The AD8 is a brief informant-based questionnaire that may also be self-administered, and which aids in identifying cognitive impairment (CI). Our goal is to assess the diagnostic accuracy (DA) of a Spanish version of that questionnaire.
Material and methodsA cross-sectional study of a clinical sample of patient/informant dyads including 330 subjects with suspected CI or dementia (DEM) and 71 controls, was conducted. We evaluated internal consistency (Cronbach's alpha) and validity (partial correlations with GDS stage, Fototest results and functional index measure [FIM]). We assessed DA for CI vs no CI (GDS stage 3–4) using the area under the ROC curve (AUC), and the cut-off with the highest Youden index was determined to be optimal.
ResultsIn the sample, 105 subjects had no CI, 99 had CI without DEM and 203 had DEM. Internal consistency was high (α 0.90, 95% confidence interval: 0.89–0.92), as were correlations with the GDS score (r=0.72, P<.001), Fototest results (r=−0.61, P<.001) and FIM (r=0.59, P<.001). The AUC for AD8 was 0.90 (95% confidence interval: 0.86–0.93), which was not significantly different from that of the Fototest (AUC 0.93, 95% confidence interval: 0.89–0.96). The optimal cut-off point was 3/4 with a sensitivity of 0.93 (95% confidence interval: 0.88–0.96) and a specificity of 0.81 (95% confidence interval: 0.72–0.88); 88.8% of the classifications were correct. Combined use of AD8 and the Fototest significantly improved the DA of both (AUC 0.96, 95% confidence interval: 0.93–0.98, P<.05).
ConclusionsThe Spanish version of the AD8 questionnaire preserves the psychometric qualities and DA of the original. Using this test in combination with the Fototest significantly increases the DA of both tests.
El AD8 es un cuestionario al informador breve que puede ser autoaplicado y facilita la identificación de deterioro cognitivo (DC); nuestro objetivo es evaluar la utilidad diagnóstica (UD) de una versión española.
Material y métodosEstudio transversal en una muestra clínica de díadas paciente/informador, 330 sujetos con sospecha de DC o demencia (DEM) y 71 controles. Se ha evaluado la consistencia interna (α de Cronbach) y la validez (correlaciones parciales con estadio GDS, Fototest e índice funcional [IF]). La UD se ha evaluado para no DC vs DC (GDS 3–4) por medio del área bajo la curva ROC (aROC) y se ha considerado mejor punto de corte aquel que hacía máximo el índice de Youden.
ResultadosEn la muestra, 105 no tenían DC, 99 tenían DC sin DEM y 203 DEM. La consistencia interna es alta (α 0.90, IC del 95%, 0,89–0,92), al igual que las correlaciones con GDS (r=0,72, p<0,001), Fototest (r=–0,61, p<0,001) e IF (r=0,59, p<0,001). El aROC del AD8 es 0,90 (IC del 95%, 0,86–0,93), sin diferencia significativa con la del Fototest (aROC 0,93, IC del 95%, 0,89–0,96); el mejor punto de corte es 3/4 con sensibilidad de 0,93 (IC del 95%, 0,88–0,96), especificidad de 0,81 (IC del 95%, 0,72–0,88) y el 88,8% de las clasificaciones correctas. El uso conjunto de AD8 y Fototest mejora de forma significativa la UD de ambos (aROC 0,96, IC del 95%, 0,93–0,98, p<0,05).
ConclusionesLa versión española del AD8 conserva las cualidades psicométricas y la UD de la versión original; su uso combinado con el Fototest mejora de forma significativa la UD de ambos.
Brief cognitive tests and informant questionnaires are the most common tools for screening for cognitive decline (CD) and dementia (DEM) in clinical practice.1 Informant questionnaires are batteries of structured questions which an informant answers to provide data about the patient's current cognitive, behavioural, and functional state as compared to his/her previous state. Informant questionnaires have an advantage over cognitive tests in that they offer a longitudinal perspective on the subject, which is essential when the subject's performance on cognitive tests approaches the upper or lower limits. They also minimise age, sex, literacy, and educational or cultural effects. Furthermore, some may be filled in directly by the informant, which saves time for medical staff.2 The main drawback to informant questionnaires is that informants are not always available or reliable; they may be poorly informed, unable to perform the necessary tasks, or have a vested interest in producing false or skewed results. Informant questionnaires are necessary in cases in which subjects refuse or do not cooperate with testing. Under other circumstances, they complement and enhance the information obtained by using cognitive tests to evaluate patients. Some evidence suggests that using the 2 methods together improves the diagnostic accuracy (DA) of both instruments.3–5
The most commonly-used informant questionnaires in Spain are the Clinical Dementia Rating6, the Blessed dementia scale,7 and the short form of the Informant Questionnaire on Cognitive Decline in the Elderly9 in its Spanish-language version.8 All 3 of these questionnaires are relatively extensive and must be administered by a professional. This eliminates 2 of the main advantages associated with informant questionnaires: brevity and the possibility of the questionnaire being filled out by an informant.
AD810 is a very brief, recently developed informant questionnaire containing just 8 yes/no questions. Its diagnostic accuracy (DA) for both CD and DEM has been subjected to rigorous validation11; a short time ago, AD8 was also validated as a diagnostic tool for Alzheimer disease when supported by LCR biomarkers (beta amyloid 1–42) and a neuroimaging study (PiB-PET).12 A study has also found that the AD8 is useful when self-administered by the patient in cases in which no informant is available.13 Lastly, evidence suggests that using AD8 along with other cognitive tests results in better CD and DEM detection.14 The above advantages explain why AD8 has become so widespread so quickly, and why adapted versions are now available in many different languages (French,15 Portuguese,16 Korean,17 and Taiwanese18), including one in Chilean Spanish.19
Our purpose is to elaborate a new version of the AD8 in a modality of Spanish that reflects the vocabulary and expressions of our region (the Chilean version includes terms such as chequera and computadora that are not common in Spain). We will assess the new version for its validity and accurate identification of subjects with mild CD and DEM, and the potential added value of associating it with a brief cognitive test (Fototest).
Materials and methodsAD8 QuestionnaireThe new peninsular Spanish version of the AD8 (Appendix B, see the questionnaire in the electronic version of this article) was drawn up using a progressive approximation approach to reach a consensus between experts in neurology (2), neuropsychology (3), internal medicine (1) and 2 professional translators, a native English speaker and a native Spanish speaker. Each participant prepared an initial translation of the original AD8 document. These translations were distributed among all group members, each of whom then prepared a second version of the translation drawing from the set of initial versions. Second versions were unified by a coordinator (C.C.P.) who drew up a single version which was then used as the working translation. Copies of the working translation were sent to all group members, who then debated those points for which there was no unanimous agreement. A final version was then generated which met with the approval of all members of the group. The final version was also approved by the author of the AD8 (J.E.G.).
DesignThis prospective, cross-sectional study included patient/informer dyads referred to our cognitive/behavioural neurology unit (CBN) between February 2011 and February 2012 due to suspected CD or DEM. We also included patient/informer dyads treated in internal medicine or general neurology units and who met the following requirements: no subjective complaints of memory loss; functionally independent; normal score on the Fototest (≥27).20 Participation in both cases was restricted to dyads in which informants were literate.
ProcedureThe AD8 was filled in by the informant in the waiting room with no instructions or clarifications other than those appearing on the form. The form was placed in an appropriately identified envelope which remained sealed until a clinical diagnosis was reached. The total score on the AD8 is equal to the number of affirmative answers.
The patients referred to the CBN unit for a consult were studied in accordance with its normal study protocol, which includes a semi-structured medical history and general and neurological examinations performed by an expert neurologist (C.C.P., R.V.C.). A neuropsychologist (S.L.A., C.M.A.) performed an extensive cognitive assessment including Fototest,20 orientation, attention (WAIS digit span), learning and episodic memory (modified CERAD word list), language (short form of the Boston naming test21 and semantic verbal fluency [SVF],22) abstraction (short form of the WAIS similarities subtest), arithmetic (Eurotest coin calculation23), motor praxis (pantomiming), and visuoconstructive abilities (CERAD drawings). A specialist nurse (E.M.G.) completed a behavioural evaluation (Spanish version of the Neuropsychiatric Inventory24) and a functional assessment (Barthel index25 and the Lawton-Brody scale26). Based on results from this assessment, patients were identified as NoCD, CDnoDEM (mild CD according to criteria established by the SEN Study Group for behavioural neurology and dementia27), or DEM (meeting DSMIV-R criteria for dementia28). This was used as the gold standard diagnosis. We calculated a simplified version of the functional index used in the PAQUID study29 which measures the subject's ability to carry out 4 instrumental tasks (use public transport, go shopping, use the telephone, and manage medications). For each of these tasks, subjects score 0 points if they are completely independent, 1 point if they need assistance or make occasional mistakes, and 2 points if they are unable to complete the task. Total scores range from 0 (total independence) to 8 (total dependence for all activities). We used the Global Deterioration Scale (GDS),30 a graduated scale that measures subjects’ cognitive state as follows: GDS 1, functionally independent subjects with no subjective concerns or CD; GDS2, subjects with subjective concerns or suspected CD and a normal cognitive assessment; GDS 3, subjects with CDnoDEM; GDS 4, subjects with mild DEM; and GDS 5–7, subjects with moderate to severe DEM.
Statistical analysisDescriptive study of sociodemographic variables and results from patients and caregivers were broken down by patient diagnosis and GDS stage; comparisons were performed using ANOVA and the chi-square test depending on whether variables were continuous or categorical. Convergent validity was measured by calculating partial correlations between the AD8 and the Fototest, and between the test construct and the FI and the GDS stage. All correlations were adjusted for age and sex. Internal consistency was evaluated using Cronbach's alpha (α). DA was measured using the area under the ROC curve (AUC) for NoCD vs CD, a group that included both CDnoDEM and mild DEM subjects (GDS 3–4) and excluded subjects with moderate to severe DEM (GDS 5–7), so as not to overestimate DA. The optimal cut-off point corresponds to the highest Youden index ([sensitivity (TPR)+specificity (SPC)]−1). The potential influence of informant characteristics on AD8 scores was evaluated using a linear regression analysis method in which the AD8 score was the dependent variable and informant characteristics and GDS were independent variables. The added value of using the Fototest and the AD8 together was evaluated using logistic regression analysis in which presence of CD was considered the dependent variable and results were predictor variables. We tested whether the model with 2 variables had better prediction value than models for either of the variables alone. In each of the models, we calculated OR for each variable, AUC, and the percentage of correct classifications (CCs). We compared the DA of the different instruments and models using the Hanley and McNeil test for comparing AUCs from the same sample.31 All parameters were estimated with 95% confidence intervals and two-way comparisons with an alpha error of 0.05. Calculations were performed using SPSS 15.0 (SPSS Inc., Chicago, IL) and MedCalc 10.0.
Ethical and formal considerationsAll subjects and informants were duly informed about the study and objectives and gave their consent to participate. The study design and manuscript preparation follow STARD recommendations for studies evaluating diagnostic tests.32
ResultsWe included a total of 407 patient/informer dyads (330 from the CBN unit and 71 from internal medicine or general neurology); 105 (25.8%) had no CD, 99 (24.3%) had CDnoDEM, and 203 (49.9%) had DEM (108 mild cases and 95 moderate to severe cases). Table 1 summarises sociodemographic information for patients and informants. Patients had an average age of 74.8±9.0 years (mean±SD) and women were clearly predominant (60.7%). Distribution among the different groups showed no sex differences and patients with CD were significantly older than subjects without CD. Informants were mainly women (75.1%) and direct relatives of the patient (spouses 56.6%; sons/daughters 25.3%; other, 18.1%). Their mean age was 54.2±13.5 years and they had an intermediate educational level (52.6% with at least secondary education).
Sociodemographic characteristics of patients and informants broken down by diagnosis.
| Total | CD | NoCD | CDnoDEM | DEM | P | |
| No. subjects | 407 | 301 | 105 | 99 | 203 | |
| Patients | ||||||
| Age (years) | 74.8±9.0 | 76.6±7.8 | 69.8±10.2 | 72.6±8.9 | 78.4±6.6 | <.05 |
| Sex (female) | 247 (60.7) | 184 (61.1) | 63 (59.4) | 50 (50.5) | 134 (66.0) | NS |
| Informants | ||||||
| Age (years) (n=379) | 54.2±13.5 | 53.3±13.1 | 56.3±14.1 | 52.1±14.6 | 53.9±12.4 | NS |
| Sex (female) (n=394) | 296 (75.1) | 217 (75.3) | 79 (74.5) | 69 (69.7) | 148 (72.9) | NS |
| Relationship n=392) | <.001 | |||||
| Spouse | 222 (56.6) | 184 (64.3) | 38 (35.8) | 49 (55.1) | 135 (68.5) | |
| Son/daughter | 99 (25.3) | 58 (20.3) | 41 (38.7) | 24 (27.0) | 34 (17.3) | |
| Other | 71 (18.1) | 44 (15.4) | 27 (25.5) | 16 (18.0) | 28 (14.2) | |
| Living arrangement (n=389) | .04 | |||||
| Same household | 168 (41.3) | 113 (39.9) | 55 (51.9) | 37 (42.0) | 76 (39.0) | |
| Daily contact | 135 (33.2) | 111 (39.2) | 24 (22.6) | 35 (39.8) | 76 (39.0) | |
| < Daily contact | 86 (25.6) | 59 (20.8) | 27 (25.5) | 16 (18.2) | 43 (22.1) | |
| Educational level (n=384) | NS | |||||
| 64 (16.7) | 43 (15.5) | 21 (19.8) | 10 (11.9) | 33 (17.0) | ||
| Primary school | 123 (32.0) | 90 (32.4) | 33 (31.1) | 26 (31.0) | 64 (33.0) | |
| Secondary school | 98 (25.5) | 67 (24.1) | 31 (29.2) | 27 (32.1) | 40 (20.6) | |
| Higher education | 99 (25.8) | 78 (28.1) | 21 (19.8) | 21 (25.0) | 57 (29.4) | |
CD: cognitive decline; No CD: no cognitive decline; CDnoDEM: cognitive decline without dementia; DEM: dementia.
Data represent number of subjects (percentage) or mean±SD.
NS: not significant.
Table 2 summarises the results (mean±SD) stratified by GDS stage. All results were poorer for higher GDS stages. Increase in AD8 score was linear (Fig. 1) and results were not significantly affected by age, sex, relationship to informant, type of living arrangement, or informant's educational level. Results on the Fototest showed similar behaviour beginning after GDS stage 2, since subjects at GDS stage 2 showed cognitive performances that were within the normal range (33.3±5.8) and not significantly different from those of subjects at GDS 1 (35.5±4.3). Meanwhile, subjects at GDS stage 2 showed an FI (0.9±1.6) that was slightly but significantly poorer than that of subjects at GDS stage 1 who were completely independent. They did score better than subjects identified as GDS stage 3 (2.1±2.0).
Results broken down by GDS stage.
| GDS | GDS 1 | GDS 2 | GDS 3 | GDS 4 | GDS 5–7 |
| No. subjects | 71 | 34 | 99 | 108 | 95 |
| Functional index | 0±0 (0) | 0.9±1.6; (0–6) | 2.1±2.0; (0–7) | 3.9±1.7; (0–8) | 6.8±1.2; (4–8) |
| AD8 | 0.7±0.9; (0–3) | 4.1±2.6; (0–8) | 5.4±1.7; (1–8) | 6.8±1.4; (3–8) | 7.5±0.8; (5–8) |
| Fototest | 35.5±4.3; (27–47) | 33.3±5.8; (17–44) | 27.6±4.2; (19–40) | 20.9±5.1; (7–30) | 15.3±5.8; (3–26) |
| Fototest-AD8 | 34.7±7.7; (26–47) | 29.2±7.1; (9–43) | 22.2±4.2; (14–37) | 14.2±5.4; (0–23) | 7.9±6.0; (0–21) |
GDS: Global Deterioration Scale.
Data are given as mean±SD (range).
AD8 scores indicate a very significant positive correlation with GDS stages (r=0.72, P<.001) and FI (r=0.59, P<.001) and a negative correlation with performance on the Fototest (r=−0.61, P<.001). This version of AD8 displays high internal consistency with α=0.90 (95% CI, 0.89–0.92).
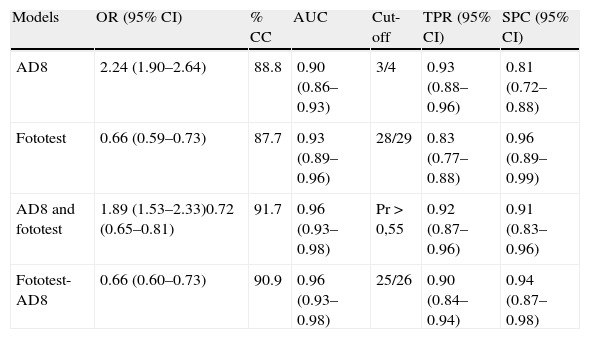
The AUC on the AD8 for NoCD vs CD (CDnoDEM+mild DEM [312 subjects] is 0.90 (95% CI, 0.86–0.93) (Table 3). The cut-off point 3/4 delivers the best discriminatory performance with a TPR=0.93 (95% CI, 0.88–0.96), SPC=0.81 (95% CI, 0.72–0.88) and a correct classification rate (CC) of 88.8%. Fototest for this sample shows an AUC of 0.93 (95% CI, 0.89–0.96). The optimum cut-off point was 28/29 with TPR=0.83 (95% CI, 0.77–0.88), SPC=0.96 (95% CI, 0.89–0.99) and a correct classification rate of 87.7%. There were no significant differences between these instruments’ diagnostic performances (difference in AUC [ΔAUC]=0.02 (95% CI, −0.03 to 0.07, P=.47).
Logistic regression models.
| Models | OR (95% CI) | % CC | AUC | Cut-off | TPR (95% CI) | SPC (95% CI) |
| AD8 | 2.24 (1.90–2.64) | 88.8 | 0.90 (0.86–0.93) | 3/4 | 0.93 (0.88–0.96) | 0.81 (0.72–0.88) |
| Fototest | 0.66 (0.59–0.73) | 87.7 | 0.93 (0.89–0.96) | 28/29 | 0.83 (0.77–0.88) | 0.96 (0.89–0.99) |
| AD8 and fototest | 1.89 (1.53–2.33)0.72 (0.65–0.81) | 91.7 | 0.96 (0.93–0.98) | Pr > 0,55 | 0.92 (0.87–0.96) | 0.91 (0.83–0.96) |
| Fototest-AD8 | 0.66 (0.60–0.73) | 90.9 | 0.96 (0.93–0.98) | 25/26 | 0.90 (0.84–0.94) | 0.94 (0.87–0.98) |
OR: odds ratio; 95% CI: 95% confidence interval; CC: correct classifications; AUC: area under the (ROC) curve; COP: cut-off point; TPR: sensitivity; SPC: specificity.
Data are given as mean±SD (range).
Diagnostic performance is better when results from both tests are used, according to the logistic regression model (Table 3) which reveals an AUC of 0.96 (95% CI, 0.93–0.98) and values of TPR=0.92 (95% CI, 0.87–0.96), SPC=0.91 (95% CI, 0.83–0.96), with 91.7% CC for cut-off point Pr>0.545. The disadvantage is that probabilities are complex (Pr=1/(1+e−(7.7+0.64.[AD8]−0.33.[Fototest])). An identical result was obtained using a practical approximation in which we corrected Fototest scores based on AD8 results by subtracting the AD8 score from the Fototest score. The best cut-off point for the corrected score is 25/26, which gives us values of TPR=0.90 (0.84–0.94), SPC=0.94, (0.87–0.98), 90.9% CC, and an AUC of 0.96 (95% CI, 0.93–0.98). There were no significant differences in diagnostic performance between the 2 models, and results from both models were superior to those delivered by each of the tools used independently (AUC≥0.03 and P<.05 for all comparisons).
DiscussionOur proposed peninsular Spanish version of the AD8 demonstrates high internal consistency (α 0.90, 95% CI, 0.86–0.93) and a significant degree of correlation to GDS stage, functional capacity, and cognitive function, which attests to its reliability, construct validity, and convergent validity. Its discriminant validity for identifying subjects with CDnoDEM and mild DEM is also high (AUC 0.90; 95% IC, 0.86–0.93), with 88.8% CC in our sample. Results from our version are nearly identical to those from the original version's validation study (α 0.86, 95% CI, 0.82–0.91; AUC 0.91, 95% CI, 0.88–0.95; 87.9% CC),11,14 which was also carried out in a clinical sample treated by a unit specialising in memory problems.
Overall diagnostic performance on the AD8 is similar to that on the Fototest in the same sample (AUC 0.93, 95% CI, 0.89–0.96; 87.7% CC). This brief cognitive test (<3min) may be taken by illiterate subjects and is not affected by level of education.20 In our region, Fototest has been shown to have better diagnostic effectiveness than the MMSE33 and better diagnostic efficiency than other tests applicable to illiterate subjects.34 While their overall DAs are similar, AD8 is more sensitive (TPR 0.93, 95% CI, 0.88–0.96; SPC 0.81, 95% CI, 0.72–0.88) at its optimum cut-off point (3/4) while the Fototest (cut-off point 28/29) is more specific (TPR 0.83, 95% CI, 0.77–0.88; SPC 0.96, 95% CI, 0.89–0.99). As in the original version, using the AD8 in conjunction with a cognitive instrument (the Fototest in our case) in a logistic regression model significantly improves its ability to discriminate between subjects with and without CD (AUC 0.96%, 95% CI, 0.93–0.98, 91.7% CC). One drawback is the complexity of the calculations involved. A more feasible and practical approach would be to correct the Fototest score using the AD8 score, which provides a consistent approximation and has a similar diagnostic performance (AUC 0.96, 95% CI, 0.93–0.98; 90.9% CC) while using a very simple calculation. For these corrected results, 25/26 is the optimum cut-off point (TPR 0.90, 95% CI, 0.84–0.94; SPC 0.94, 95% CI, 0.87–0.98).
In our sample, the optimum AD8 cut-off point for discriminating between subjects with and without CD is 3/4, which is clearly higher than the cut-off recommended in the original version (≥2). This difference may be explained by there being distinct gold standards for diagnosing CD. In the original version, the CDR scale was used as the gold standard diagnostic method,6 while we used clinical diagnosis categorised by the GDS scale.30 The CDR scale is basically a detailed, structured informant questionnaire that classifies the subject based on an informant's assessment of that subject's cognitive and functional state, without evaluating the subject's cognitive performance. However, our use of the GDS scale30 is based on results from the cognitive evaluation. Subjects with subjective concerns or suspected CD whose cognitive performance was within normal limits were classified as GDS 2. Subjects with documented cognitive changes were classed as GDS 3. It is likely that many subjects identified as GDS 2 could in fact be classified as CDR 0.5; although their cognitive performance is within normal ranges, their functional capacity is slightly but significantly lower than that of subjects in GDS 1. The FI score of 35.3% of subjects classified as GDS 2 (12 of 34) is ≥1, meaning that they are not fully independent. Fototest scores for these subjects (28.0±6.0) are significantly lower than for subjects with GDS 2 and FI=0 (35.4±4.6) and similar to the scores of subjects classified as GDS 3 for this test. This probably indicates a subgroup of individuals in very early stages of CD whose cognitive performance remains within the normal range, but who suffer from CD with respect to their previous levels of performance. This decline cannot be detected by an initial cognitive evaluation, but it is clear and obvious for a trained informant, and this increases the patient's score on the AD8. Another aspect that may explain the disparity between the cut-off points in the original version and our own is that our validation was performed in a clinical sample with a high prevalence of CD and DEM (74.0%). Furthermore, subjects without CD were patients who made appointments because of subjective concerns or suspicions of CD or due to other medical problems. In contrast, the original version was validated in a sample of volunteers who were part of a longitudinal ageing study; in this group, subjects without CD were not necessarily ill, and the prevalence of CD was clearly lower (53.0%).10
The strong points of our study include its large sample size and its natural reflection of clinical practice. Its weaknesses include the fact that it was carried out in a clinical sample taken from a specialist CBN unit. This limits extrapolation of our results not only on the municipal and regional levels, but also with respect to other non-specialist clinical settings such as primary care. Other limitations include the fact that we did not verify the informant's cognitive state, knowledge of the patient, or reading level and ability to comprehend the questions on the form. As a result, we cannot determine to what extent these variables may have influenced results.
In conclusion, our version of the AD8 possesses similar psychometric characteristics to those of the original. It is a brief, easy-to-use tool which informants may fill out themselves, and it helps identify subjects suffering from CD or mild dementia. Joint use of the AD8 and the Fototest delivers a higher DA than that of either test employed alone. We recommend conducting additional multi-country studies and including other care levels such as primary care, in order to extend this method to different settings.
FundingDr Galvin's contribution to this project was funded by a grant from the U.S. National Institutes of Health (R01 AG040211).
Conflicts of interestC. Carnero Pardo created the Fototest and has received professional fees for academic and consulting activities for Janssen Cilag, Pfizer, Eisai, Esteve, Novartis, Lundbeck, and Grunenthal.
Under the terms of its Creative Commons licence, Fototest may be used and distributed for non-commercial purposes provided that it is not modified and its authorship is explicitly recognised.
María Espinosa García, Elvira Guerrero Moral, and Richard Davies collaborated in the translation process.
An intermediate analysis of this study was presented orally at the 63rd Annual Meeting of the Spanish Society of Neurology.
Please cite this article as: Carnero Pardo C, et al. Evaluación de la utilidad diagnóstica de la versión española del cuestionario al informador «AD8». Neurología. 2013;28:88–94.