Currently there is no tool to quantify buccophonatory apraxia to stratify, compare and monitor patients longitudinally in an objective manner. Our aim in this study is to create a quantitative scale for buccophonatory apraxia and evaluate it in patients with the non-fluent/grammatical variant of primary progressive aphasia (nfvPPA) and other neurodegenerative diseases that occur with speech and/or language problems.
MethodsThe scale was designed based on useful elements in the assessment of buccophonatory apraxia and the total was quantified in seconds. The scale was administered to 64 participants with diagnoses of: nfvPPA, semantic variant of primary progressive aphasia (svPPA), logopenic variant of primary progressive aphasia (lvPPA), Huntington’s disease, Parkinson’s disease, as well as a group of healthy controls.
ResultsPatients showed a significantly higher score compared to controls. The nfvPPA group had the highest mean score on the scale (429 seconds ± 278). The scale was useful to differentiate vnfPPA from svPPA and Parkinson’s disease (area under curve [AUC] of 0.956 and 0.989, respectively), but less to differentiate it from Huntington’s disease (AUC = 0.67) and lvPPA. There was a statistically significant relationship between total score and disease severity in nfvPPA (P < .029).
ConclusionsThe Barcelona scale for buccophonatory apraxia could be useful to quantitatively evaluate buccophonatory apraxia in different neurodegenerative diseases, and compare patients, especially in nfvPPA.
Actualmente no existe una herramienta que permita cuantificar la apraxia bucofonatoria para estratificar, comparar y monitorizar longitudinalmente a los pacientes de manera objetiva. Nuestro objetivo en el presente trabajo es crear una escala cuantitativa para la apraxia bucofonatoria y evaluarla en pacientes con la variante no fluente/agramatical de la Afasia Progresiva Primaria (vnfAPP) y otras enfermedades neurodegenerativas que cursan con problemas del habla y/o lenguaje.
MétodosSe diseñó una escala a partir de elementos útiles en la exploración de la apraxia bucofonatoria y se cuantificó el total en segundos. Se administró la escala a 64 participantes con diagnósticos de: vnfAPP, variante semántica de la Afasia Progresiva Primaria (vsAPP), variante logopénica de la Afasia Progresiva Primaria (vlAPP), enfermedad de Huntington, enfermedad de Parkinson, así como a un grupo de controles sanos.
ResultadosLos pacientes mostraron una puntuación significativamente mayor respecto a los controles. El grupo de vnfAPP presentó la puntuación media más alta en la escala (429 segundos ± 278). La escala resultó útil para diferenciar la vnfAPP de la vsAPP y de la enfermedad de Parkinson (área bajo la curva [AUC] de 0.956 y 0.989 respectivamente), pero menos para diferenciarla de la enfermedad de Huntington (AUC = 0.67) y de la vlAPP (AUC = 0.772). Existió una relación estadísticamente significativa entre la puntuación total y la gravedad de la enfermedad en la vnfAPP (P < .029).
ConclusionesLa escala Barcelona para la apraxia bucofonatoria podría ser útil para evaluar cuantitativamente la apraxia bucofonatoria en diferentes enfermedades neurodegenerativas, y comparar pacientes, en especial en la vnfAPP.
Primary progressive aphasia (PPA) is a neurodegenerative disorder characterised by progressive language impairment.1 The condition is classified into 3 clinical variants according to the clinical characteristics of language impairment2: 1) semantic variant PPA (svPPA), characterised by progressive loss of semantic knowledge, leading to problems with naming and understanding of isolated words; 2) logopenic variant PPA (lvPPA), characterised by word-finding difficulties both in naming tasks and in spontaneous speech, as well as difficulties with sentence repetition; and 3) nonfluent/agrammatic PPA (nfvPPA), characterised by agrammatism in language production and/or verbal apraxia, causing slowed, effortful speech, with frequent speech sound errors and distortions. Some cases of PPA cannot be classified as any of these variants or present mixed characteristics.
Verbal apraxia, also known as apraxia of speech, is an impairment of motor programming of speech resulting from neurological injury. It causes impairment in spatial and temporal planning and programming of the movements of the phonatory muscles that produce vocal sounds.3 It also causes articulatory inconsistency, dysprosody, and slowed rate of speech.4 Slowed speech, prolonged words or segments, and sound distortions have frequently been described in these patients.5
Patients with nfvPPA may also present difficulty imitating or performing nonverbal movements or gestures. This is known as nonverbal apraxia, or buccofacial apraxia, and is defined as the inability to efficiently produce or imitate movements involving the face, tongue, mouth, jaw, or palate on command. Impairment involves respiratory and oral structures, with relative preservation of the ability to produce semiautomatic actions and reflex movements.6 Verbal apraxia and nonverbal apraxia frequently co-present in patients with nfvPPA.
In clinical practice, several instruments have been developed for the assessment of verbal apraxia with a view to assisting in differential diagnosis with such other speech disorders as aphasia and dysarthria. According to our literature search, no validated Spanish-language versions of these tools have been published. Most of the clinical tools available are targeted at English-speaking populations. The Motor Speech Evaluation assesses different speech tasks, including vowel prolongation; syllable, word, and phrase repetition; reading of a text; and picture description.7 The Apraxia Battery for Adults-Second Edition is a standardised instrument including 6 subtests evaluating the diadochokinetic rate, repetition of words of different lengths, and oral movements, among other tasks.8 The Apraxia of Speech Rating Scale evaluates the presence, relative frequency, and severity of characteristics associated with verbal apraxia.9 Most of these scales were conceived as diagnostic tools. However, they are all based on the evaluator’s subjective judgement. As a result, they are less useful for evaluating disease progression or treatment response. Such other tools as articulation rate involve sophisticated analysis of voice recordings.10–12 Regarding the assessment of nonverbal apraxia, the nonverbal oral agility test included in the oral agility domain of the Boston Diagnostic Aphasia Examination (BDAE) quantifies nonverbal agility of the tongue and lips, scoring the number of repetitions in 5 seconds.13 The Apraxia Battery for Adults-Second Edition, mentioned previously, also includes a subtest for the assessment of nonverbal apraxia, scored from 0 to 5, with 0 indicating inability to perform a movement and 5 indicating correct movement performance. In some studies, nonverbal apraxia has been evaluated with an ad hoc protocol.14
Despite the assessment tools mentioned above, we still lack a practical, simple, objective scale for the quantitative rating of verbal and nonverbal apraxia, which is particularly necessary for nfvPPA. We decided to design a Spanish-language scale, as well as a Catalan-language version, for the global assessment and monitoring of buccofacial apraxia. In view of the high frequency of other speech and language disorders, such as dysarthria and aphasia, in other neurodegenerative diseases, we also administered the scale to patients with speech alterations secondary to other neurodegenerative diseases.
Material and methodsParticipantsThe study was approved by the ethics committee at Hospital Clínic de Barcelona. All patients were recruited at the day hospital for neurodegenerative diseases of Hospital Clínic de Barcelona.
To be included in the study, participants had to be diagnosed with PPA, Huntington disease (HD), or Parkinson’s disease (PD) by an expert neurologist, according to the current diagnostic criteria.2,15,16 In order to ensure the clinical applicability of the scale to patients with speech and language disorders, each diagnostic group included only individuals presenting these disorders. Diagnosis of these disorders was confirmed by a speech therapist or neuropsychologist using the BDAE and the Frenchay Dysarthria Assessment (FDA).13,17 We excluded all patients with normal results on the FDA. To prevent the inclusion of patients with advanced dementia, we only selected patients scoring above 15 points on the Mini–Mental State Examination. The stage of the disease was determined as follows. For PPA, we used the BDAE to evaluate the severity of speech impairment (BDAE 4 = mild, BDAE 3-2 = moderate, BDAE 1 = severe).13 For PD, we used the Hoehn and Yahr Scale (scores of 1-2 points = mild, 3 = moderate, 4-5 = severe).18 For HD, we used Myers’ functional scale, with scores > 80 indicating mild HD, scores 80-50 indicating moderate HD, and scores < 50 indicating severe HD.19 A neurologist and a neuropsychologist specialising in dementia evaluated patients with PPA using language and speech tasks (spontaneous speech, picture description, and sentence repetition)13 to obtain a profile of language and speech characteristics.
The control group included healthy volunteers specifically recruited for this study; they had no history of psychiatric or neurological disease, and presented normal spontaneous speech.
Assessment scaleThe Barcelona scale was designed using items included in different assessment tools for verbal and nonverbal apraxia: the Motor Speech Evaluation7 and its non-validated Spanish-language version, the FDA,17 the nonverbal agility subtest of the BDAE,13 the buccofacial apraxia test of the Ducarne Aphasia Battery,20 and the aphasia examination test published by González and Borregón.21 We reviewed the different items, and included those evaluating articulation and buccofacial movements. We also reviewed the scoring system of each item. The following basic principles of item construction were considered: representativeness, relevance, diversity, clarity, simplicity, and comprehensibility. To select the best items, we considered the difficulty index (verbal and nonverbal), the discrimination index, and differential item functioning. We used the Crosslinguistic Overlap Scale for Phonology22 to analyse the phonological overlap between both languages. The Barcelona scale for buccofacial apraxia was administered by a speech therapist specialising in neurodegenerative diseases (NM). The scale provides a total score (in seconds) and includes 2 subscales (one for nonverbal apraxia and the other for verbal apraxia). The nonverbal apraxia subscale evaluates the time taken for the participant to perform 5 repetitions of different oral sounds and gestures with the face, tongue, and lips (13 items). The verbal apraxia subscale contains 14 items, evaluating 5 domains: phonation (1 item), simple diadochokinesis (3), alternating diadochokinesis (3), repetition of multisyllabic words (5), and sentence reading (2). The instructions were clear and concise, and were given either verbally or as a model for repetition. All participants understood the instructions. Performance time was measured with a stopwatch, starting at the time when the participant began to perform the first gesture and stopping when they completed the last repetition. We recorded the number of seconds needed to complete the 5/10 repetitions required for each item (Appendix A, Supplementary data).
Two versions of the scale were created: one in Spanish and the other in Catalan. The scale was administered in the native or most dominant language of each participant (in Catalan for 36 patients and 15 controls, and in Spanish for 28 patients and 4 controls).
ScoringThe total score is expressed in seconds, and is calculated by combining the scores of each item. It indicates the number of seconds a participant needed to complete all the tasks; therefore, higher scores indicate poorer performance. The stopwatch used for measurements had a precision of one-tenth of a second; values were rounded up to the nearest whole number. All 64 patients completed the 30 items of the scale, resulting in a total of 1920 items. Four patients (3 with nfvPPA and one with lvPPA) failed to complete one item (6.25% of patients and 0.21% of items); therefore, the percentage of failed items was extremely low. In these cases, a score of 50 was assigned to the item; this is twice the worst score recorded among all the patients who completed the task. Therefore, it is unlikely that a patient who completes the task will score above 50 and, consequently, score higher than patients unable to complete it. We established this procedure in order to enable comparison of scores for patients unable to complete a task.
Statistical analysisStatistical analysis was performed with the RStudio software (version 4.0.2). Comparisons between groups were performed with non-parametric tests. Statistical significance was set at P < .05 for all analyses. Qualitative variables were analysed with Fisher’s exact test. The Kruskal-Wallis test was used to compare quantitative values between 3 or more groups. Comparisons between pairs of values were performed with the Wilcoxon test, adjusting the P-value for multiple comparisons with the Bonferroni correction. We plotted ROC curves and calculated the area under the curve (AUC) to compare test performance between diagnostic groups. We correlated verbal and nonverbal apraxia scores using the non-parametric Spearman’s correlation coefficient. To control for the effects of other variables on the results of the scale, we performed a multivariate analysis including total scale score as the dependent variable and group, age, sex, and disease severity as independent variables.
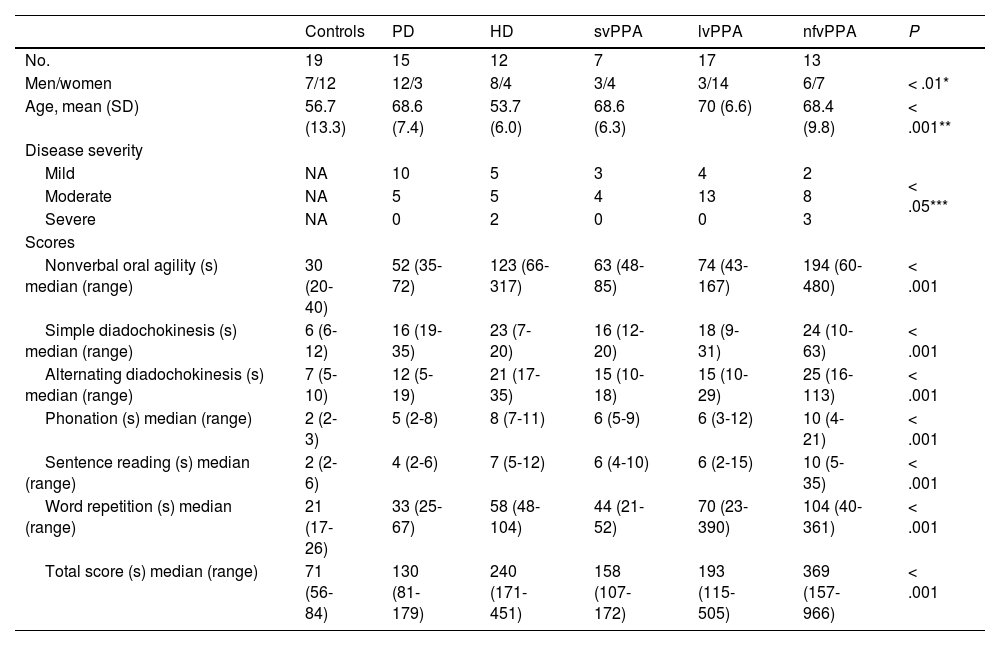
ResultsParticipantsA total of 64 individuals participated in the study: 7 with svPPA, 17 with lvPPA, 13 with nfvPPA, 12 with HD, and 15 with PD. We also included 19 neurologically healthy controls. Table 1 provides a summary of the sample’s demographic and clinical characteristics.
Demographic and clinical characteristics of the sample.
| Controls | PD | HD | svPPA | lvPPA | nfvPPA | P | |
|---|---|---|---|---|---|---|---|
| No. | 19 | 15 | 12 | 7 | 17 | 13 | |
| Men/women | 7/12 | 12/3 | 8/4 | 3/4 | 3/14 | 6/7 | < .01* |
| Age, mean (SD) | 56.7 (13.3) | 68.6 (7.4) | 53.7 (6.0) | 68.6 (6.3) | 70 (6.6) | 68.4 (9.8) | < .001** |
| Disease severity | |||||||
| Mild | NA | 10 | 5 | 3 | 4 | 2 | < .05*** |
| Moderate | NA | 5 | 5 | 4 | 13 | 8 | |
| Severe | NA | 0 | 2 | 0 | 0 | 3 | |
| Scores | |||||||
| Nonverbal oral agility (s) median (range) | 30 (20-40) | 52 (35-72) | 123 (66-317) | 63 (48-85) | 74 (43-167) | 194 (60-480) | < .001 |
| Simple diadochokinesis (s) median (range) | 6 (6-12) | 16 (19-35) | 23 (7-20) | 16 (12-20) | 18 (9-31) | 24 (10-63) | < .001 |
| Alternating diadochokinesis (s) median (range) | 7 (5-10) | 12 (5-19) | 21 (17-35) | 15 (10-18) | 15 (10-29) | 25 (16-113) | < .001 |
| Phonation (s) median (range) | 2 (2-3) | 5 (2-8) | 8 (7-11) | 6 (5-9) | 6 (3-12) | 10 (4-21) | < .001 |
| Sentence reading (s) median (range) | 2 (2-6) | 4 (2-6) | 7 (5-12) | 6 (4-10) | 6 (2-15) | 10 (5-35) | < .001 |
| Word repetition (s) median (range) | 21 (17-26) | 33 (25-67) | 58 (48-104) | 44 (21-52) | 70 (23-390) | 104 (40-361) | < .001 |
| Total score (s) median (range) | 71 (56-84) | 130 (81-179) | 240 (171-451) | 158 (107-172) | 193 (115-505) | 369 (157-966) | < .001 |
HD: Huntington disease; lvPPA: logopenic variant primary progressive aphasia; NA: not applicable; nfvPPA: nonfluent/agrammatic primary progressive aphasia; PD: Parkinson’s disease; SD: standard deviation; svPPA: semantic variant primary progressive aphasia. P-values correspond to the Fisher exact test for qualitative variables and the Kruskal-Wallis test for quantitative variables.
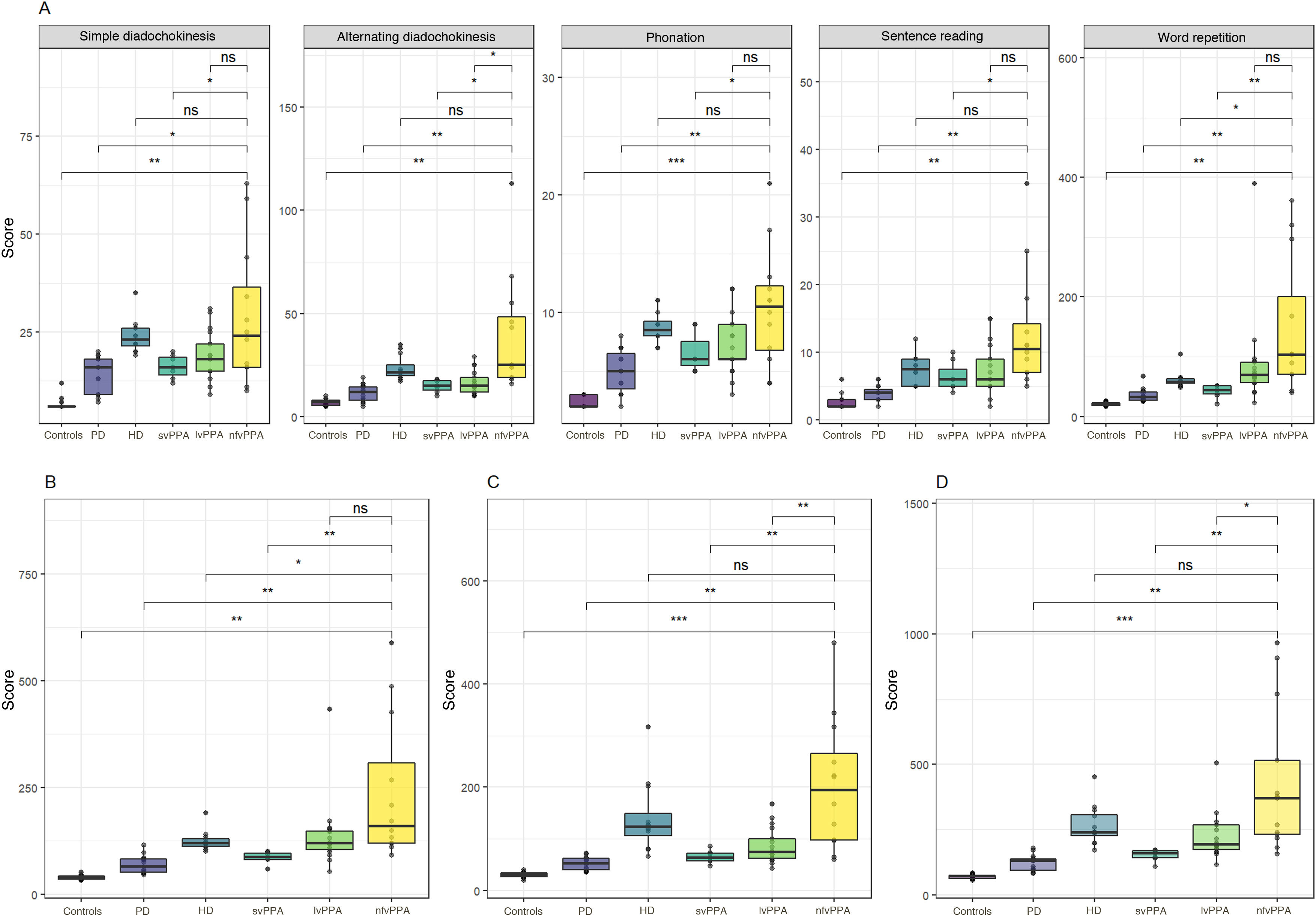
Fig. 1 and Table 1 present verbal apraxia subtest scores, total verbal apraxia score, nonverbal apraxia score, and total scale scores for each group. Mean (SD) total score in the control group was 69 seconds (8). Patients presented significantly higher mean scores than controls both for total scale score (242 s [173]) and for subtest scores (P < .01 for all comparisons).
Total scale scores, by group and subtest. A) Verbal apraxia subtest scores; B) total verbal apraxia scores; C) nonverbal apraxia score; D) total scale score.
HD: Huntington disease; lvPPA: logopenic variant primary progressive aphasia; nfvPPA: nonfluent/agrammatic primary progressive aphasia; ns: not significant; PD: Parkinson’s disease; svPPA: semantic variant primary progressive aphasia.
*P < .05; **P < .01; ***P < .001; ****P < .0001.
The nfvPPA group displayed the highest scores (429 s [278]), scoring significantly higher for the total scale score as compared with the remaining groups, except the HD group. However, we did find significant differences between patients with nfvPPA and those with HD in total verbal apraxia score (P < .05). We also observed significant differences between the 3 PPA subtypes, with the nfvPPA group showing the poorest performance and the svPPA group presenting the best performance (P < .001 for all comparisons).
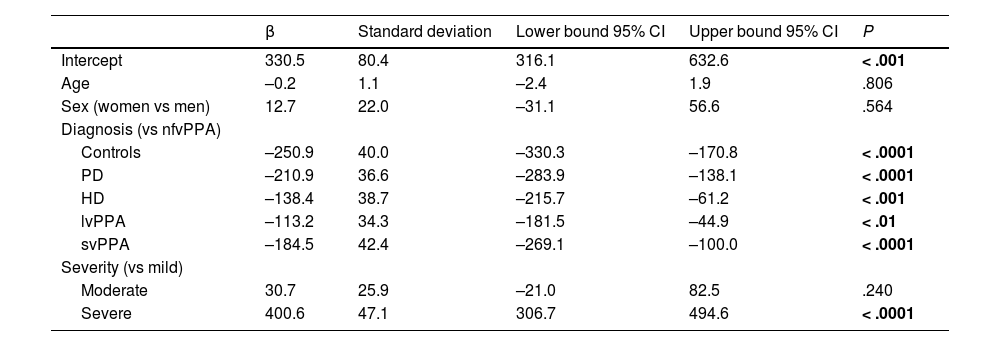
A multivariate linear regression analysis including the control variables age, sex, and disease severity also revealed significant differences in total scale score between the nfvPPA group and the remaining groups (Table 2).
Multivariate linear regression analysis.
| β | Standard deviation | Lower bound 95% CI | Upper bound 95% CI | P | |
|---|---|---|---|---|---|
| Intercept | 330.5 | 80.4 | 316.1 | 632.6 | < .001 |
| Age | –0.2 | 1.1 | –2.4 | 1.9 | .806 |
| Sex (women vs men) | 12.7 | 22.0 | –31.1 | 56.6 | .564 |
| Diagnosis (vs nfvPPA) | |||||
| Controls | –250.9 | 40.0 | –330.3 | –170.8 | < .0001 |
| PD | –210.9 | 36.6 | –283.9 | –138.1 | < .0001 |
| HD | –138.4 | 38.7 | –215.7 | –61.2 | < .001 |
| lvPPA | –113.2 | 34.3 | –181.5 | –44.9 | < .01 |
| svPPA | –184.5 | 42.4 | –269.1 | –100.0 | < .0001 |
| Severity (vs mild) | |||||
| Moderate | 30.7 | 25.9 | –21.0 | 82.5 | .240 |
| Severe | 400.6 | 47.1 | 306.7 | 494.6 | < .0001 |
CI: confidence interval; HD: Huntington disease; lvPPA: logopenic variant primary progressive aphasia; nfvPPA: nonfluent/agrammatic primary progressive aphasia; PD: Parkinson’s disease; svPPA: semantic variant primary progressive aphasia.
Bold values correspond to statistically significant values.
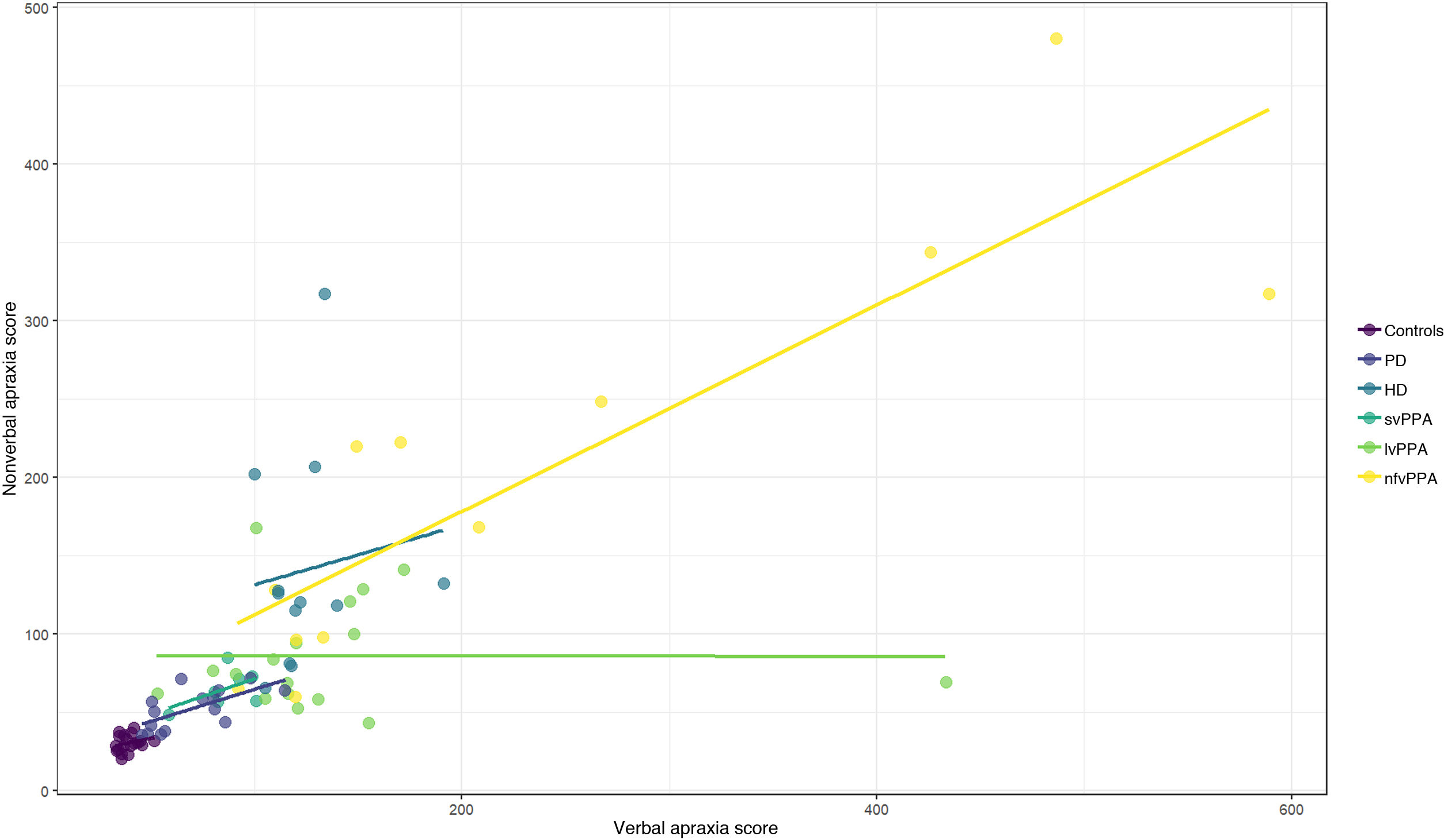
A correlation was found between total verbal apraxia score and nonverbal apraxia score (Spearman rho = 0.86; P < .0001) (Fig. 2).
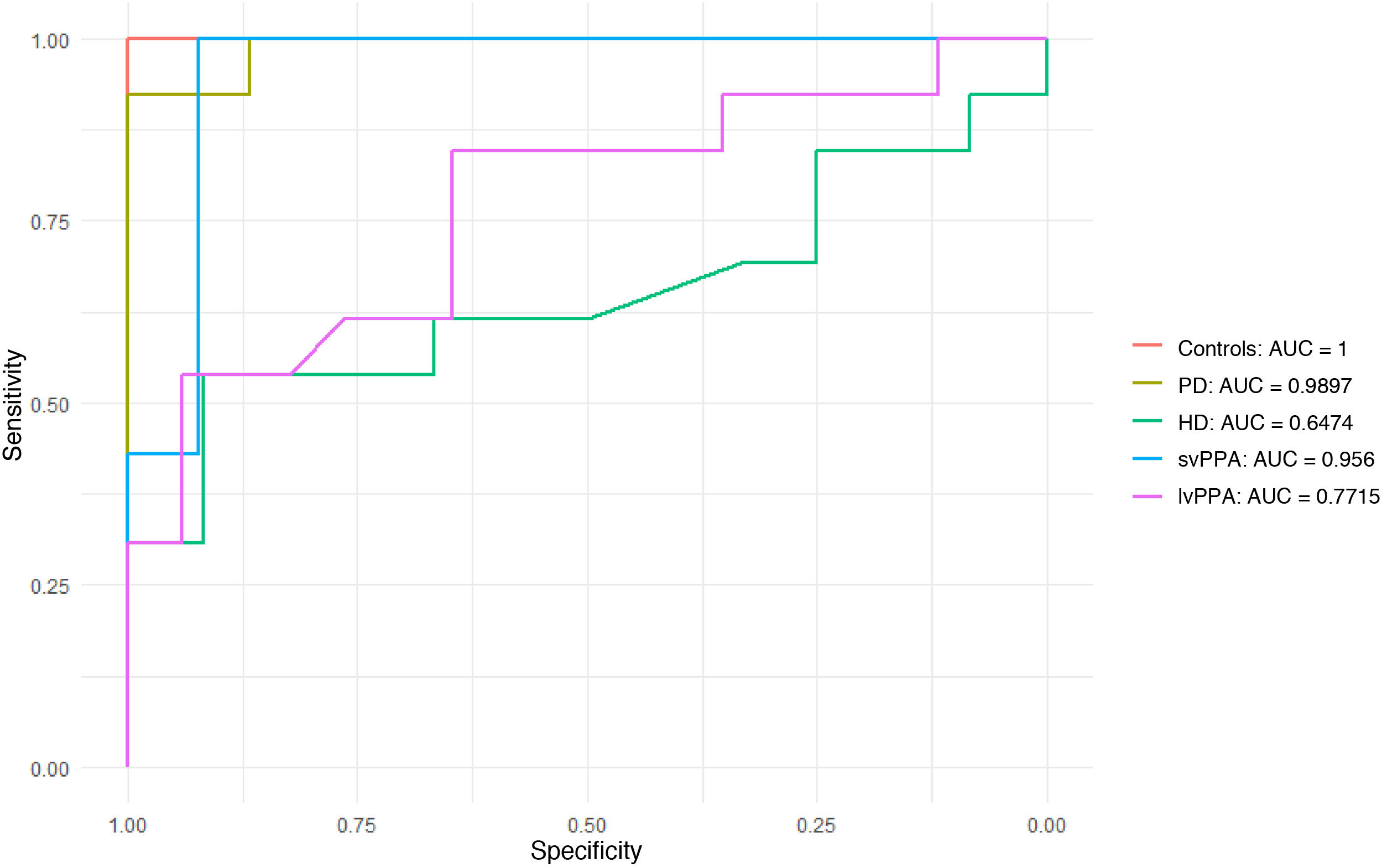
ROC curves for discrimination between diseasesWe evaluated the scale’s ability to discriminate between nfvPPA (the group with the highest scores) and the remaining diseases (Fig. 3). ROC curves for discrimination of nfvPPA from svPPA and PD showed very good AUC values (0.956 and 0.989, respectively). The scale was found to be less useful for discriminating between nfvPPA and HD (AUC = 0.647) and between nfvPPA and lvPPA (AUC = 0.771).
ROC curves for comparisons of nonfluent/agrammatic primary progressive aphasia against the remaining patient groups and controls.
AUC: area under the curve; HD: Huntington disease; lvPPA: logopenic variant primary progressive aphasia; PD: Parkinson’s disease; svPPA: semantic variant primary progressive aphasia.
The optimal cut-off point for differentiating nfvPPA from the remaining diseases was 215 seconds, with a sensitivity of 84% and a specificity of 78% for identifying buccofacial apraxia in patients with nfvPPA.
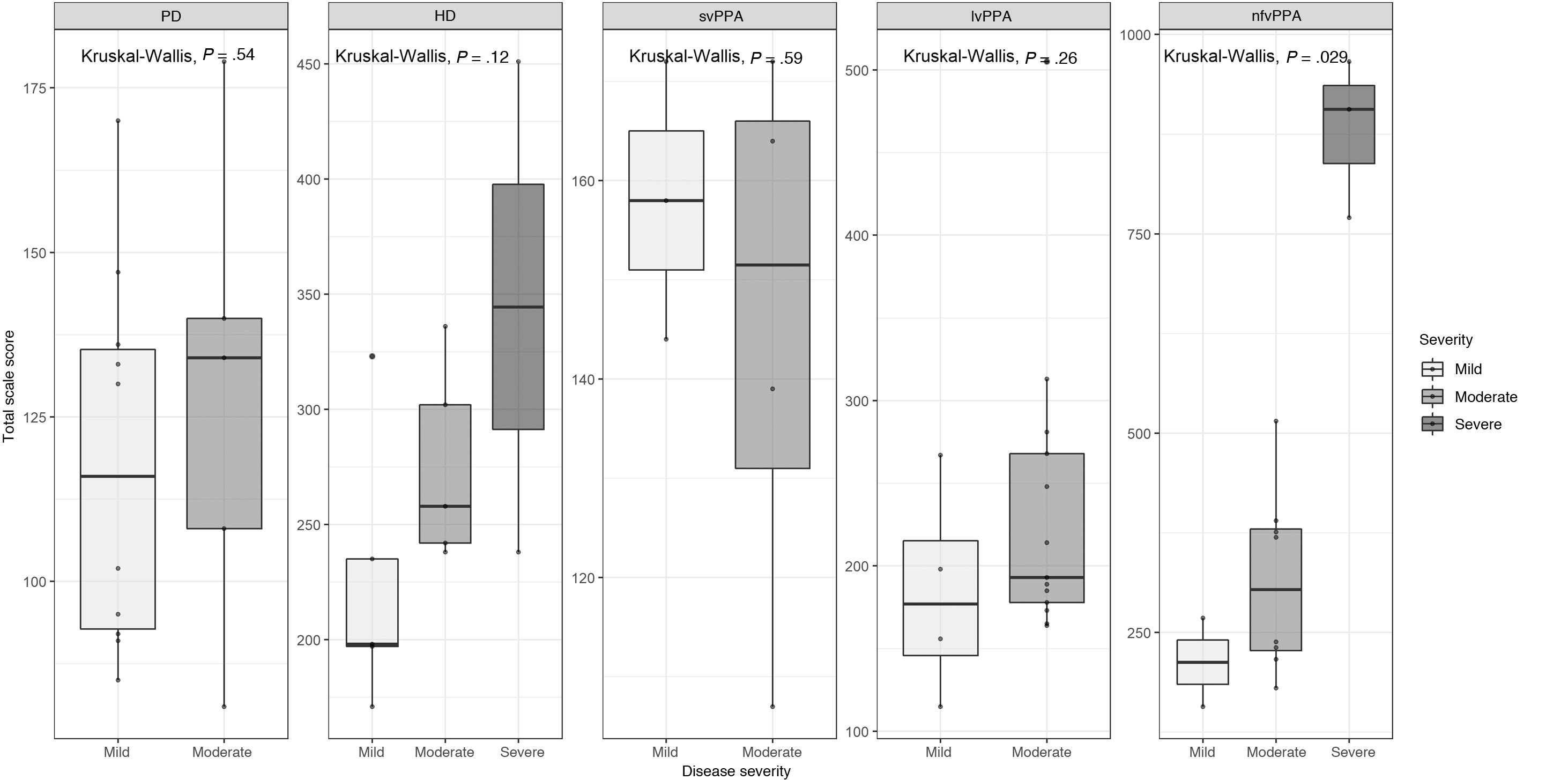
Total scale scores by disease severityFig. 4 shows the mean total scale scores for each patient group by severity. We observed a trend toward higher total scale scores in patients with greater disease severity in all groups except for the svPPA group; this association was statistically significant in the nfvPPA group (P < .029).
DiscussionWe designed a tool for the assessment of buccofacial apraxia with a view to objectively quantifying this alteration among patients with speech alterations and establishing comparisons between different patient profiles.
Most diagnostic and assessment scales for verbal apraxia, including the Apraxia of Speech Rating Scale and the Motor Speech Evaluation, are based on qualitative or semiquantitative data, and consequently depend on the rater’s subjective judgement.7,9 Our scale, in contrast, provides a quantitative measure, that is the time taken for a subject to complete a series of tasks, thus avoiding rater subjectivity. Future studies should aim to analyse the scale’s inter-rater reliability and usefulness for monitoring the progression of apraxia. Regarding nonverbal apraxia, the nonverbal oral agility test of the BDAE does quantify nonverbal agility by recording the number of repetitions completed in 5 seconds (eg, contracting and relaxing the lips, opening and closing the mouth, sticking the tongue out and back in).13 However, this test does not evaluate verbal agility, but rather focuses on movements of the tongue and lips.
The Barcelona scale for buccofacial apraxia was found to be useful for discriminating between nfvPPA and PD (AUC = 0.989): although patients with PD also present speech alterations (eg, dysarthria), buccofacial and verbal volitional movements are frequently better preserved. The scale was also useful in differentiating nfvPPA from svPPA (AUC = 0.956) due to preservation of motor function in the latter, with patients presenting more alterations in language than in speech production. However, the scale was less useful for discriminating between nfvPPA and HD (AUC = 0.647) due to the greater oral motor difficulties and slowed speech observed in the latter patient group. It is also less useful for differentiating between nfvPPA and lvPPA (AUC = 0.771), probably due to the high number of repetitions these patients make with increasing length and phonetic complexity of utterances, especially in the item evaluating word repetition. Patients with lvPPA present high total scale scores, similarly to patients with nfvPPA.
Our results showed that patients at more advanced stages of the disease achieved poorer scores, with a trend toward higher scores in patients with more severe disease across groups, with the exception of svPPA; this trend was statistically significant for the nfvPPA group (P < .029). This suggests that the Barcelona scale may be used to evaluate clinical progression and response to therapeutic interventions.
The main limitation of our study is the small sample size, which is explained by the low prevalence of some of the diseases included. Although the sample size enabled us to detect significant differences between groups, subgroup comparisons (eg, by disease severity) are greatly limited. Another important limitation is derived from the fact that, although the scale was designed to quantify verbal and nonverbal apraxia, scores also seem to be influenced by speech and language alterations other than apraxia, as participants without apraxia (eg, patients with lvPPA or PD) presented higher scores than controls. Likewise, we did not analyse inter-rater reliability. Finally, although our results suggest that scores increase with disease progression, no longitudinal data are available.
In conclusion, our results suggest that the Barcelona scale may be a useful tool for the quantitative assessment of buccofacial apraxia in different neurodegenerative diseases, helping to discriminate between these diseases, especially in the case of nfvPPA. Quantitative data may help to measure longitudinal changes and response to potential therapeutic interventions. These findings should be validated in larger samples, and ideally in longitudinal studies.
Sources of fundingSBE received funding from the BBVA Foundation – Joan Rodés-Josep Baselga and NM received a grant from Hospital Clínic de Barcelona to complete a year of training at a foreign centre.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank all the volunteers who participated in the study.