Risk factors for dementia include genetic factors, aging, environmental factors, certain diseases, and unhealthy lifestyle; most types of dementia share a common chronic systemic inflammatory phenotype. Psoriasis is also considered to be a chronic systemic inflammatory disease. It has been suggested that psoriasis may also contribute to the risk of dementia. The aim of this study was to systematically review the literature on the association between psoriasis and dementia.
DevelopmentArticles were selected according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We searched the PubMed and Web of Science databases to identify articles published in peer-reviewed journals and studying the association between psoriasis and dementia. Studies meeting the inclusion criteria were reviewed. We used the Newcastle–Ottawa Scale to assess the quality of each study. After applying the inclusion and exclusion criteria, we included 8 studies for review, 3 of which were found to present a higher risk of bias. Six of the 8 studies supported the hypothesis that prior diagnosis of psoriasis increases the risk of dementia; one study including only a few cases reported that psoriasis decreased the risk of dementia, and one study including relatively young patients found no significant association between psoriasis and the risk of dementia.
ConclusionMost studies included in this review supported the hypothesis that psoriasis constitutes a risk factor for dementia. However, well-designed stratified cohort studies assessing both psoriasis severity and treatment status are still required to determine the real effect of psoriasis on the risk of dementia and its subtypes.
Entre los factores de riesgo de la demencia se incluyen algunas características genéticas, el envejecimiento, factores medioambientales, determinadas enfermedades y estilos de vida poco saludables. La mayoría de los tipos de demencia comparten un fenotipo de carácter inflamatorio, sistémico y crónico. La psoriasis también se considera una enfermedad inflamatoria, sistémica y crónica. Se ha especulado que la psoriasis podría aumentar el riesgo de demencia. El objetivo de este estudio es realizar una revisión sistemática de la literatura disponible sobre la posible asociación entre la psoriasis y el desarrollo de demencia.
DesarrolloSeleccionamos los artículos siguiendo las directrices de la declaración Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), utilizando las bases de datos PubMed y Web of Science para localizar artículos publicados en revistas científicas que analizaran la asociación entre psoriasis y demencia. Incluimos en nuestra revisión los artículos que cumplían los criterios de inclusión. Para valorar la calidad de los estudios, usamos la escala Newcastle-Ottawa. Tras aplicar los criterios de inclusión y exclusión, seleccionamos 8 estudios, de los cuales 3 presentaban un mayor riesgo de sesgo. Seis de los 8 estudios postulan la hipótesis de que el diagnóstico de psoriasis aumenta el riesgo de desarrollar demencia posteriormente. Por otro lado, un estudio que incluía solo algunos casos describe que la psoriasis disminuye el riesgo de demencia y un estudio con pacientes relativamente jóvenes no encontró asociación significativa entre la psoriasis y el riesgo de desarrollar demencia.
ConclusionesLa mayoría de los estudios incluidos en esta revisión apoyan la hipótesis de que la psoriasis representa un factor de riesgo de desarrollar demencia. Sin embargo, se necesitan más estudios de cohortes con un diseño adecuado que analicen tanto la gravedad de la psoriasis como el estado del tratamiento para determinar el efecto real de la psoriasis sobre el riesgo de desarrollar demencia y sus subtipos.
Dementia is one of the most common causes of disability in the elderly, and has become a major issue that requires urgent measures. According to the age of onset, dementia can be divided into early onset and late onset dementia. Early onset dementia presents before 65 years and is rare, in which genetic factors are the main pathogenic factors.1 In contrast, late onset dementia, which is often thought to be the result of multifactorial interactions, has an onset above 65 years and is much more common.2,3
Risk factors for dementia include genetic factors,3 aging, environmental factors,4–7 some types of diseases,8–11 and poor lifestyles.12,13 Through analysis, it can be seen that most of these risk factors share a common systemic chronic inflammatory phenotype. In terms of genetic factors, apolipoprotein E ɛ4 allele (APOE4) is by far the greatest genetic risk factor of late onset Alzheimer's disease (AD), the most common form of dementia. Most studies reveal that APOE4 is associated with an enhanced innate immune response.14 For aging, there is consistent evidence that systemic chronic low-grade inflammation is one of its main characteristics.15 In environmental terms, pathogen infection and head trauma, two well-established environmental risk factors for AD, are usually associated with inflammation triggering.16 Some diseases, including cardiovascular diseases, diabetes, hyperhomocysteinemia and depressive illness, are risk factors for AD, and it happens that these diseases are all associated with a state of systemic chronic inflammation.17–20 Lifestyle risk factors for AD, such as obesity and physical inactivity, have also been reported to be closely associated with systemic chronic inflammation.21,22
In addition to the fact that most of the risk factors for dementia share a common systemic chronic inflammatory phenotype, accumulating evidence has shown that amyloid-β pathological hallmark of AD is induced under inflammatory condition,23–28 and that anti-inflammatory approaches show protective effects on AD,29–35 which suggests that chronic inflammation caused by multiple factors may be a major cause of dementia development.
Studies indicate that psoriasis is not only a local inflammatory disease of the skin, but also a systemic chronic inflammatory disease.36 In addition, psoriasis usually occurs much earlier than dementia, therefore, there are reasons to speculate that psoriasis may also be a risk factor for dementia. In recent years, especially in the last three years, researchers have begun to focus on the association between psoriasis and the risk of subsequent dementia. The aim of this paper is to assess the relationship between psoriasis and the risk of subsequent dementia by a systematic review.
DevelopmentLiterature search strategyA systematic literature search of PubMed and Web of Science was conducted on August 29, 2020 to identify observational studies on the association between psoriasis and the risk of dementia. A combination of Medical Subject Heading terms and free terms, including “AND” and “OR” Boolean operators, was utilized to retrieve the targeted literatures. The search string was psoriasis AND (dementia OR “vascular dementia” OR “frontotemporal dementia” OR “Lewy body disease” OR “cognitive dysfunction” OR “cognitive impairment” OR “mental disorder” OR “Alzheimer's disease”).
Study selection and data extractionAll of the following criteria were required for literature inclusion in the review: (1) studies examining the association between psoriasis and subsequent dementia; (2) observational studies published in peer-reviewed journals; (3) literatures with full text. Studies were excluded if they met any of the following criteria: (1) published non-full-text literatures; (2) non-observational studies; (3) studies that did not specify the temporal sequence of psoriasis and dementia.
Two authors independently reviewed the search results of literatures. After removing the duplicate literatures, full-text observational studies published in peer-reviewed journals and providing information on the association between psoriasis and dementia were selected by browsing titles and abstracts. The full text of screened articles was further examined to exclude the studies that did not reflect the causality of psoriasis and subsequent dementia. Selection differences between the two authors were resolved through consultation. Finally, the included studies were scanned to extract data, including the first author, publication year, region, study design, number of cases and controls, follow-up period and main findings.
Risk of bias assessmentNewcastle–Ottawa Scale was used to assess the quality of each observational study.37 Briefly, each study was assigned a maximum of 9 asterisks: 4 for selection, 2 for comparability, and 3 for outcome or exposure. The total number of asterisks greater than 6 was considered low risk of bias. Two authors independently extracted the data and gave each study a quality assessment. Assessment differences were resolved through discussion to reach an agreement.
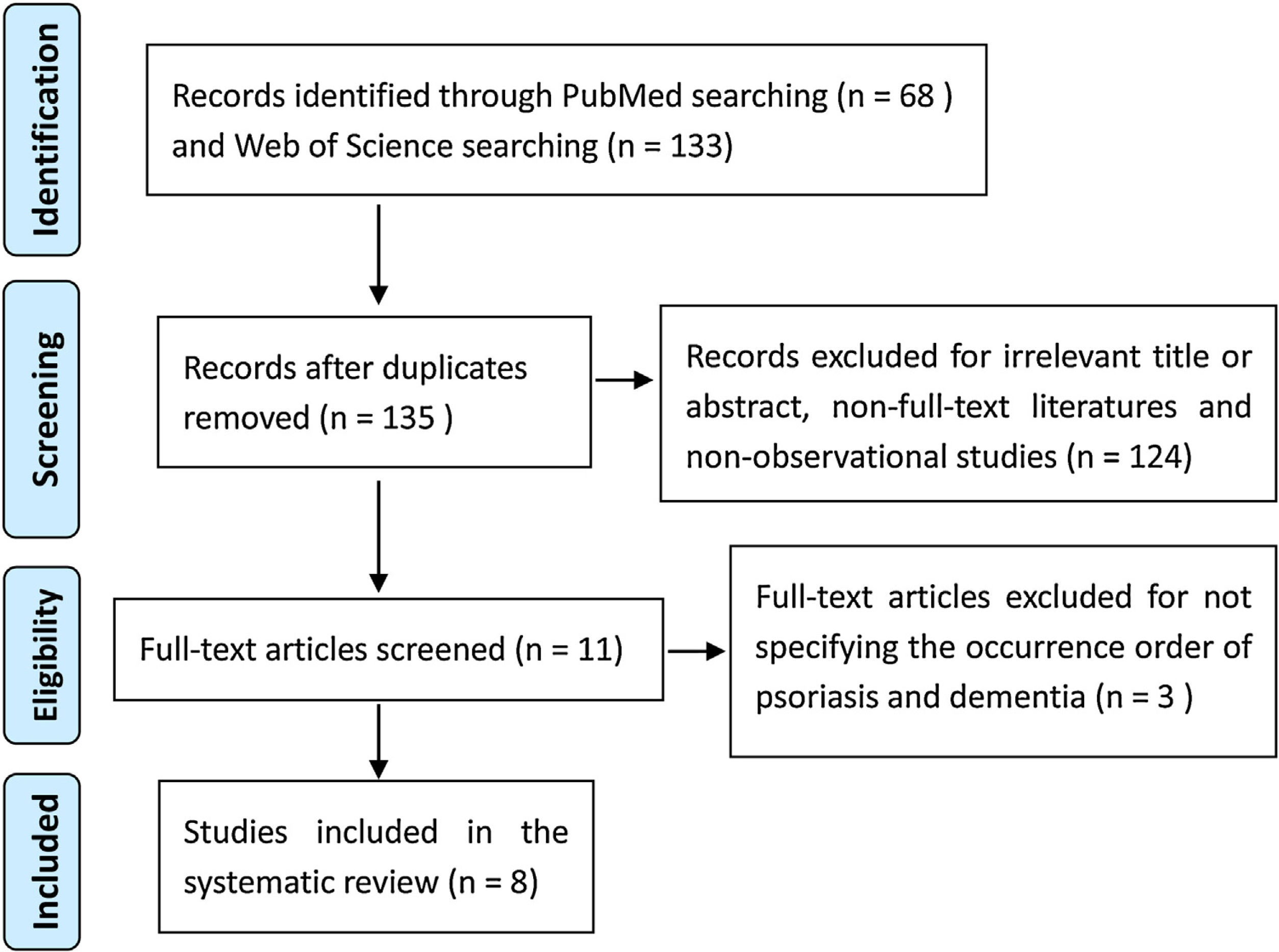
ResultsOverview of the included studiesThe flow diagram (Fig. 1) presents the results of the screening and selection process of the articles included. A total of 201 articles were identified in the original search, with 68 from PubMed and 133 from Web of Science. After screening by applying the inclusion and exclusion criteria, eight relevant observational studies were eventually selected for the systematic review.
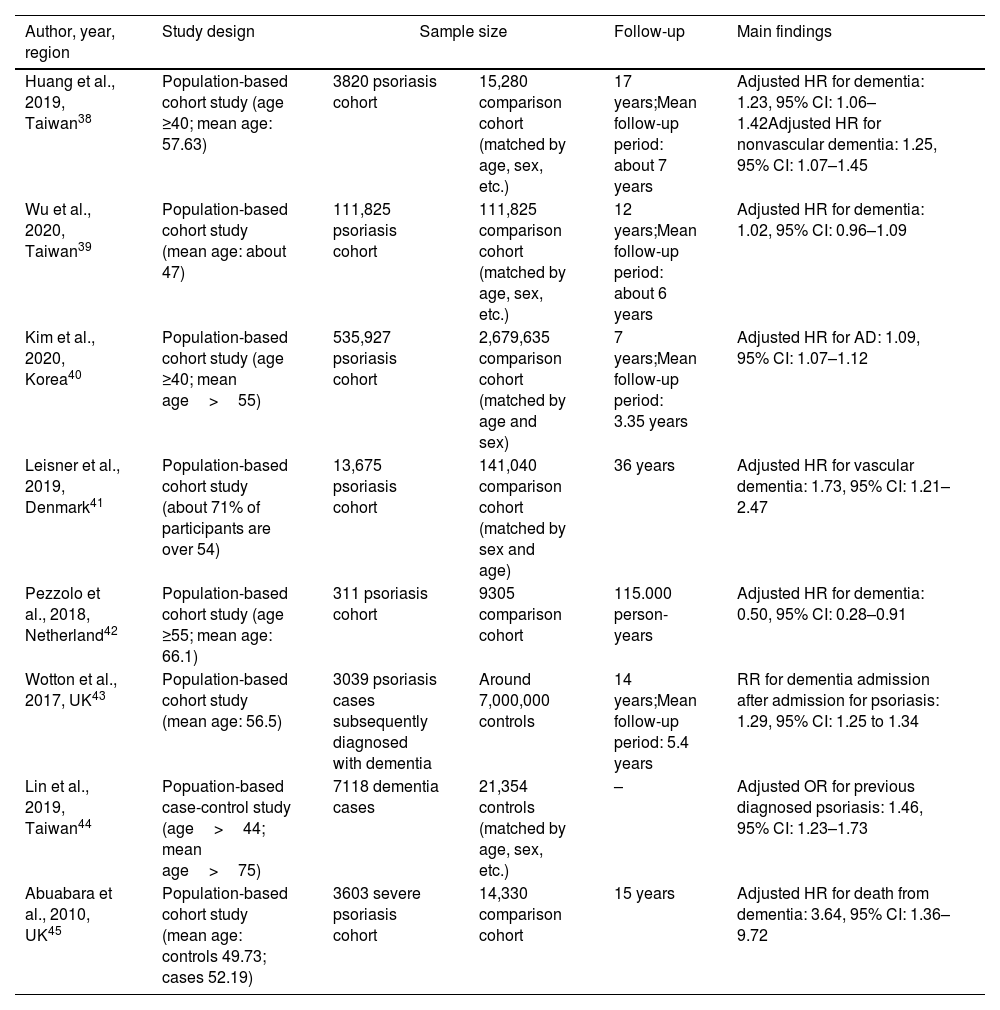
The main characteristics of the studies included in this review were summarized in Table 1. Of the eight eligible observational studies, seven were population-based cohort studies and one was a case-control study. Six of the eight observational studies found a significant positive association between psoriasis and subsequent dementia directly or indirectly,38,40,41,43–45 one study reported a null association,39 and one showed an inverse association.42
Characteristics of the eight eligible observational studies included.
| Author, year, region | Study design | Sample size | Follow-up | Main findings | |
|---|---|---|---|---|---|
| Huang et al., 2019, Taiwan38 | Population-based cohort study (age ≥40; mean age: 57.63) | 3820 psoriasis cohort | 15,280 comparison cohort (matched by age, sex, etc.) | 17 years;Mean follow-up period: about 7 years | Adjusted HR for dementia: 1.23, 95% CI: 1.06–1.42Adjusted HR for nonvascular dementia: 1.25, 95% CI: 1.07–1.45 |
| Wu et al., 2020, Taiwan39 | Population-based cohort study (mean age: about 47) | 111,825 psoriasis cohort | 111,825 comparison cohort (matched by age, sex, etc.) | 12 years;Mean follow-up period: about 6 years | Adjusted HR for dementia: 1.02, 95% CI: 0.96–1.09 |
| Kim et al., 2020, Korea40 | Population-based cohort study (age ≥40; mean age>55) | 535,927 psoriasis cohort | 2,679,635 comparison cohort (matched by age and sex) | 7 years;Mean follow-up period: 3.35 years | Adjusted HR for AD: 1.09, 95% CI: 1.07–1.12 |
| Leisner et al., 2019, Denmark41 | Population-based cohort study (about 71% of participants are over 54) | 13,675 psoriasis cohort | 141,040 comparison cohort (matched by sex and age) | 36 years | Adjusted HR for vascular dementia: 1.73, 95% CI: 1.21–2.47 |
| Pezzolo et al., 2018, Netherland42 | Population-based cohort study (age ≥55; mean age: 66.1) | 311 psoriasis cohort | 9305 comparison cohort | 115.000 person-years | Adjusted HR for dementia: 0.50, 95% CI: 0.28–0.91 |
| Wotton et al., 2017, UK43 | Population-based cohort study (mean age: 56.5) | 3039 psoriasis cases subsequently diagnosed with dementia | Around 7,000,000 controls | 14 years;Mean follow-up period: 5.4 years | RR for dementia admission after admission for psoriasis: 1.29, 95% CI: 1.25 to 1.34 |
| Lin et al., 2019, Taiwan44 | Popuation-based case-control study (age>44; mean age>75) | 7118 dementia cases | 21,354 controls (matched by age, sex, etc.) | – | Adjusted OR for previous diagnosed psoriasis: 1.46, 95% CI: 1.23–1.73 |
| Abuabara et al., 2010, UK45 | Population-based cohort study (mean age: controls 49.73; cases 52.19) | 3603 severe psoriasis cohort | 14,330 comparison cohort | 15 years | Adjusted HR for death from dementia: 3.64, 95% CI: 1.36–9.72 |
CI: confidence interval; HR: hazard ratio; RR: rate ratio
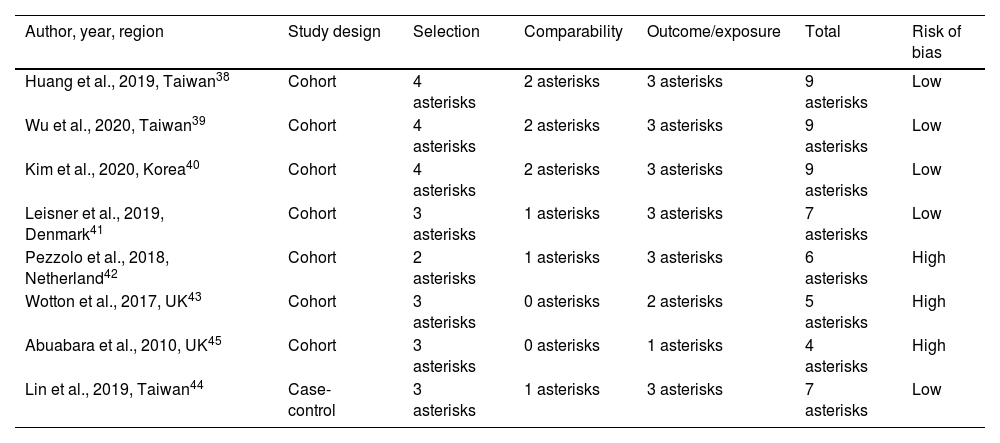
Of the eight studies included in this systematic review, seven had a sample size of more than 3000, and one had less than 350 cases of psoriasis.42 The studies were categorized as low or high risk of bias. Overall, the risk of bias was low in five studies38–41,44 and high in three ones42,43,45 (Table 2).
Quality assessment of the included studies.
| Author, year, region | Study design | Selection | Comparability | Outcome/exposure | Total | Risk of bias |
|---|---|---|---|---|---|---|
| Huang et al., 2019, Taiwan38 | Cohort | 4 asterisks | 2 asterisks | 3 asterisks | 9 asterisks | Low |
| Wu et al., 2020, Taiwan39 | Cohort | 4 asterisks | 2 asterisks | 3 asterisks | 9 asterisks | Low |
| Kim et al., 2020, Korea40 | Cohort | 4 asterisks | 2 asterisks | 3 asterisks | 9 asterisks | Low |
| Leisner et al., 2019, Denmark41 | Cohort | 3 asterisks | 1 asterisks | 3 asterisks | 7 asterisks | Low |
| Pezzolo et al., 2018, Netherland42 | Cohort | 2 asterisks | 1 asterisks | 3 asterisks | 6 asterisks | High |
| Wotton et al., 2017, UK43 | Cohort | 3 asterisks | 0 asterisks | 2 asterisks | 5 asterisks | High |
| Abuabara et al., 2010, UK45 | Cohort | 3 asterisks | 0 asterisks | 1 asterisks | 4 asterisks | High |
| Lin et al., 2019, Taiwan44 | Case-control | 3 asterisks | 1 asterisks | 3 asterisks | 7 asterisks | Low |
Overall, seven cohort studies with varying follow-up periods and one case-control study were eventually selected for the systematic review, and six of them showed a positive association.
In a 17-year cohort study with 3820 psoriasis patients and 15,280 sex-, age-, and urbanization-matched comparisons from Taiwan National Health Insurance Research Database, Huang et al. reported that psoriasis was a risk factor for dementia after adjusting for age, sex and other confounders including cardiovascular risk factors (HR=1.23; 95% CI, 1.06–1.42), particularly for the nonvascular type (HR=1.25; 95% CI, 1.07–1.45) but not for vascular dementia (HR=1.27; 95% CI, 0.83–1.93).38 When comparing psoriasis patients who had received systemic therapy for ≥90 days with no systemic therapy, adjusted HR for dementia was 0.66 (95% CI, 0.45–0.97). Similarly, adjusted HR for dementia was 0.69 (95% CI, 0.50–0.97) when comparing psoriasis patients who had received anti-rheumatic drugs and/or biologics with no systemic therapy. In this study, the age of all participants was ≥40, and the mean age was 57.63. The mean follow-up period was approximately 7 years.
Using the same database as Huang et al.,38 Wu et al. investigated the subsequent dementia risk of newly diagnosed psoriasis patients in a 12-year follow-up study.39 A total of 111,825 patients with psoriasis and 111,825 age-, sex-, and index date-matched controls were recruited. The mean ages of the psoriasis and the control group were 47.3 and 47.2 years, respectively. The mean follow-up period was 6.1 years. Contrary to the results of Huang et al., this large sample cohort study of Wu et al. reported that psoriasis was not associated with subsequent dementia (adjusted HR=1.02; 95% CI, 0.96–1.09).
Based on the Korean National Health Insurance System database from January 2008 to December 2014, Kim et al. investigated the subsequent risk of AD in patients newly diagnosed with psoriasis.40 A total of 535,927 subjects with psoriasis and 2,679,635 age- and sex-matched controls were selected for the follow-up study. The mean age of all participants was over 55. Results showed that the psoriasis patients had a significantly increased risk of AD compared to the controls after adjusting for income level, diabetes, hypertension, dyslipidemia, and depression (HR=1.09; 95% CI, 1.07–1.12, p<0.0001). Of note, AD risk was significantly increased in psoriasis patients not receiving systemic therapy compared to those receiving it (HR, 1.10; 95% CI: 1.08–1.12 vs. HR, 0.99; 95% CI: 0.90–1.09, p<0.0001).
From database between 1977 and 2012 covering all Danish hospitals, Leisner et al. identified 13,675 individuals with psoriasis and 141,040 controls matched on sex and birth year to examine the association between psoriasis and subsequent development of mental disorder including dementia.41 In this study, more than 70% of participants were over 54 years of age. The HR for vascular dementia was 1.73 (95% CI, 1.21–2.47) after adjusting for birth year and sex.
Pezzolo et al. identified 311 psoriatic and 9305 non-psoriatic participants in a population-based Rotterdam Study to explore subsequent dementia risk in psoriasis patients.42 After 115.000 person-years of follow-up, a lower risk of developing dementia was observed after adjusting for age, gender, education and cardiovascular risk factors (HR=0.50; 95% CI, 0.28–0.91).
Based on UK national hospital care and mortality administrative data between 1999 and 2012, Wotton et al. investigated whether hospital admission for autoimmune disease, including psoriasis, was associated with an elevated risk of future admission for dementia after a mean follow-up of 5.4 years.43 The exact number of psoriasis patients enrolled in this study was not disclosed by the authors. Since a total of 3039 psoriasis cases were subsequently diagnosed with dementia, it was estimated that the sample size of psoriasis patients was much larger than 3039. The mean age of all psoriasis patients at entry into cohort was 56.5. Analysis of results showed that hospital admission for psoriasis was significantly positively associated with that for dementia at p<0.05 (RR=1.29; 95% CI, 1.25–1.34). The RR for dementia diagnosis was consistent in sub-analysis for both AD and vascular dementia.
Using Taiwan Longitudinal Health Insurance Database 2000, Lin et al. identified 7118 individuals with dementia and 21,354 sex- and age-matched controls to explore the association between previously diagnosed psoriasis and dementia.44 Considering that the prevalence of dementia in patients aged ≤44 years was low, only patients aged >44 years were enrolled in this study. The results showed that more patients with dementia had prior psoriasis (n=210, 3.0%) than patients in the control group (n=422, 1.5%). The adjusted OR was 1.46 (95% CI, 1.23–1.73; p<0.001). Even after excluding the patients diagnosed with psoriasis within the 1, 2, or 3 years preceding the index date, dementia was still significantly related to a prior psoriasis diagnosis with adjusted ORs 1.47, 1.57, and 1.62, respectively.
Using the General Practice Research Database of UK between 1987 and 2002, Abuabara et al. identified 3603 patients with severe psoriasis and 14,330 patients with no history of psoriasis to study cause-specific mortality in patients with severe psoriasis.45 The mean age of patients with severe psoriasis was 52.19 and that of control group was 49.73. The findings suggested that patients with severe psoriasis were at increased risk of death from dementia (age- and sex-adjusted HR=3.64, 95% CI, 1.36–9.72), which indirectly supported that there was an association between psoriasis and subsequent dementia.
DiscussionThis paper provided a systematic review of the association between psoriasis and subsequent dementia. Most observational studies included in this review supported that prior diagnosed psoriasis was associated with an increased risk of subsequent dementia. It should be noted that in this review, we excluded three studies that explored the association between psoriasis and dementia rather than between psoriasis and subsequent dementia. Of the three excluded studies, one showed a positive association,29 another reported a reverse association46 and the other gave an insignificant association.47 As mentioned by Kridin et al.46 in the paper, the cross-sectional design used in the study made it impossible to obtain the occurrence order of psoriasis and dementia, so the results could not reflect the causal relationship between psoriasis and dementia. Therefore, according to the criteria of literature selection and exclusion, we excluded the study by Kridin et al. in the systematic review. Similarly, since the study by Zhou et al.29 as well as the study by Feldman et al.47 did not disclose the temporal sequence of psoriasis and dementia, we excluded them in the review.
In the study to explore the causal relationship between psoriasis and subsequent dementia, the age and follow-up period of participants were two key factors affecting the results of the experiment. In this review, Huang et al.38 and Wu et al.39 respectively used the same database and close mean follow-up period to carry out a large sample cohort study on the association between psoriasis and subsequent dementia, but the results were different, with the former reporting a positive association and the latter a null association. The main difference between these two studies was the age difference of participants. In the study of Wu et al., about 37% of participants were under 40 years of age, while in Huang et al., individuals younger than 40 years of age were excluded due to insufficient follow-up period for the development of dementia. Since dementia mainly occurs after 65 years of age, the follow-up period should be long enough to allow dementia to occur, and the younger the participants, the longer the follow-up. The younger participants and the insufficient follow-up period in the study of Wu et al. may be responsible for the null association between psoriasis and subsequent dementia.
The lack of stratified analysis based on the severity of psoriasis is a common limitation of the eight studies included in this review. According to the size and location of lesions, psoriasis can be divided into three types: mild, moderate and severe. The inflammatory response varies according to the type of psoriasis, and the risk of dementia may vary accordingly. In the study of Pezzolo et al.,42 different from most studies included in this review, a lower risk of developing dementia was observed. In addition to the limited number of psoriasis patients (n=311) leading to very few dementia cases among psoriasis patients (15 among psoriasis patients), 76.7% of all psoriasis patients belonging to mild type may be a main cause of reduced risk of subsequent dementia.
Large sample stratified analysis based on dementia subtypes should also be considered in future studies. Of the eight studies included in the review, four examined the association between psoriasis and subsequent vascular dementia,38,39,41,43 and two of them showed a positive association41,43; three investigated the association between psoriasis and subsequent AD,40,41,43 and two of them reported a positive association.40,43 Overall, these studies are not sufficient to conclude whether psoriasis is associated with subsequent AD or vascular dementia. More stratified studies based on dementia subtypes are needed to analyze the association between psoriasis and dementia subtypes.
In this review, three of the eight studies included also investigated the effect of systemic therapy for psoriasis on the risk of dementia,38–40 two of which supported that systemic therapy, especially long-term systemic therapy, reduced the risk of dementia,38,40 which indicated that stratified analysis based on treatment status was necessary generally to avoid underestimation of the real risk of dementia or its subtypes.
Regarding the mechanism of psoriasis increasing the risk of dementia, it is speculated that psoriasis may play a role by promoting the systemic chronic inflammatory response. The findings that anti-inflammatory therapy for psoriasis reduces the risk of dementia and improves cognitive functions in psoriasis patients seem to support this speculation.29,48
ConclusionIn conclusion, most studies included in this review supported that psoriasis was associated with an increased risk of subsequent dementia. However, large-scale stratified research based on both psoriasis severity and treatment status is still required to better investigate the real effect of psoriasis on the risk of subsequent dementia and its subtypes.
FundingThis study was funded by the Initiation Funds for High-level Talents Program of Xi’an International University [grant numbers XAIU202005].
Conflicts of interestThe authors declare that there are no conflicts of interest regarding the publication of this article.