Cancer and degenerative diseases share some pathogenic mechanisms which act in opposition to one another to produce either uncontrolled cell proliferation or cell death. According to several studies, patients with Alzheimer disease have a lower risk of neoplasia, and vice versa. This study describes the prevalence of tumours (active or successfully treated) in a series of patients with and without a dementing degenerative disease treated at a cognitive neurology unit.
Patients and methodWe analysed the frequency and topography of tumours and the presence or absence of a neurodegenerative disease in a group of 1164 patients. Neurodegenerative diseases were classified in 4 groups: Alzheimer disease, synucleinopathies, Pick complex, and polyglutamine complex. We subsequently compared tumour frequency in patients with and without a degenerative disease, and prevalence of neurodegenerative diseases in patients with and without tumours.
ResultsTumours were detected in 12.1% of the patients with a neurodegenerative disease and in 17.3% of the remaining patients. Around 14.8% of the patients with a history of neoplasia and 20.8% of the patients with no history of neoplasia were diagnosed with a neurodegenerative disease. Except for these differences and the differences between subgroups (type of degenerative disease and tumour location) were not statistically significant, except when comparing neurodegenerative diseases to central nervous system tumours, and synucleinopathies to neoplasms.
ConclusionDementing degenerative diseases and neoplastic disorders are not mutually exclusive. Nevertheless, the rate of co-occurrence is lower than would be expected given the prevalence rate for each group.
El cáncer y las enfermedades degenerativas constituyen trastornos con algunos mecanismos compartidos que actúan en sentido opuesto, produciendo un fenómeno incontrolado de proliferación o pérdida de células. Observaciones diversas apuntan que los pacientes con enfermedad de Alzheimer tienen menor riesgo de desarrollar tumores y vice versa. En este artículo se expone la prevalencia de tumores (activos o superados) en pacientes de neurología cognitiva con y sin una enfermedad degenerativa demenciante.
Pacientes y métodoEn 1.164 pacientes se analizó la frecuencia y topografía de tumores y la presencia o ausencia de enfermedad neurodegenerativa, que se clasificó en 4 grupos (enfermedad de Alzheimer, sinucleinopatía, enfermedad del complejo Pick y del complejo de poliglutamina). Se comparó la frecuencia de tumor en los subgrupos con y sin enfermedad degenerativa, y de esta en los pacientes con y sin trastorno tumoral.
ResultadosSe registró proceso tumoral en el 12,1% de los pacientes con enfermedad neurodegenerativa y en el 17,3% del resto del grupo. En el grupo del estudio, un 14,8% de los que tienen antecedente tumoral fue diagnosticado de enfermedad neurodegenerativa, frente al 20,8% entre los que no tienen ese antecedente. Estas diferencias y las observadas en la comparación de subgrupos (tipo de enfermedad degenerativa y topografía del tumor) no alcanzaron significación estadística, excepto al contrastar enfermedades neurodegenerativas con tumores del sistema nervioso central y sinucleinopatías con neoplasias.
ConclusionesLas enfermedades neoplásicas y las neurodegenerativas demenciantes no son excluyentes, aunque muestran menor asociación de la esperada por su respectiva prevalencia.
In the same way as the 20th century saw a huge step forward in the field of infectious diseases, degenerative and neoplastic diseases constitute the great challenge of 21st century medicine. According to worldwide data from the World Health Organization's International Agency for Research on Cancer, there were 15206036 new cases of cancer (excluding non-melanoma skin cancer; 2753183 cases in the European Union and 227076 in Spain) and 8885195 deaths due to cancer (1341938 in the European Union and 108390 in Spain) in 2015.1 The present study focuses on degenerative diseases that cause dementia. Despite focusing on a specific subgroup, incidence of these diseases is high. According to published epidemiological data, 7 million to 15 million new cases of degenerative diseases were recorded worldwide in 2015 (1.6 million in Europe and 100000 in Spain).2–5 Degenerative diseases constitute the third leading cause of moderate to severe disability in people older than 60 in developed countries, after sensory processing disorders and osteoarticular disorders.6 The incidence of the most frequent neoplastic and neurodegenerative diseases increases with age7,8; the increase in life expectancy will therefore result in greater incidence, unless we develop treatments capable of reverting this trend.
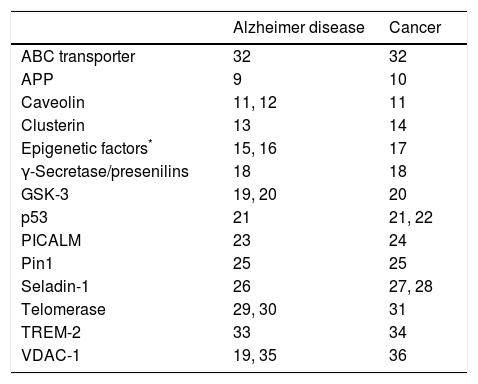
Cancer is characterised by uncontrolled cell proliferation, while degenerative diseases are associated with spontaneous cell loss at a faster rate than that observed in normal ageing. This opposition suggests the possibility of common underlying biological mechanisms. Alterations in these regulatory mechanisms may promote either cell death or cell proliferation. Multiple pathophysiological circumstances are known to be involved in both degenerative diseases and cancer. Table 1 lists some of the factors associated with Alzheimer disease (AD),9–36 although other degenerative diseases are associated with additional factors.37
Proteins and genetic factors involved in the pathogenesis of Alzheimer disease and cancer, and bibliographical references of studies explaining their action mechanisms.
| Alzheimer disease | Cancer | |
|---|---|---|
| ABC transporter | 32 | 32 |
| APP | 9 | 10 |
| Caveolin | 11, 12 | 11 |
| Clusterin | 13 | 14 |
| Epigenetic factors* | 15, 16 | 17 |
| γ-Secretase/presenilins | 18 | 18 |
| GSK-3 | 19, 20 | 20 |
| p53 | 21 | 21, 22 |
| PICALM | 23 | 24 |
| Pin1 | 25 | 25 |
| Seladin-1 | 26 | 27, 28 |
| Telomerase | 29, 30 | 31 |
| TREM-2 | 33 | 34 |
| VDAC-1 | 19, 35 | 36 |
DNA methylation, histone acetylation, non-coding RNA. ABC: ATP-binding cassette; APP: amyloid precursor protein; GSK-3: glycogen synthase kinase 3; PICALM: phosphatidylinositol binding clathrin assembly protein; Pin1: peptidyl-prolyl cis/trans isomerase 1; seladin-1: 24-dehydrocholesterol reductase; TREM-2: triggering receptor expressed on myeloid cells 2; VDAC-1: voltage-dependent anion channel 1.
Recent studies report reduced frequencies of tumours in patients with AD and lower rates of AD in patients with history of cancer38–44; this supports the hypothesis of shared aetiopathogenic mechanisms.45–47 Although most studies focus on AD, some researchers describe the same phenomenon in patients with non-Alzheimer degenerative dementias.48–50 This paves the way for research into treatments to prevent or modulate the progression of both degenerative diseases and cancer.
This study explores the prevalence of a wide range of neoplastic processes (either active or successfully treated) in a group of patients attending a cognitive neurology unit. Our patients had varying degrees of cognitive impairment with a wide range of causes (including neurodegenerative diseases); this allowed us to determine whether the presence of a neoplastic process (generic or specific) may modify the risk of developing neurodegenerative diseases, and vice versa.
Patients and methodsWe analysed data from a register of patients attending a neurology clinic which primarily treats patients with cognitive impairment. At the initial consultation, we gathered data on age, sex, aetiological diagnosis, and history of tumours, whether active or successfully treated; this information is collected systematically. We excluded patients with uncertain aetiological diagnosis and those for whom information on history of tumours was imprecise or unreliable.
Patients were classified according to whether or not they had been diagnosed with a neurodegenerative disease with potential to cause dementia. For patients who did have neurodegenerative disease, we also recorded whether the diagnosis was AD, a disease of the Pick complex (frontotemporal dementia and its variants, corticobasal syndrome, and progressive supranuclear palsy), α-synucleinopathies (Parkinson's disease dementia, Lewy body dementia, multiple system atrophy),51 or polyglutamine diseases (Huntington disease and some types of spinocerebellar atrophy).52 All patients showed the typical cognitive, behavioural, and neuroimaging alterations (MRI, CT, and/or 18F-fludeoxyglucose PET) of the symptomatic phase of each disease.
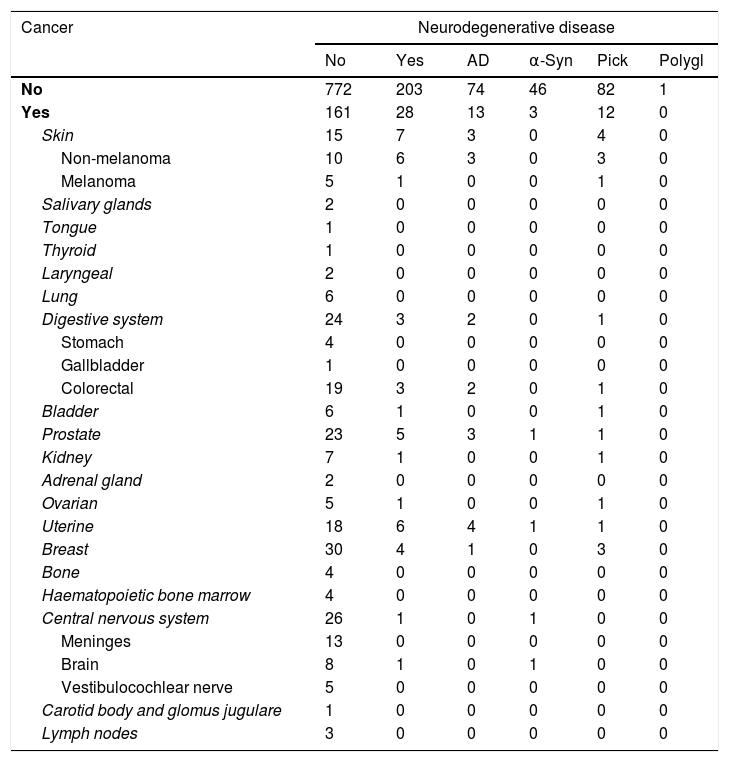
We established a topographical classification of tumours for those patients with cancer (Table 2). Central nervous system (CNS) tumours were grouped according to the 2016 classification of the World Health Organization.53
Distribution of the patient sample by type of neurodegenerative disease and cancer.
| Cancer | Neurodegenerative disease | |||||
|---|---|---|---|---|---|---|
| No | Yes | AD | α-Syn | Pick | Polygl | |
| No | 772 | 203 | 74 | 46 | 82 | 1 |
| Yes | 161 | 28 | 13 | 3 | 12 | 0 |
| Skin | 15 | 7 | 3 | 0 | 4 | 0 |
| Non-melanoma | 10 | 6 | 3 | 0 | 3 | 0 |
| Melanoma | 5 | 1 | 0 | 0 | 1 | 0 |
| Salivary glands | 2 | 0 | 0 | 0 | 0 | 0 |
| Tongue | 1 | 0 | 0 | 0 | 0 | 0 |
| Thyroid | 1 | 0 | 0 | 0 | 0 | 0 |
| Laryngeal | 2 | 0 | 0 | 0 | 0 | 0 |
| Lung | 6 | 0 | 0 | 0 | 0 | 0 |
| Digestive system | 24 | 3 | 2 | 0 | 1 | 0 |
| Stomach | 4 | 0 | 0 | 0 | 0 | 0 |
| Gallbladder | 1 | 0 | 0 | 0 | 0 | 0 |
| Colorectal | 19 | 3 | 2 | 0 | 1 | 0 |
| Bladder | 6 | 1 | 0 | 0 | 1 | 0 |
| Prostate | 23 | 5 | 3 | 1 | 1 | 0 |
| Kidney | 7 | 1 | 0 | 0 | 1 | 0 |
| Adrenal gland | 2 | 0 | 0 | 0 | 0 | 0 |
| Ovarian | 5 | 1 | 0 | 0 | 1 | 0 |
| Uterine | 18 | 6 | 4 | 1 | 1 | 0 |
| Breast | 30 | 4 | 1 | 0 | 3 | 0 |
| Bone | 4 | 0 | 0 | 0 | 0 | 0 |
| Haematopoietic bone marrow | 4 | 0 | 0 | 0 | 0 | 0 |
| Central nervous system | 26 | 1 | 0 | 1 | 0 | 0 |
| Meninges | 13 | 0 | 0 | 0 | 0 | 0 |
| Brain | 8 | 1 | 0 | 1 | 0 | 0 |
| Vestibulocochlear nerve | 5 | 0 | 0 | 0 | 0 | 0 |
| Carotid body and glomus jugulare | 1 | 0 | 0 | 0 | 0 | 0 |
| Lymph nodes | 3 | 0 | 0 | 0 | 0 | 0 |
α-Syn: α-synucleinopathies; AD: Alzheimer disease; Pick: diseases of the Pick complex; polygl: polyglutamine diseases.
We performed a statistical analysis to detect any differences in the frequency of neoplastic diseases in general or any of the specific types recorded, between patients with and without a dementia-related neurodegenerative disease or any of its variants. The chi-square test and the Fisher exact test were used for this analysis; P-values <.05 were considered statistically significant. Odds ratios and 95% confidence intervals (CI) were used as the measure of association. Data were analysed using version 19.0 of the SPSS statistical software for Windows.
ResultsTables 2 and 3 summarise the clinical characteristics of our patient sample. Patients were mainly women, both in the total sample and in all subgroups. Our sample's mean age was above 70.
Characteristics of patients included in the study.
| Degenerative disease | No degenerative disease | Total | |
|---|---|---|---|
| Cancer | 28(12.1; 14.8; 60.7; 76.8) | 161(17.3; 85.2; 53.4; 77.7) | 189(16.2; 100; 54.5; 77.6) |
| No cancer | 203(87.9; 20.8; 59.6; 74.6) | 772(82.7; 79.2; 63.9; 72.3) | 975(83.8; 100; 63.0; 72.8) |
| Total | 231(100; 19.8; 59.7; 74.9) | 933(100; 80.2; 62.1; 73.3) | 1164(100; 100; 61.6; 73.6) |
Data are presented as number of cases (% of column; % of row; % women; mean age).
History of cancer (active or successfully treated) was recorded in 12.1% of patients with dementia-related neurodegenerative diseases and 17.3% of patients without. In our sample, 14.8% of patients with history of cancer and 20.8% of patients without had a diagnosis of neurodegenerative disease (Table 3); these differences were not statistically significant (P=.059).
We found significant differences in the frequency of CNS tumours between patients with and without neurodegenerative diseases as a whole (P=.028) (Table 4), and in the frequency of tumours between patients with and without α-synucleinopathies (P=.047) (Tables 5 and 6).
Presence of central nervous system tumours in patients with and without dementia-related neurodegenerative diseases.
| Degenerative disease | No degenerative disease | Total | |
|---|---|---|---|
| CNS tumour | 1(0.4; 3.7) | 26(2.8; 96.3) | 27(2.3; 100) |
| No CNS tumour | 230(99.6; 20.2) | 907(97.2; 79.8) | 1137(97.7; 100) |
| Total | 231(100; 19.8) | 933(100; 80.2) | 1164(100; 100) |
CNS: central nervous system.
Data are presented as number of cases (% of column; % of row).
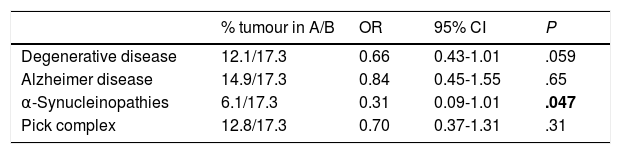
Presence of tumours in patients with different types of neurodegenerative disease.
| % tumour in A/B | OR | 95% CI | P | |
|---|---|---|---|---|
| Degenerative disease | 12.1/17.3 | 0.66 | 0.43-1.01 | .059 |
| Alzheimer disease | 14.9/17.3 | 0.84 | 0.45-1.55 | .65 |
| α-Synucleinopathies | 6.1/17.3 | 0.31 | 0.09-1.01 | .047 |
| Pick complex | 12.8/17.3 | 0.70 | 0.37-1.31 | .31 |
95% CI: 95% confidence interval; A: patients with the disease indicated in the row; B: patients without degenerative diseases; OR: odds ratio.
The only statistically significant result is shown in bold (P<.05).
Presence of tumours in patients diagnosed with α-synucleinopathies or no degenerative disease.
| α-Synucleinopathies | No degenerative disease | Total | |
|---|---|---|---|
| Tumour | 3(6.1; 1.8) | 161(17.3; 98.2) | 164(16.7; 100) |
| No tumour | 46(93.9; 5.6) | 772(82.7; 94.4) | 818(83.3; 100) |
| Total | 49(100; 5.0) | 933(100; 95.0) | 982(100; 100) |
Data are presented as number of cases (% of column; % of row).
We studied a sample of 1164 patients to analyse differences in the frequency of tumours in patients with and without dementia-related neurodegenerative diseases, and in the frequency of this type of diseases between patients with and without history of tumours.
The patients included were predominantly women (61.6%), which may be explained by the higher percentage of women in the study population (51.8%) and the higher incidence of dementia among women2 (our sample was drawn from a cognitive neurology unit). However, the percentage of women differs significantly between the groups with and without history of tumours (54.5% vs 63%). This may be due to the higher incidence of cancer among men.1,54
Patients with neurodegenerative diseases were older than those without (P<.034); the same was true for patients with history of cancer compared to those without (P<.001). This was to be expected given the age-dependent increase in the incidences of AD and Lewy body dementia (which account for more than half of cases of dementia) and cancer.8,55
Our results show a lower frequency of tumours among patients with dementia-related neurodegenerative diseases, and vice versa (Table 3). These data show a trend towards significance (P=.059). In the subgroup analysis, however, differences show a much less clear trend towards significance due to the smaller size of each subgroup. CNS tumours were significantly less frequent among patients with neurodegenerative diseases (one in 231 patients) than among those without (26 in 933 patients) (Table 4).
Our results are consistent with findings reported in the literature. Most studies report a lower incidence of tumours in patients with dementia-related neurodegenerative diseases and a lower incidence of the latter in patients with a history of cancer. Our results, however, show that these diseases are not mutually exclusive; some observations even reveal an association between them. Although results are heterogeneous (between studies and types of tumour), a meta-analysis of multiple studies shows a significantly weaker association between neurodegenerative diseases and cancer.38–40
With respect to type of neurodegenerative diseases, both our results and those of other studies show a negative association between cancer and AD.48–50 Our study also found a lower prevalence of tumours among patients with α-synucleinopathies. In addition to its role in the development of dementia-related α-synucleinopathies, α-synuclein is expressed in several types of tumour, including brain tumours with a neuronal component,56 schwannomas,57 melanomas and benign nevi,58 ovarian tumours,59 and myelodysplastic syndromes.60 This is not always reflected by epidemiological data, however. Some series of patients with Parkinson's disease show a higher incidence of melanoma,50,61 while others report the opposite.50,62 Some studies have found a higher incidence of brain tumours50,63 and breast cancer50,61 and a lower risk of lung and colorectal cancer in patients with Parkinson's disease.64 Tumours are generally less frequent in patients with Parkinson's disease62 or Lewy body dementia.48 In any case, the inconsistent results suggest that mutual protection is neither strong nor uniform, probably due to the involvement of other factors.
Analysis by tumour type shows less marked, more heterogeneous differences: Ou et al.42 found a significantly lower incidence of lung cancer in men with AD; Realmuto et al.65 found AD to be associated with significant differences in cancer risk in women and for endocrine tumours; and Roe et al.44 and Behrens et al.66 found differences for skin tumours. Our study found significant differences only when assessing CNS tumours as a single category. Classifying patients by tumour location results in small sample sizes, which prevents detection of statistically significant differences unless effect sizes are large. According to our results, only 2 types of tumour (non-melanoma skin cancer and uterine cancer) were more frequent among patients with degenerative diseases (2.6% for both types of cancer) than in the remaining participants (1.1% and 1.9%, respectively); the differences were not significant, however (Table 2). The analysis by tumour type resulted in small subgroups. The lack of significant differences prevents us from concluding that the presence of any of these tumours protects against neurodegenerative diseases.
The differences observed for CNS tumours merit some consideration. Hypothetically, the potential interference between the mechanisms of cell proliferation and cell death would seem to be more likely to occur in diseases affecting the same organ or system (e.g., the brain, the CNS). Therefore, development of a degenerative disease of the brain would be more compatible with a proliferative process affecting the lung or any other organ not associated with the nervous system. It should be noted that the genetic alterations known to cause cancer and degenerative diseases (causal mutations and risk genotypes) do not overlap. A review article by Richterová et al.67 lists over 60 genes involved in the pathogenesis of brain tumours, none of which coincide with any of the 30 genes currently known to be associated with AD.68 A single person may present genetic and environmental factors associated with degenerative diseases and cancer, and may therefore develop both. Neither our findings nor those of previous studies suggest that either type of disease precludes the other; rather, they show that the association between degenerative diseases and cancer is less frequent than the occurrence of these entities separately. This relative protection may be due to shared pathogenic mechanisms acting in opposite directions (Table 1).
Subgroup analysis by type of tumour or degenerative disease finds less clear differences due to the smaller sample size. The small number of patients with polyglutamine diseases and the low frequency of some types of tumour may be responsible for their low prevalence at advanced ages. In any case, the small number of cases prevents us from determining whether there is a relationship of intense cross-protection between the 2 entities. Some highly frequent types of cancer, including lung, stomach, liver, and oesophageal cancer, may be underrepresented due to the high associated mortality rates.1 This means that a large percentage of patients with these tumours would die before reaching the risk age for developing dementia-related neurodegenerative diseases. We may hypothesise that if it were not for this increase in the mortality rate, the association observed in our study would be even less marked. To minimise this bias, all patients with tumours and long survival times should be followed up.
The same is true of patients with degenerative diseases, which are associated with increased mortality rates (1.4 to 2.7 times higher).69,70 This means that the incidence of tumours associated with advanced age (which would develop later in life) will be lower in these patients than in individuals of the same age but without degenerative diseases.
According to the literature and to our own results, neurodegenerative diseases and cancer are not mutually exclusive, although the rate of co-occurrence is lower than expected considering the prevalence of each type of disease separately. This phenomenon is therefore of little use in clinical practice, since the presence of one of these diseases does not rule out the other when the patient shows compatible signs or symptoms. However, the low incidence of the combination of both types of disease points to the involvement of biases associated with the high mortality rates of both types of diseases, as well as common pathogenic mechanisms acting in opposite directions. Our findings underscore the importance of exploring treatments targeting these mechanisms, as these studies have greater translational value, and may result in the development of drugs capable of treating the 2 types of diseases with the highest mortality and disability rates worldwide.
FundingThis study has not been presented at any scientific conference or received funding from any institution.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Robles Bayón A, Gude Sampedro F. Nueva observación de la protección relativa que las enfermedades neurodegenerativas y el cáncer se confieren entre sí. Neurología. 2019;34:283–290.