=−2.165 | 29.33 | 6.22 | z=−0.817 |
| Widowed | 44 | 35.18 | 6.19 | P=.760 | 30.32 | 5.42 | P=.030 | 31.20 | 10.82 | P=.417 |
| MMSEa | ||||||||||
| ≥18 | 50 | 35.12 | 7.33 | z=0.211 | 29.80 | 5.46 | z=0.049 | 31.44 | 7.58 | z=0.885 |
| <18 | 52 | 34.73 | 5.60 | P=.736 | 29.73 | 4.39 | P=.944 | 28.50 | 10.39 | P=.208 |
| Caregiver factors | ||||||||||
| Gendera | ||||||||||
| Female | 72 | 34.39 | 6.51 | z=1.664 | 30.25 | 5.43 | z=2.136 | 30.24 | 9.75 | z=−0.226 |
| Male | 30 | 36.20 | 6.31 | P=.200 | 28.67 | 3.35 | P=.142 | 30.23 | 6.38 | P=.821 |
| Relationshipb | ||||||||||
| Spouse | 42 | 36.10 | 6.16 | X2=3.970P=.137 | 29.00 | 4.92 | X2=1.380P=.502 | 29.70 | 7.11 | X2=0.626P=.731 |
| Offspring | 52 | 33.38 | 6.94 | 30.58 | 4.91 | 31.06 | 10.12 | |||
| Not related | 8 | 38.75 | 1.70 | 28.50 | 5.74 | 27.67 | 9.07 | |||
HM: homemaker; MMSE: mini-mental state examination; NSW: non-skilled worker; QoL-AD-c: assessments by caregivers; QoL-AD-p: assessments by patients; QoL-AD-pr: assessments by professionals; SD: standard deviation; SW: skilled worker.
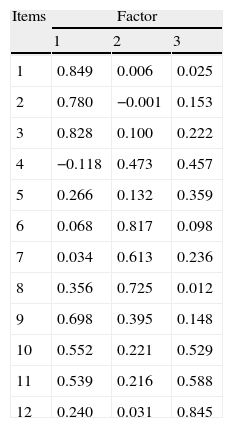
Bartlett's test of sphericity was significant (P=.000) and the Kaiser–Meyer–Olkin test value was 0.785, which ensured the adequacy of the sample for factor analysis. Principal component analysis obtained 3 factors with eigenvalues greater than 1, which explained 61.30% of the total variance; once rotated, this correlated with the original variables as presented in Table 3. Item 5 (memory) was weakly correlated with the 3 isolated factors and had a 7.71% explanatory power. The factors obtained had good internal consistency (alpha coefficient≥0.70) and were interpreted as follows: health factor (r2=29.57%), social relations and environment factor (r2=17.27%) and functional capacity factor (r2=14.46%).
Correlations between the scores of each item and factor scores.
| Items | Factor | ||
| 1 | 2 | 3 | |
| 1 | 0.849 | 0.006 | 0.025 |
| 2 | 0.780 | −0.001 | 0.153 |
| 3 | 0.828 | 0.100 | 0.222 |
| 4 | −0.118 | 0.473 | 0.457 |
| 5 | 0.266 | 0.132 | 0.359 |
| 6 | 0.068 | 0.817 | 0.098 |
| 7 | 0.034 | 0.613 | 0.236 |
| 8 | 0.356 | 0.725 | 0.012 |
| 9 | 0.698 | 0.395 | 0.148 |
| 10 | 0.552 | 0.221 | 0.529 |
| 11 | 0.539 | 0.216 | 0.588 |
| 12 | 0.240 | 0.031 | 0.845 |
This study supports the thesis that patients with mild to moderate AD can, and therefore should, evaluate their own QoL. In order to do so, it is essential to have a simple, easy instrument that is quick to administer, so as to avoid bias due to lack of understanding of questions or decreased attention. This version of the QoL-AD meets the necessary requirements for its application in this population,12 and its time of application and completion rate are similar to the original version.4,18 Some authors have questioned the validity of the assessments by patients of their own QoL, due to their cognitive impairment and to a lack of real awareness about their deficits.19,20 In our sample, which included patients with MMSE of up to 9 points and total lack of insight, the QoL-AD scale presented high levels of internal consistency, which did not differ in those patients with the highest levels of cognitive impairment or lowest levels of insight. The scores of the items could therefore be added to obtain a total score. The reliability of the scale in patients with moderate cognitive impairment has been reported in the literature,4,21,22 whilst there is more controversy about the consequences of lack of insight. Both Ready et al.17 and Berwig et al.23 reported a negative effect of anosognosia on the internal consistency of the assessments of patients about their own QoL. Nevertheless, both studies were performed with larger and more complex scales than the QoL-AD scale. The excellent test–retest reliability of the scale is remarkable. This shows that, despite the mnemonic condition of patients, the measurements were not influenced by transient changes in mood, health and other factors. Our results agree with those published in the literature4,24–26 and support the usefulness of the QoL-AD scale to carry out a temporal monitoring of QoL in these patients. In this study, we verified that the QoL-AD scale measures the construct QoL, given the significant correlation observed with the overall measurement of QoL and with a generic health-related quality of life (HRQoL) scale, according to reports by other authors.24,25,27 On the other hand, the absence of a relationship between the scores in the QoL-AD scale and the socio-demographic factors, a fact reported in some studies, supports an adequate divergent validity.21,22 Although not all studies have observed this independence,28,29 since QoL is a concept covering all the major aspects in the life of an individual, it is likely to reflect associations that are explained by the existence of a colinearity between variables.30 However, the observation of an apparently contradictory fact is common; the weak impact of cognitive function on the QoL of patients.24,28 It is likely that patients in very early stages give some importance to the involvement of cognitive functions but as the disease progresses, this importance generally shifts to neuropsychiatric symptoms and functional involvement.31,32 Factor analysis results are in agreement with the results published by Thorgrimsen et al.24 The rotated factors refer to the sections of the QoL included in the Lawton model: health, functional capacity and environment.3 Revell et al.33 isolated a factor of psychological wellbeing, which coincided in our study with the health factor. However, they did not consider the dimension of functional capacity. These differences could be due to the fact that their target population consisted of healthy elderly people without cognitive impairment and functional disability. However, the results of this work have to be corroborated by confirmatory factor analysis.
The fact that the target population was composed of patients treated at outpatient day care centres could be considered as a limitation of this study. In the future, it should be verified whether the modification of the scale makes it useful in care centre environments and whether it maintains adequate psychometric properties for patients in advanced stages. Although the reliability and validity of assessments by caregivers and professionals have been contrasted, studying the agreement shown with the assessments by patients is needed to ensure that its use is adequate in advanced stages.
Conflict of interestsThe authors declare that the present work was funded exclusively by their wages and completed with the infrastructure of the institutions to which they belong. None of the authors received funding from the biomedical industry.
The authors wish to thank Dr. R.G. Logsdon for authorising the validation of the scale, and the patients, caregivers and day care centre professionals for their selfless collaboration in this work.
Please cite this article as: Gómez-Gallego M, et al. Validación de la versión española de la escala QoL-AD en pacientes con enfermedad de Alzheimer, cuidadores y profesionales sanitarios. Neurología. 2011;27:4–10.
Some results of this study were presented at the 62nd Annual Meeting of the Spanish Neurology Society.