Acute cerebellitis is a rare inflammatory disease with a highly variable clinical course that ranges from benign self-limiting symptoms to a fulminant presentation associated with a high risk of death due to compression of the posterior fossa, acute hydrocephalus, and intracranial hypertension.
MethodsWe reviewed clinical, laboratory, and radiological findings from children diagnosed with acute cerebellitis between May 2007 and November 2016. We analysed treatments and clinical and radiological progression.
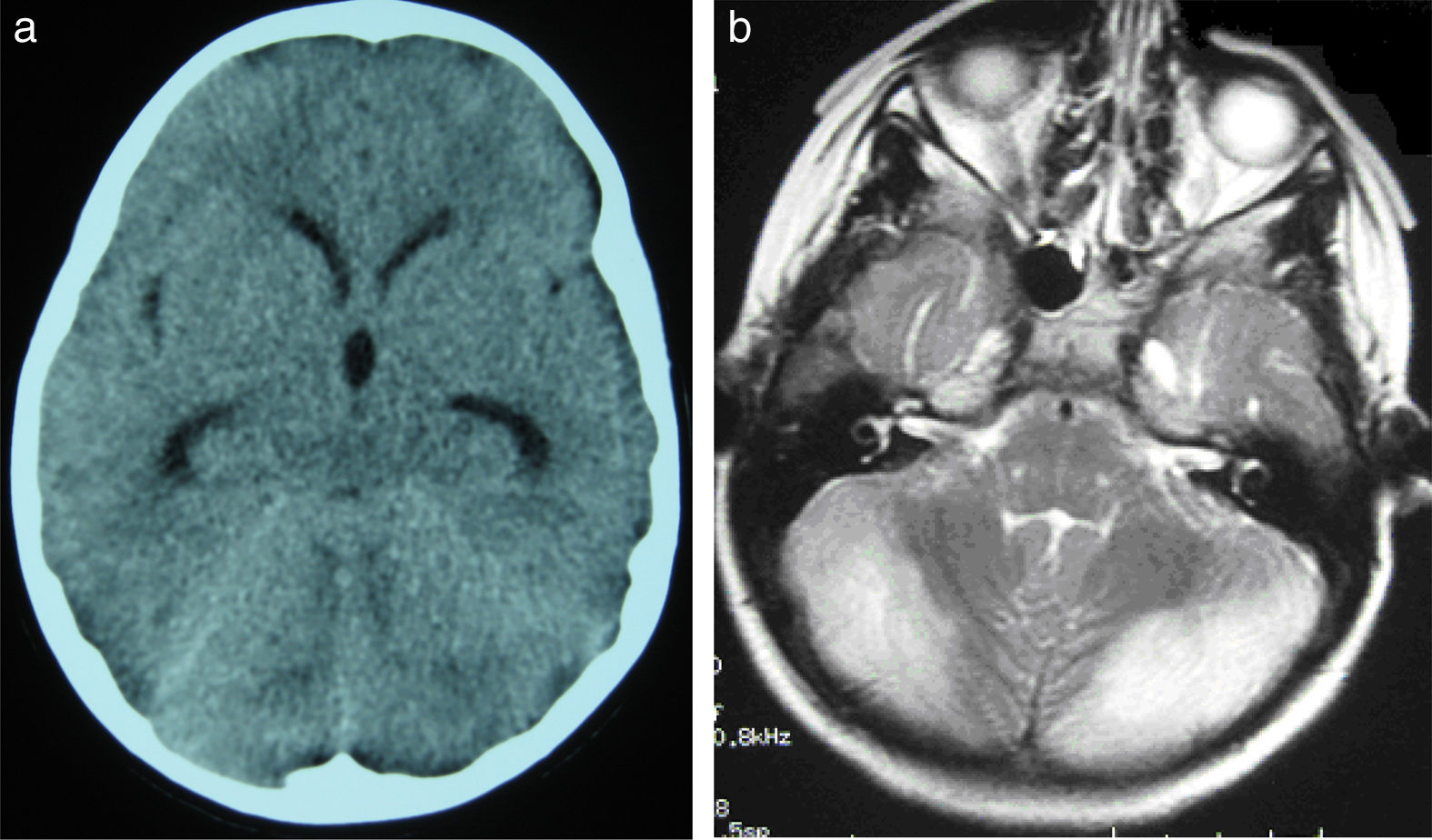
ResultsNine children met the diagnostic criteria for cerebellitis. Headache, vomiting, and drowsiness were the most frequent initial symptoms; ataxia, dysarthria, and dysmetria were the most common cerebellar signs. Cerebellitis was diagnosed with magnetic resonance imaging, which revealed cerebellar involvement (unilateral or bilateral); computerised tomography images either were normal or showed indirect signs such as triventricular hydrocephalus due to extrinsic compression of the aqueduct of Sylvius. Corticosteroids were the most commonly used treatment (6 patients). One patient required surgery due to triventricular hydrocephalus. Eight patients recovered completely, whereas the ninth displayed neurological sequelae.
ConclusionsCerebellitis is a medical and surgical emergency; diagnosis requires a high level of suspicion and an emergency brain magnetic resonance imaging study. It is a clinical-radiological syndrome characterised by acute or subacute encephalopathy with intracranial hypertension and cerebellar syndrome associated with T2-weighted and FLAIR hyperintensities in the cerebellar cortex (unilaterally or bilaterally) and possible triventricular dilatation. Treatment is based on high-dose corticosteroids and may require external ventricular drain placement and decompressive surgery.
La cerebelitis aguda es una rara afección inflamatoria con curso clínico muy variable: desde proceso autolimitado benigno hasta presentación fulminante con riesgo vital por compresión de fosa posterior, hidrocefalia aguda e hipertensión endocraneal.
MétodosRevisión de los hallazgos clínicos, analíticos y radiológicos de niños diagnosticados de cerebelitis aguda en el periodo comprendido entre mayo del 2007 y noviembre del 2016. Se analizan los tratamientos empleados y la evolución clínica y radiológica.
ResultadosNueve niños cumplían criterios de cerebelitis. La cefalea, los vómitos y la somnolencia fueron los síntomas de presentación más frecuentes; la ataxia, la disartria y la dismetría fueron los signos cerebelosos más frecuentes. La resonancia magnética fue el método diagnóstico mostrando afectación cerebelosa (uni o bilateral), mientras que la tomografía computarizada fue normal o solo mostraba signos indirectos como hidrocefalia triventricular por compresión extrínseca del acueducto de Silvio. Los corticoides fueron el tratamiento más empleado, administrados en 6 de los pacientes. Un paciente requirió intervención quirúrgica por hidrocefalia triventricular. Ocho pacientes tuvieron recuperación completa, mientras que uno presenta déficits neurológicos.
ConclusionesLa cerebelitis es una urgencia médico-quirúrgica. Precisa un alto índice de sospecha y la realización de resonancia magnética cerebral urgente. Es un síndrome clínico-radiológico: encefalopatía aguda o subaguda, con hipertensión endocraneal y síndrome cerebeloso junto a hiperintensidad en córtex cerebeloso (uni o bilateral) en secuencias T2 y FLAIR y posible dilatación triventricular. El tratamiento es con corticoides a dosis altas y puede precisar derivación ventricular externa y cirugía descompresiva.
Acute cerebellitis, a rare, recently described disease, constitutes the most severe form of cerebellar infectious/inflammatory disease.1,2 This clinical/radiological syndrome may be associated with acute or subacute encephalopathy, intracranial hypertension (headache, vomiting, and decreased level of consciousness), and cerebellar syndrome (ataxia, dysmetria, dysarthria, and vertigo).3 From a radiological viewpoint, T2-weighted and FLAIR MRI sequences reveal unilateral or bilateral cerebellar cortex hyperintensities, while CT may show mild dilatation of the third and lateral ventricles, marked hydrocephalus, a small fourth ventricle, and compression of posterior fossa structures.2 Acute cerebellitis usually develops during the course of a primary infection of the cerebellum or following infection or vaccination.4 The clinical course varies greatly, ranging from a relatively benign, self-limited course to an extremely severe presentation5–7 whose associated complications (posterior fossa compression, acute hydrocephalus, intracranial hypertension) may endanger the patient's life. This medical and surgical emergency may respond to high-dose corticosteroids; in more severe cases, it may require an external ventricular drain or even decompressive surgery.
We present our experience with acute cerebellitis in paediatric patients, focusing on symptoms, diagnostic tools, treatment, and progression.
Patients and methodsOur study included all patients diagnosed with cerebellitis and included in the database of the neuropaediatrics department at Hospital Universitario Miguel Servet, in Zaragoza (first case, May 2007; last case, November 2016). The database, which has been used in other studies,8,9 includes detailed follow-up data on all patients attended at the neuropaediatrics department. Patient data were gathered from the database and the patients’ medical histories.
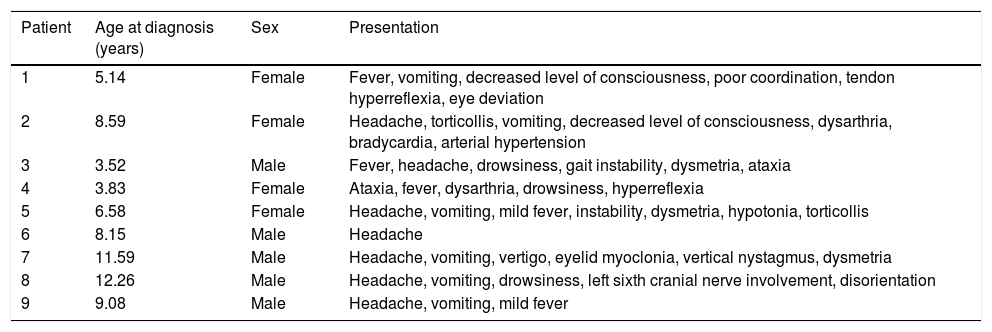
ResultsNine children (5 boys and 4 girls), aged 3.5-12.2 years old, met our inclusion criteria. Tables 1–4 summarise clinical, laboratory, and radiological findings; treatments used; and long-term follow-up data.
Clinical and demographic data for the 9 patients with acute cerebellitis.
| Patient | Age at diagnosis (years) | Sex | Presentation |
|---|---|---|---|
| 1 | 5.14 | Female | Fever, vomiting, decreased level of consciousness, poor coordination, tendon hyperreflexia, eye deviation |
| 2 | 8.59 | Female | Headache, torticollis, vomiting, decreased level of consciousness, dysarthria, bradycardia, arterial hypertension |
| 3 | 3.52 | Male | Fever, headache, drowsiness, gait instability, dysmetria, ataxia |
| 4 | 3.83 | Female | Ataxia, fever, dysarthria, drowsiness, hyperreflexia |
| 5 | 6.58 | Female | Headache, vomiting, mild fever, instability, dysmetria, hypotonia, torticollis |
| 6 | 8.15 | Male | Headache |
| 7 | 11.59 | Male | Headache, vomiting, vertigo, eyelid myoclonia, vertical nystagmus, dysmetria |
| 8 | 12.26 | Male | Headache, vomiting, drowsiness, left sixth cranial nerve involvement, disorientation |
| 9 | 9.08 | Male | Headache, vomiting, mild fever |
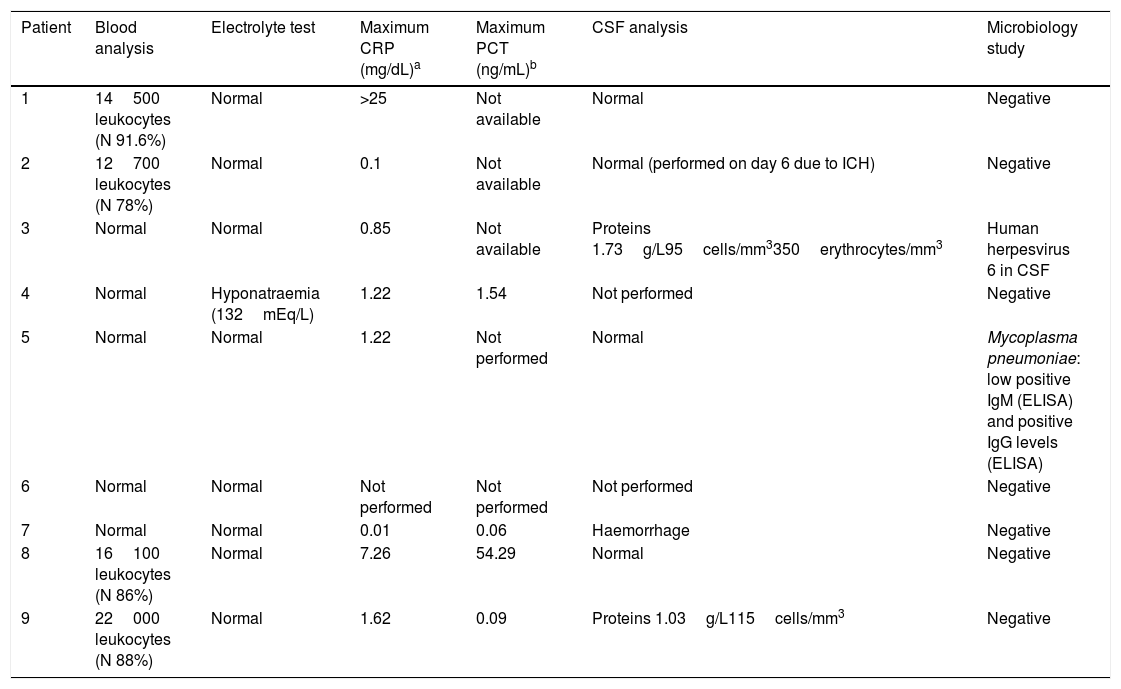
Laboratory test results for the 9 patients with acute cerebellitis.
| Patient | Blood analysis | Electrolyte test | Maximum CRP (mg/dL)a | Maximum PCT (ng/mL)b | CSF analysis | Microbiology study |
|---|---|---|---|---|---|---|
| 1 | 14500 leukocytes (N 91.6%) | Normal | >25 | Not available | Normal | Negative |
| 2 | 12700 leukocytes (N 78%) | Normal | 0.1 | Not available | Normal (performed on day 6 due to ICH) | Negative |
| 3 | Normal | Normal | 0.85 | Not available | Proteins 1.73g/L95cells/mm3350erythrocytes/mm3 | Human herpesvirus 6 in CSF |
| 4 | Normal | Hyponatraemia (132mEq/L) | 1.22 | 1.54 | Not performed | Negative |
| 5 | Normal | Normal | 1.22 | Not performed | Normal | Mycoplasma pneumoniae: low positive IgM (ELISA) and positive IgG levels (ELISA) |
| 6 | Normal | Normal | Not performed | Not performed | Not performed | Negative |
| 7 | Normal | Normal | 0.01 | 0.06 | Haemorrhage | Negative |
| 8 | 16100 leukocytes (N 86%) | Normal | 7.26 | 54.29 | Normal | Negative |
| 9 | 22000 leukocytes (N 88%) | Normal | 1.62 | 0.09 | Proteins 1.03g/L115cells/mm3 | Negative |
CRP: C-reactive protein; CSF: cerebrospinal fluid; ICH: intracranial hypertension; IgG: immunoglobulin G; IgM: immunoglobulin M; N: neutrophils; PCT: procalcitonin.
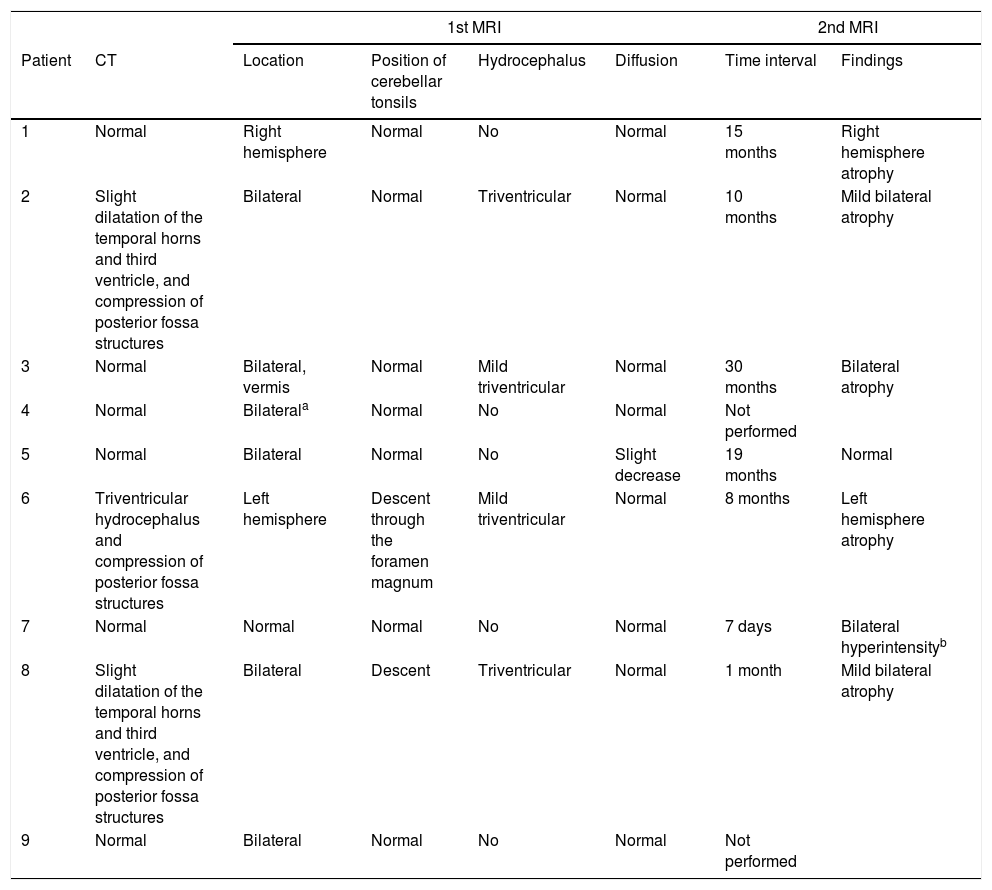
Neuroimaging findings for the 9 patients with acute cerebellitis.
| 1st MRI | 2nd MRI | ||||||
|---|---|---|---|---|---|---|---|
| Patient | CT | Location | Position of cerebellar tonsils | Hydrocephalus | Diffusion | Time interval | Findings |
| 1 | Normal | Right hemisphere | Normal | No | Normal | 15 months | Right hemisphere atrophy |
| 2 | Slight dilatation of the temporal horns and third ventricle, and compression of posterior fossa structures | Bilateral | Normal | Triventricular | Normal | 10 months | Mild bilateral atrophy |
| 3 | Normal | Bilateral, vermis | Normal | Mild triventricular | Normal | 30 months | Bilateral atrophy |
| 4 | Normal | Bilaterala | Normal | No | Normal | Not performed | |
| 5 | Normal | Bilateral | Normal | No | Slight decrease | 19 months | Normal |
| 6 | Triventricular hydrocephalus and compression of posterior fossa structures | Left hemisphere | Descent through the foramen magnum | Mild triventricular | Normal | 8 months | Left hemisphere atrophy |
| 7 | Normal | Normal | Normal | No | Normal | 7 days | Bilateral hyperintensityb |
| 8 | Slight dilatation of the temporal horns and third ventricle, and compression of posterior fossa structures | Bilateral | Descent | Triventricular | Normal | 1 month | Mild bilateral atrophy |
| 9 | Normal | Bilateral | Normal | No | Normal | Not performed | |
CT: computed tomography; MRI: magnetic resonance imaging.
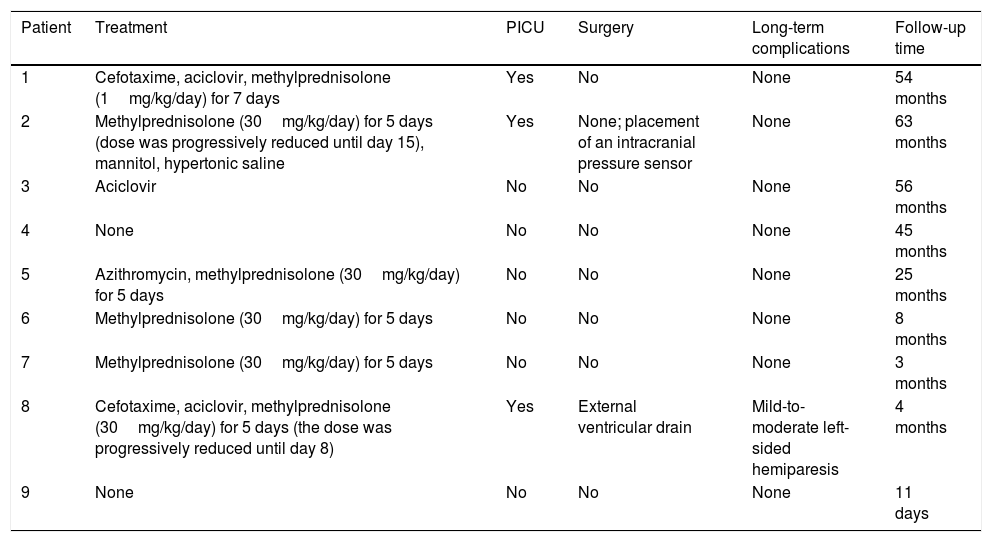
Treatment, clinical course, and long-term progression of the 9 patients with acute cerebellitis.
| Patient | Treatment | PICU | Surgery | Long-term complications | Follow-up time |
|---|---|---|---|---|---|
| 1 | Cefotaxime, aciclovir, methylprednisolone (1mg/kg/day) for 7 days | Yes | No | None | 54 months |
| 2 | Methylprednisolone (30mg/kg/day) for 5 days (dose was progressively reduced until day 15), mannitol, hypertonic saline | Yes | None; placement of an intracranial pressure sensor | None | 63 months |
| 3 | Aciclovir | No | No | None | 56 months |
| 4 | None | No | No | None | 45 months |
| 5 | Azithromycin, methylprednisolone (30mg/kg/day) for 5 days | No | No | None | 25 months |
| 6 | Methylprednisolone (30mg/kg/day) for 5 days | No | No | None | 8 months |
| 7 | Methylprednisolone (30mg/kg/day) for 5 days | No | No | None | 3 months |
| 8 | Cefotaxime, aciclovir, methylprednisolone (30mg/kg/day) for 5 days (the dose was progressively reduced until day 8) | Yes | External ventricular drain | Mild-to-moderate left-sided hemiparesis | 4 months |
| 9 | None | No | No | None | 11 days |
PICU: admission to paediatric intensive care unit.
The most common initial manifestation was headache (7 patients), followed by vomiting (6) and decreased level of consciousness or drowsiness (5). Four patients displayed signs and symptoms of cerebellar involvement (ataxia, dysmetria, dysarthria, and vertigo) (Table 1). Regarding laboratory findings, 5 patients presented fever or mild fever, 4 patients had leukocytosis (neutrophilia), and 6 had high C-reactive protein levels (2 of these also had high procalcitonin levels) (Table 2).
In the microbiological studies, CSF polymerase chain reaction detected human herpesvirus 6 DNA in patient 3, while patient 5 tested positive for Mycoplasma pneumoniae (IgM and IgG) in a serology study.
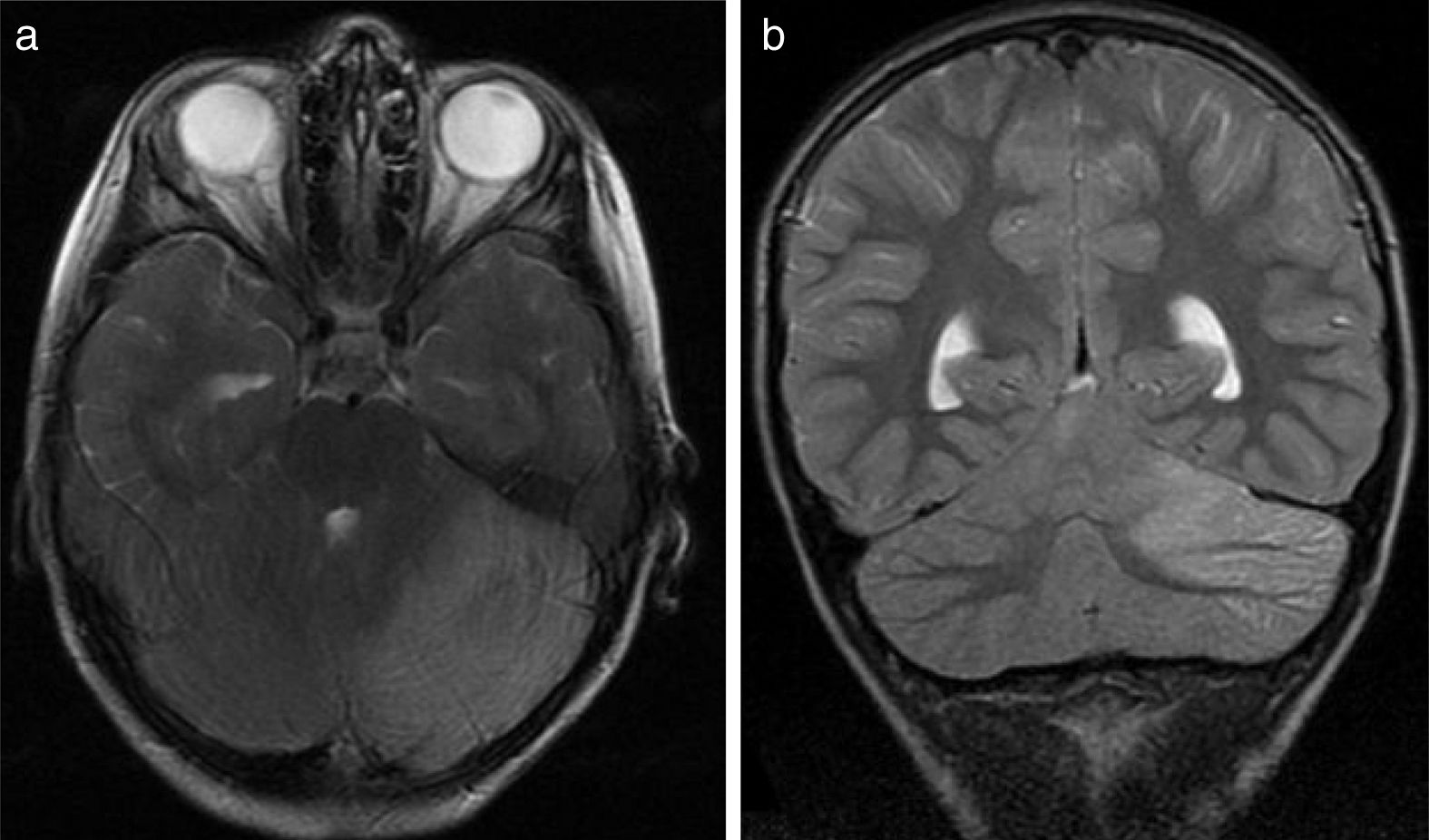
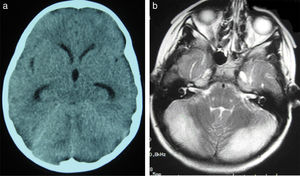
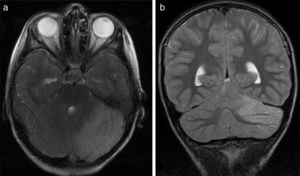
All patients underwent CT and MRI studies (Table 3). Three patients displayed CT alterations indicating posterior fossa compression and triventricular hydrocephalus, which was in the initial stages in 2 patients (Fig. 1a). All patients displayed MRI alterations: 7 patients showed bilateral cerebellar hyperintensities (Fig. 1b), associated with cerebellar vermis hyperintensity in one patient, and 2 showed unilateral cerebellar hyperintensities (one on the left hemisphere and the other on the right) (Fig. 2a and b). Two patients displayed downward displacement of the cerebellar tonsils through the foramen magnum. MRI also revealed triventricular hydrocephalus in 4 patients. Patient 4 underwent an MRI study as part of routine follow-up for Gorlin syndrome, 3 years after the first manifestations of cerebellitis. The MRI study revealed hyperintense areas in the cerebellar cortex bilaterally. CT and MRI scan results were initially normal in patient 7, with bilateral cerebellar hyperintensities detected in an additional MRI scan performed 7 days later due to persistent symptoms and diagnostic suspicion of the disorder. Patient 9 showed normal CT results and underwent an MRI scan 11 days after symptom onset (6 days after admission to hospital), at which point he was asymptomatic and receiving no treatment.
(a) Cranial CT scan of patient 2. Slight dilatation of the temporal horns and the third ventricle, which appears more rounded than is normal. Compression of structures of the posterior fossa; the fourth ventricle is not visible. (b) Axial T2-weighted brain MRI sequence from patient 2. Grey matter hyperintensity in both cerebellar hemispheres.
(a) Axial T2-weighted brain MRI scan of patient 6. Signal hyperintensity in the left cerebellar cortex. Hemicerebellitis. (b) Coronal T2-weighted brain MRI scan of patient 6. Hyperintensities affect the left cerebellar cortex exclusively; no white matter hyperintensities are observed.
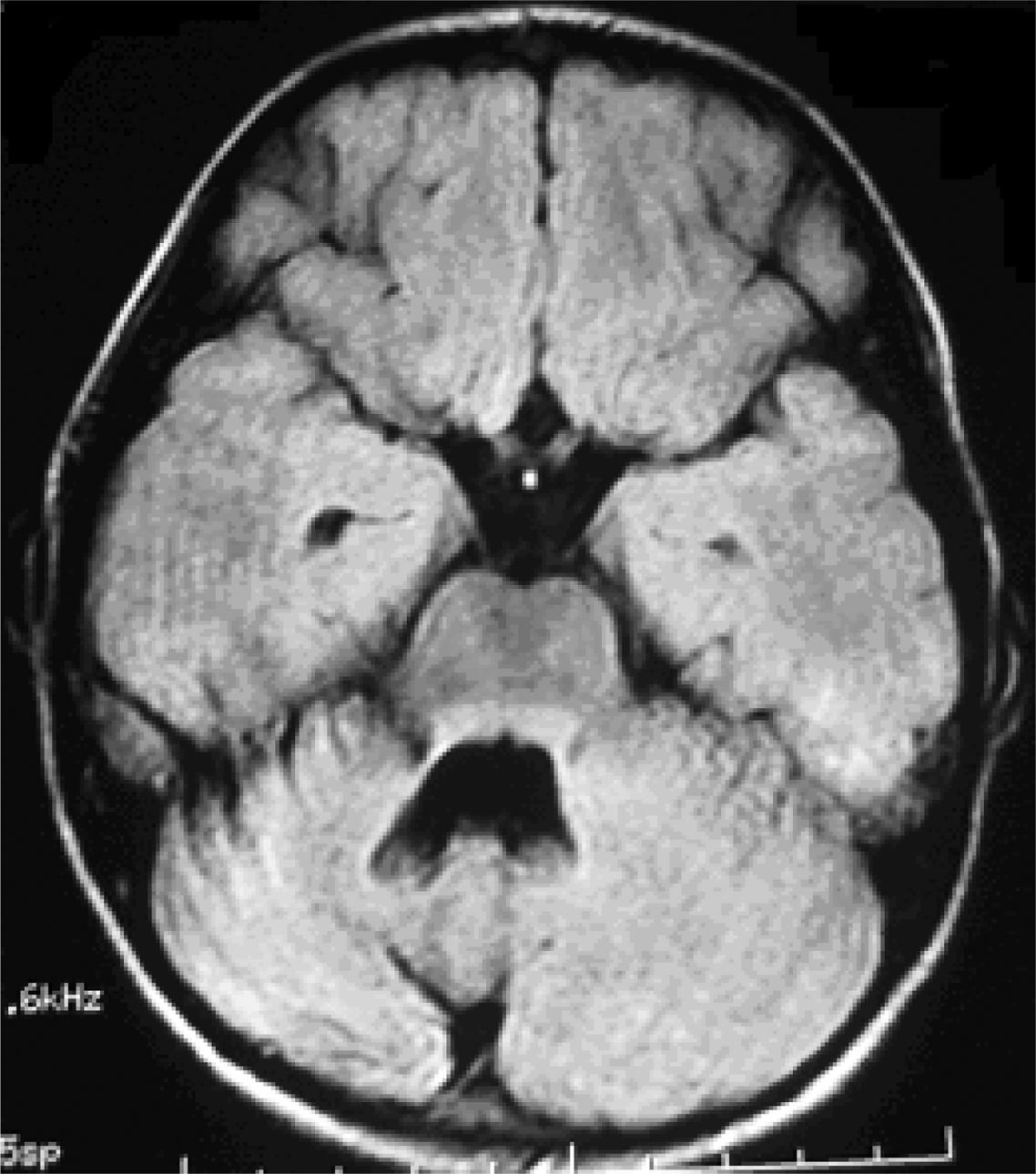
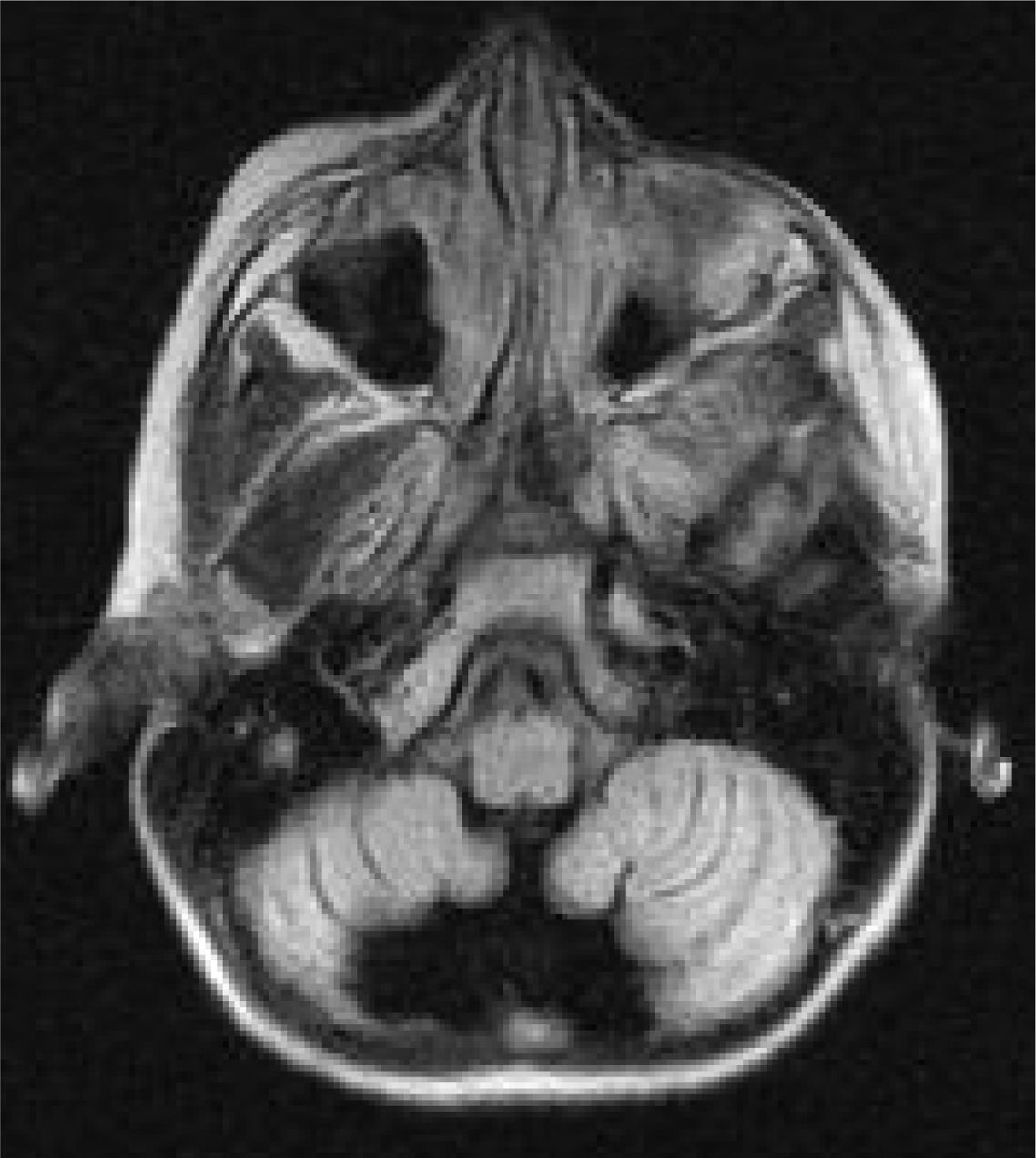
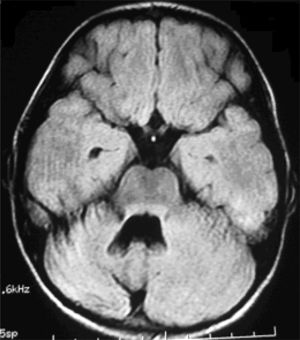
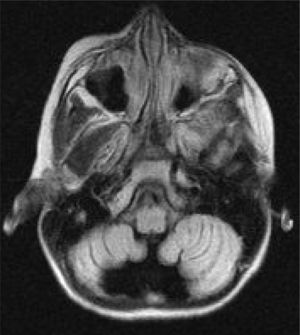
Six patients underwent follow-up MRI scans between 1 and 30 months after symptom onset. Five patients displayed cerebellar atrophy, which was bilateral in 3 and unilateral in 2 (Figs. 3 and 4). Patients 7 and 9 have not yet undergone a follow-up MRI study.
Six patients received corticosteroids (Table 4): one received 1mg/kg/day for 7 days and 5 received 30mg/kg/day for 5 days; the dose was subsequently decreased in 2 patients (8 and 15 days). Three patients received antibiotics (2 cefotaxime and 1 azithromycin) and 3 aciclovir. Three patients were admitted to the paediatric intensive care unit, one of whom (patient 2) required intubation and mechanical ventilation due to decreased level of consciousness and signs of brain herniation (arterial hypertension and severe bradycardia), in addition to intracranial pressure sensor placement and antioedema therapy. Patient 8 received surgery for hydrocephalus; after an unsuccessful ventriculostomy, an external ventricular drain was placed.
Progression was favourable in 8 patients (follow-up time ranged from 3 to 63 months, except for patient 9, who was asymptomatic 11 days after symptom onset, having received no treatment). At 4 months of follow-up, patient 8 showed mild-to-moderate left-sided hemiparesis following intraparenchymal haemorrhage involving the internal capsule and thalamus, a surgical complication.
DiscussionAcute cerebellitis, whose incidence is unknown, appears to be underdiagnosed rather than rare.1,4 Our series includes 9 patients diagnosed over a period of 9 years, although some cases (especially mild cases, as in patient 6) could easily have remained undetected, as identification of the condition requires a high level of suspicion and in most cases an MRI study.
Although some authors distinguish acute cerebellitis from postinfectious acute cerebellar ataxia (ACA),4 others regard it as the most severe form of the same autoimmune/inflammatory process involved in ACA.10
ACA is characterised by sudden onset of gait instability without meningeal symptoms, seizures, altered level of consciousness, or neurological signs not explained by alterations in the rhombencephalon.11 Acute cerebellitis can present with a wider range of symptoms, including cerebellar symptoms, ataxia, vomiting, headache, fever, meningeal symptoms, seizures, and altered level of consciousness. MRI shows normal results or non-specific findings in patients with ACA,11–13 but reveals unilateral or bilateral cerebellar cortex hyperintensities in patients with acute cerebellitis. Cerebellitis cannot be ruled out without an MRI study.14
Its clinical course is highly variable, ranging from a relatively benign self-limited process to fulminant presentation with acute hydrocephalus, severe intracranial hypertension, and risk of death. In our series, some patients progressed favourably without specific treatment (patients 4 and 9), whereas other, more severe cases (patients 2 and 8) involved a high vital risk. No patient died, although patient 8 was left with severe sequelae due to the gradual progression of the disease and the surgical complications.
Clinical presentation of acute cerebellitis varies greatly13; the main form of presentation includes intracranial hypertension (associated with headache, nausea, vomiting, decreased level of consciousness, and/or drowsiness), although patients may present other, less specific manifestations, including fever. Intracranial hypertension may be caused by obstructive hydrocephalus secondary to the compression exerted by inflammation on the aqueduct of Sylvius and the fourth ventricle. Patients may present cerebellar symptoms: ataxia, dysarthria, dysmetria, or intention tremor. Five patients from our series showed signs of intracranial hypertension and 4 presented cerebellar symptoms.
Neuroimaging is essential for diagnosis. Our results (Table 3) show that MRI is the diagnostic technique of choice, as it is the tool that best identifies central nervous system inflammation. If symptoms persist despite normal initial MRI results, an additional MRI scan should be performed; in patient 7, a second MRI scan performed 7 days after the initial study led to diagnosis of acute cerebellitis. CT is ineffective in diagnosing inflammatory processes in general, and this entity in particular. Its usefulness is limited to detecting indirect signs of posterior fossa involvement, such as triventricular hydrocephalus and morphological alterations of the structures located in the region, as seen in 3 of our patients.
Laboratory tests contributed little, which confirms that diagnosis is mainly clinical and radiological (Table 2). According to the literature, up to 24% of cases of acute cerebellitis are associated with a wide range of infectious agents, including measles virus, rubella virus, mumps virus, varicella-zoster virus, Epstein-Barr virus, herpes simplex virus 1, rotavirus, cytomegalovirus, poliovirus, influenza virus, respiratory syncytial virus, coxsackievirus, Salmonella, Borrelia, Bordetella, Coxiella, Streptococcus pneumoniae, and Mycoplasma pneumoniae.1,15–17 In our series, infection was detected in only 2 patients (human herpesvirus 6 in patient 3 and Mycoplasma pneumoniae in patient 5). Interestingly, these 2 patients displayed no leukocytosis and only slightly increased C-reactive protein levels (0.85 and 1.22mg/dL). CSF analysis (lumbar puncture was performed only in patients with no contraindication for the procedure due to suspected intracranial hypertension) revealed no abnormalities, except for elevated protein levels in the patient with human herpesvirus 6 infection (1.73g/L) and in patient 9 (1.03g/L); this is consistent with the literature.2
Although few studies have addressed the pathogenic mechanisms of this entity, the available evidence suggests oedema mediated by autoimmune mechanisms and associated with lymphocytic and eosinophilic infiltration with no signs of demyelination; this is the main difference between acute cerebellitis and acute disseminated encephalomyelitis. The disease is thought to be mediated by autoimmune mechanisms, as it usually manifests after an infection and is associated with presence of antibodies against Purkinje cells, centrosomes, glutamate receptors, gangliosides, cardiolipin, and glutamic acid decarboxylase.15,18–21 However, the pathophysiology of acute cerebellitis is yet to be fully understood.
Antimicrobial therapy should be considered in these patients since acute cerebellitis may be associated with a number of pathogens: ataxia may be a sign of viral encephalitis or bacterial meningitis. Empirical antibiotic treatment is essential when lumbar puncture cannot be performed due to the risk of cerebral herniation.22–24
Acute cerebellitis is a medical and surgical emergency; high-dose corticosteroids seem to be the cornerstone of effective treatment. However, the suitability of these drugs and the role of other adjuvant therapies is much debated; no consensus guidelines for the treatment of this entity have been published. In addition to reducing cerebral oedema, which may play a crucial role in reducing ventricular dilatation and the resulting intracranial hypertension, corticosteroids have an immunomodulatory effect.25 In a series of 7 patients with cerebellitis, Göhlich-Ratmann et al.26 report that the 3 patients receiving high-dose corticosteroids achieved complete recovery, whereas none of the 4 patients who died or displayed sequelae had been treated with corticosteroids. Other series, such as those published by Roldan et al.25 and Noguera-Julián et al.,27 show better long-term prognosis and shorter disease duration in association with corticosteroid treatment.
Other immunomodulatory treatments, including intravenous immunoglobulins and plasmapheresis, have been proposed for the treatment of acute cerebellitis3,4 and even ACA.28–30
More aggressive approaches may be necessary, including external ventricular drain placement31 and posterior fossa decompression surgery. In our series, only one patient required an external ventricular drain (ventriculostomy was attempted initially).
Acute cerebellitis seems to be a more frequent condition than previously thought. Diagnosis requires a high level of suspicion and an emergency brain MRI scan.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García-Iñiguez JP, López-Pisón FJ, Madurga Revilla P, Montejo Gañán I, Domínguez Cajal M, Monge Galindo L, et al. Cerebelitis aguda en pediatría: nuestra experiencia. Neurología. 2019;34:291–299.