To compare the characteristics of patients undergoing treatment with continuous intestinal infusion of levodopa–carbidopa (CIILC) for advanced Parkinson's disease and the data on the effectiveness and safety of CIILC in the different autonomous communities (AC) of Spain.
MethodsA retrospective, longitudinal, observational study was carried out into 177 patients from 11 CAs who underwent CIILC between January 2006 and December 2011. We analysed data on patients’ clinical and demographic characteristics, variables related to effectiveness (changes in off time/on time with or without disabling dyskinesia; changes in Hoehn and Yahr scale and Unified Parkinson's Disease Rating Scale scores; non-motor symptoms; and Clinical Global Impression scale scores) and safety (adverse events), and the rate of CIILC discontinuation.
ResultsSignificant differences were observed between CAs for several baseline variables: duration of disease progression prior to CIILC onset, off time (34.9%–59.7%) and on time (2.6%–48.0%; with or without disabling dyskinesia), Hoehn and Yahr score during on time, Unified Parkinson's Disease Rating Scale-III score during both on and off time, presence of =4 motor symptoms, and CIILC dose. Significant differences were observed during follow-up (>24 months in 9 of the 11 CAs studied) for the percentage of off time and on time without disabling dyskinesia, adverse events frequency, and Clinical Global Impression scores. The rate of CIILC discontinuation was between 20% and 40% in 9 CAs (78% and 80% in remaining 2 CAs).
ConclusionsThis study reveals a marked variability between CAs in terms of patient selection and CIILC safety and effectiveness. These results may have been influenced by patients’ baseline characteristics, the availability of multidisciplinary teams, and clinical experience.
Comparar las características de los pacientes con enfermedad de Parkinson avanzada en tratamiento con infusión intestinal continua de levodopa-carbidopa (IICLC) y los datos de efectividad y seguridad de IICLC entre diferentes comunidades autónomas (CC. AA.).
MétodosEstudio longitudinal observacional y retrospectivo. Se incluyeron 177 pacientes de 11 CC. AA. que iniciaron tratamiento con IICLC entre enero de 2006 y diciembre de 2011. Se compararon las características clínicas y demográficas, las variables de efectividad (cambios en el tiempo OFF, ON con y sin discinesias discapacitantes, cambios en la escala de Hoehn y Yahr y puntuación de la Unified Parkinson's Disease Rating Scale, síntomas no motores e Impresión Clínica Global) y seguridad (acontecimientos adversos), y la tasa de suspensión de IICLC.
ResultadosSe hallaron diferencias significativas entre las CC. AA. en diversas variables basales: duración de la enfermedad hasta el inicio de IICLC, tiempo OFF (34,9-59,7%) y ON (con o sin discinesias; 2,6-48,0%), Hoehn y Yahr en ON, Unified Parkinson's Disease Rating Scale-III en ON y OFF, presencia de=4 síntomas motores y dosis de IICLC. En el seguimiento (>24 meses en 9 de 11 CC. AA.) hubo diferencias significativas en el porcentaje de tiempo OFF, tiempo ON sin discinesias discapacitantes, frecuencia de acontecimientos adversos e Impresión Clínica Global. La tasa de suspensión fue de entre 20-40% en todas las CC. AA., excepto en 2 (78 y 80%).
ConclusionesEste estudio muestra una amplia variabilidad en la selección de los pacientes y en la efectividad y seguridad de IICLC entre las diferentes CC. AA. Podrían influir las características basales de los pacientes, la disponibilidad de un equipo multidisciplinar y la experiencia clínica.
Levodopa continues to be the gold standard of pharmacological treatment for Parkinson's disease (PD).1 The drug has repeatedly been shown to increase independence in the activities of daily living and to improve quality of life in patients with PD. However, patients develop such complications as motor fluctuations and dyskinesia several years after treatment onset.2–4 These are difficult to manage with the conventional treatments currently available, and considerably worsen patients’ quality of life.5 Continuous infusion of levodopa–carbidopa intestinal gel (LCIG) was developed to reduce adverse drug reactions resulting from levodopa-induced pulsatile stimulation of dopamine receptors.6 LCIG therapy has been found to be efficacious,7,8 effective,9–11 and safe,7–11 and to improve patients’ quality of life7–11 and independence,7–9 constituting an interesting treatment alternative to reduce the motor complications associated with advanced PD.5,8,9,12
In Spain, LCIG was approved in 2006. Although several sets of guidelines have been published on its use for advanced PD,13 the treatment is not widespread; indication depends on multiple factors, including experience with the procedure, resources available, internal protocols, and healthcare management factors (each Spanish autonomous community has its own healthcare system). Therefore, the treatment varies greatly in clinical practice. Few data are available on the characteristics of patients with advanced PD treated with LCIG in Spain, or on the treatment's effectiveness, safety, and tolerability. Our study was intended as a subanalysis of the E-DUO study,14 a multicentre retrospective study of the effectiveness and safety of LCIG therapy in Spain: we aimed to compare the characteristics of the patients included and the effectiveness and safety of the procedure between the participating autonomous communities.
Patients and methodsWe conducted a retrospective, observational, longitudinal, multicentre, open-label study of 18 hospitals in 11 autonomous communities. The study was approved by the research ethics committees of the participating hospitals and complies with the principles of the Declaration of Helsinki. We obtained informed consent from all participants except those who died before the study began.
We included consecutive patients diagnosed with advanced PD according to the MRC London Neurodegenerative Diseases Brain Bank criteria15 who had started receiving LCIG therapy between January 2006 and December 2011 (whether or not they continued with the treatment at the time of inclusion). A minimum of 11 months’ follow-up by a neurologist at the same centre was required for inclusion in the study. Participating centres were required to have experience with LCIG therapy with at least 5 patients.
Variables and data collection proceduresBaseline was considered to be the time point when patients started LCIG therapy; participants were included in the study at the inclusion visit (V1).
Patients’ medical histories were used to gather baseline data on sociodemographic characteristics, disease status, previous treatments for PD, concomitant treatments, and data on LCIG therapy (dose and hours of infusion per day). The researcher responsible for patient assessment at each centre determined whether patients may have been eligible at baseline for other second-line treatments. At V1, data were collected on disease progression and LCIG therapy (dose, hours of infusion per day, effectiveness, and safety) through clinical assessment and administering questionnaires.
To define disease characteristics and treatment effectiveness, we gathered the following information both at baseline and at V1: Hoehn and Yahr (H&Y) stage during “on” and “off” periods; percentage of waking day in “on” state with no disabling dyskinesia, percentage of waking day in “on” state with disabling dyskinesia, and percentage of waking day in “off” state as measured with the Unified Parkinson's Disease Rating Scale [UPDRS]17 part IV; UPDRS part III score during “on” and “off” periods; changes in motor and non-motor symptoms (V1 compared to baseline) on a 7-point linear scale (1: great improvement; 2: moderate improvement; 3: mild improvement; 4: no changes; 5: mild worsening; 6: moderate worsening; 7: severe worsening); and changes in Clinical Global Impression (CGI) scale scores at V1 compared to baseline according to the patient and the neurologist.
To evaluate treatment safety we collected data on drop-outs and adverse reactions to the treatment; our results focus on severe adverse reactions. Adverse reactions were classified by the participating neurologists using the following categories: (1) related to levodopa; (2) related to the procedure (gastrostomy); and (3) related to the device. For adverse reactions related to levodopa, neurologists had to indicate the type of causal relationship (definite, probable, or possible).
Statistical analysisEffectiveness variables were analysed at V1 in the sample of patients receiving LCIG therapy, whereas safety data were analysed in the initial sample (including patients dropping out before V1). We performed a descriptive analysis of the data, with categorical and discrete variables expressed as frequencies (%) and continuous variables as means (SD) or medians (IQR), as appropriate. Minimum and maximum values were compared with the Fisher exact test, the Wilcoxon test, or the t test, as appropriate. Comparisons were made between the 11 autonomous communities participating in the study. The primary variable of treatment effectiveness was a decrease in the mean percentage of “off” time; this was estimated using the mean of the range of UPDRS item 39 (part IV): (1) 0%; (2) 1%–25%; (3) 26%–50%; (4) 51%–75%; (5) 76%–100%. The level of improvement in motor and non-motor symptoms (7-point scale) was categorised as follows: 1–3 points=improvement; 4 points=no change; and 5–7=worsening. All tests were two-tailed; statistical significance was set at P<.05. All statistical analyses were performed using the SAS software, version 9.2 (SAS Institute, Cary, NC, USA).
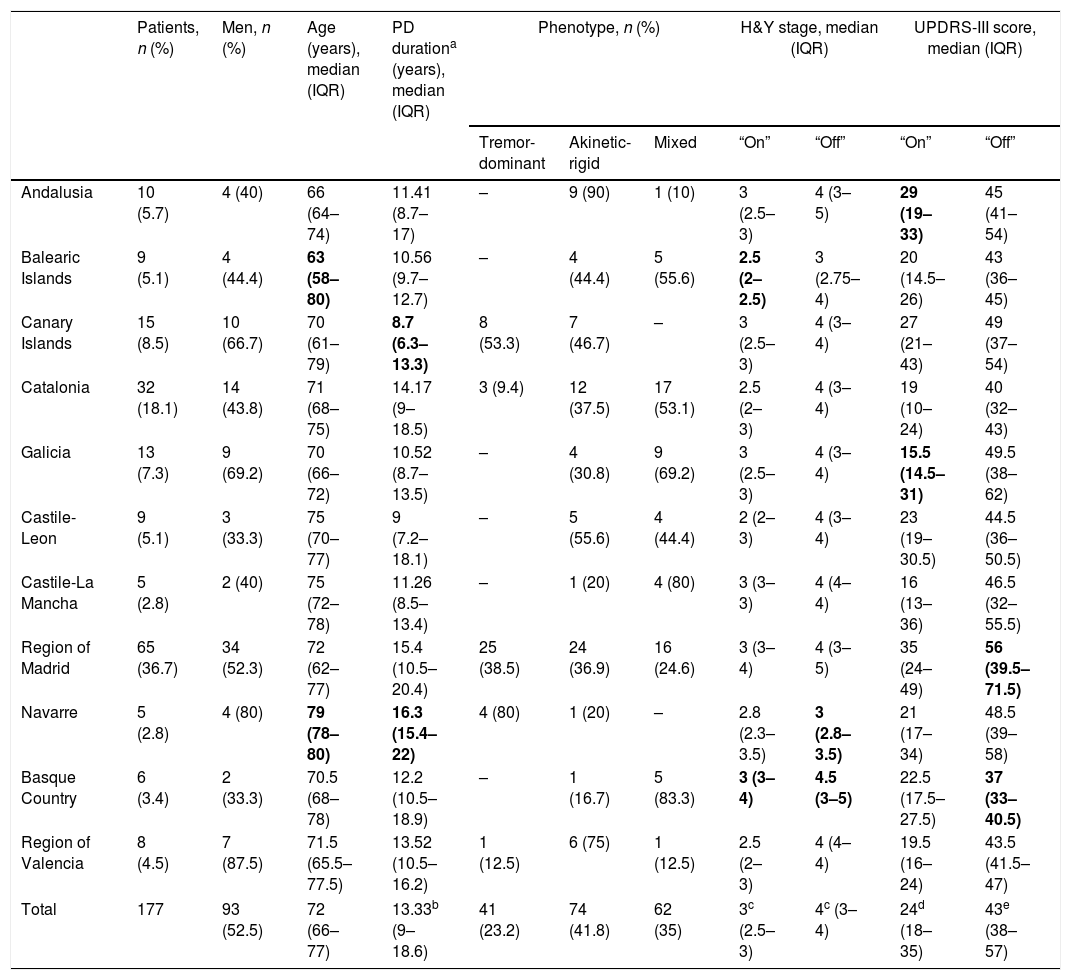
ResultsWe initially selected 185 patients with advanced PD; 8 were excluded for not adhering to the protocol. The final sample comprised 177 patients who had started LCIG therapy during the study period. At the time of inclusion, patients had been receiving treatment for a median of 34.7 months (IQR, 18.9–49 months). Patient distribution by autonomous community and baseline clinical and demographic characteristics are shown in Table 1. Age differences between autonomous communities were not significant (P=.204). Median disease progression time at the onset of LCIG therapy was >10 years in 9 of the 11 autonomous communities; significant differences were observed between Navarre and the Canary Islands (P=.0015).
Clinical and demographic data of the study sample at the time of onset of continuous infusion of levodopa–carbidopa intestinal gel.
| Patients, n (%) | Men, n (%) | Age (years), median (IQR) | PD durationa (years), median (IQR) | Phenotype, n (%) | H&Y stage, median (IQR) | UPDRS-III score, median (IQR) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tremor-dominant | Akinetic-rigid | Mixed | “On” | “Off” | “On” | “Off” | |||||
| Andalusia | 10 (5.7) | 4 (40) | 66 (64–74) | 11.41 (8.7–17) | – | 9 (90) | 1 (10) | 3 (2.5–3) | 4 (3–5) | 29 (19–33) | 45 (41–54) |
| Balearic Islands | 9 (5.1) | 4 (44.4) | 63 (58–80) | 10.56 (9.7–12.7) | – | 4 (44.4) | 5 (55.6) | 2.5 (2–2.5) | 3 (2.75–4) | 20 (14.5–26) | 43 (36–45) |
| Canary Islands | 15 (8.5) | 10 (66.7) | 70 (61–79) | 8.7 (6.3–13.3) | 8 (53.3) | 7 (46.7) | – | 3 (2.5–3) | 4 (3–4) | 27 (21–43) | 49 (37–54) |
| Catalonia | 32 (18.1) | 14 (43.8) | 71 (68–75) | 14.17 (9–18.5) | 3 (9.4) | 12 (37.5) | 17 (53.1) | 2.5 (2–3) | 4 (3–4) | 19 (10–24) | 40 (32–43) |
| Galicia | 13 (7.3) | 9 (69.2) | 70 (66–72) | 10.52 (8.7–13.5) | – | 4 (30.8) | 9 (69.2) | 3 (2.5–3) | 4 (3–4) | 15.5 (14.5–31) | 49.5 (38–62) |
| Castile-Leon | 9 (5.1) | 3 (33.3) | 75 (70–77) | 9 (7.2–18.1) | – | 5 (55.6) | 4 (44.4) | 2 (2–3) | 4 (3–4) | 23 (19–30.5) | 44.5 (36–50.5) |
| Castile-La Mancha | 5 (2.8) | 2 (40) | 75 (72–78) | 11.26 (8.5–13.4) | – | 1 (20) | 4 (80) | 3 (3–3) | 4 (4–4) | 16 (13–36) | 46.5 (32–55.5) |
| Region of Madrid | 65 (36.7) | 34 (52.3) | 72 (62–77) | 15.4 (10.5–20.4) | 25 (38.5) | 24 (36.9) | 16 (24.6) | 3 (3–4) | 4 (3–5) | 35 (24–49) | 56 (39.5–71.5) |
| Navarre | 5 (2.8) | 4 (80) | 79 (78–80) | 16.3 (15.4–22) | 4 (80) | 1 (20) | – | 2.8 (2.3–3.5) | 3 (2.8–3.5) | 21 (17–34) | 48.5 (39–58) |
| Basque Country | 6 (3.4) | 2 (33.3) | 70.5 (68–78) | 12.2 (10.5–18.9) | – | 1 (16.7) | 5 (83.3) | 3 (3–4) | 4.5 (3–5) | 22.5 (17.5–27.5) | 37 (33–40.5) |
| Region of Valencia | 8 (4.5) | 7 (87.5) | 71.5 (65.5–77.5) | 13.52 (10.5–16.2) | 1 (12.5) | 6 (75) | 1 (12.5) | 2.5 (2–3) | 4 (4–4) | 19.5 (16–24) | 43.5 (41.5–47) |
| Total | 177 | 93 (52.5) | 72 (66–77) | 13.33b (9–18.6) | 41 (23.2) | 74 (41.8) | 62 (35) | 3c (2.5–3) | 4c (3–4) | 24d (18–35) | 43e (38–57) |
H&Y: Hoehn and Yahr scale; IQR: interquartile range; PD: Parkinson's disease; UPDRS: Unified Parkinson's Disease Rating Scale.
The highest and lowest values of some variables are highlighted in bold type.
In the group of patients dropping out of treatment, time to drop-out ranged from a median of 39.1 (IQR, 23.5–60.3) in the region of Valencia (n=8) to a median of 2.8 months (IQR, 2.3–3.3) in the Basque Country (n=6) (P=.08).
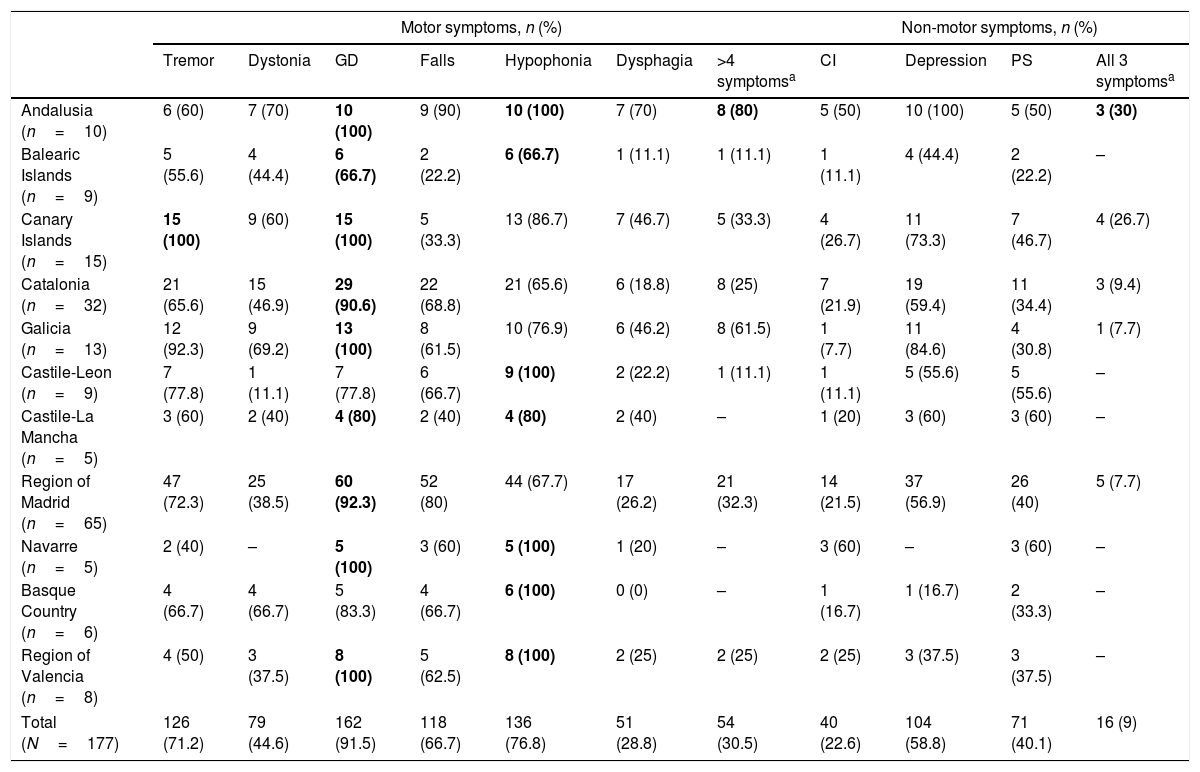
Clinical characteristics of patients receiving LCIG therapyAt baseline, statistically significant differences were observed between autonomous communities for H&Y stage during “on” periods (P=.0136) and for UPDRS-III scores during “on” and “off” periods (P<.0001) (Table 1). The highest H&Y stages during “on” and “off” periods were found in the Basque Country. Table 2 shows the percentage of patients with motor and non-motor symptoms at baseline. In 5 autonomous communities, all patients had gait disorders (the most frequent symptom in all regions) and hypophonia. Andalusia was the autonomous community with the highest proportion of patients with more than 4 motor symptoms (80%), whereas the Balearic Islands had the lowest proportion (11.1%); differences between the 2 were statistically significant (P=.0325). Andalusia was also the autonomous community where most patients presented all 3 of the non-motor symptoms analysed (cognitive impairment, depression, and psychiatric symptoms) at baseline. In all autonomous communities, patients had received at least 3 oral drugs before starting LCIG therapy (median: 3; IQR, 3–4; Supplementary Table 1); the most frequent reason for starting LCIG therapy was increased duration of “off” periods (mean, 85.9%), followed by lack of response to oral treatment (mean, 63.3%). A highly variable percentage of patients met eligibility criteria for treatment with apomorphine or deep brain stimulation (Supplementary Table 1). The mean dose of LCIG showed no remarkable changes between baseline and V1, although we did find statistically significant differences between autonomous communities. The mean (SD) dose at baseline ranged from 91.5mL (25.7) in Navarre to 61.1mL (17.3) in Castile-Leon (P=.0277); the mean final dose ranged from 92.7mL (23.9) in Navarre to 65.1mL (10.5) in Castile-Leon (P=.0459).
Motor and non-motor symptoms at baseline, before onset of continuous infusion of levodopa–carbidopa intestinal gel.
| Motor symptoms, n (%) | Non-motor symptoms, n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tremor | Dystonia | GD | Falls | Hypophonia | Dysphagia | >4 symptomsa | CI | Depression | PS | All 3 symptomsa | |
| Andalusia (n=10) | 6 (60) | 7 (70) | 10 (100) | 9 (90) | 10 (100) | 7 (70) | 8 (80) | 5 (50) | 10 (100) | 5 (50) | 3 (30) |
| Balearic Islands (n=9) | 5 (55.6) | 4 (44.4) | 6 (66.7) | 2 (22.2) | 6 (66.7) | 1 (11.1) | 1 (11.1) | 1 (11.1) | 4 (44.4) | 2 (22.2) | – |
| Canary Islands (n=15) | 15 (100) | 9 (60) | 15 (100) | 5 (33.3) | 13 (86.7) | 7 (46.7) | 5 (33.3) | 4 (26.7) | 11 (73.3) | 7 (46.7) | 4 (26.7) |
| Catalonia (n=32) | 21 (65.6) | 15 (46.9) | 29 (90.6) | 22 (68.8) | 21 (65.6) | 6 (18.8) | 8 (25) | 7 (21.9) | 19 (59.4) | 11 (34.4) | 3 (9.4) |
| Galicia (n=13) | 12 (92.3) | 9 (69.2) | 13 (100) | 8 (61.5) | 10 (76.9) | 6 (46.2) | 8 (61.5) | 1 (7.7) | 11 (84.6) | 4 (30.8) | 1 (7.7) |
| Castile-Leon (n=9) | 7 (77.8) | 1 (11.1) | 7 (77.8) | 6 (66.7) | 9 (100) | 2 (22.2) | 1 (11.1) | 1 (11.1) | 5 (55.6) | 5 (55.6) | – |
| Castile-La Mancha (n=5) | 3 (60) | 2 (40) | 4 (80) | 2 (40) | 4 (80) | 2 (40) | – | 1 (20) | 3 (60) | 3 (60) | – |
| Region of Madrid (n=65) | 47 (72.3) | 25 (38.5) | 60 (92.3) | 52 (80) | 44 (67.7) | 17 (26.2) | 21 (32.3) | 14 (21.5) | 37 (56.9) | 26 (40) | 5 (7.7) |
| Navarre (n=5) | 2 (40) | – | 5 (100) | 3 (60) | 5 (100) | 1 (20) | – | 3 (60) | – | 3 (60) | – |
| Basque Country (n=6) | 4 (66.7) | 4 (66.7) | 5 (83.3) | 4 (66.7) | 6 (100) | 0 (0) | – | 1 (16.7) | 1 (16.7) | 2 (33.3) | – |
| Region of Valencia (n=8) | 4 (50) | 3 (37.5) | 8 (100) | 5 (62.5) | 8 (100) | 2 (25) | 2 (25) | 2 (25) | 3 (37.5) | 3 (37.5) | – |
| Total (N=177) | 126 (71.2) | 79 (44.6) | 162 (91.5) | 118 (66.7) | 136 (76.8) | 51 (28.8) | 54 (30.5) | 40 (22.6) | 104 (58.8) | 71 (40.1) | 16 (9) |
CI: cognitive impairment; GD: gait disorders; PS: psychiatric symptoms.
The most common symptom in each autonomous community is indicated in bold type.
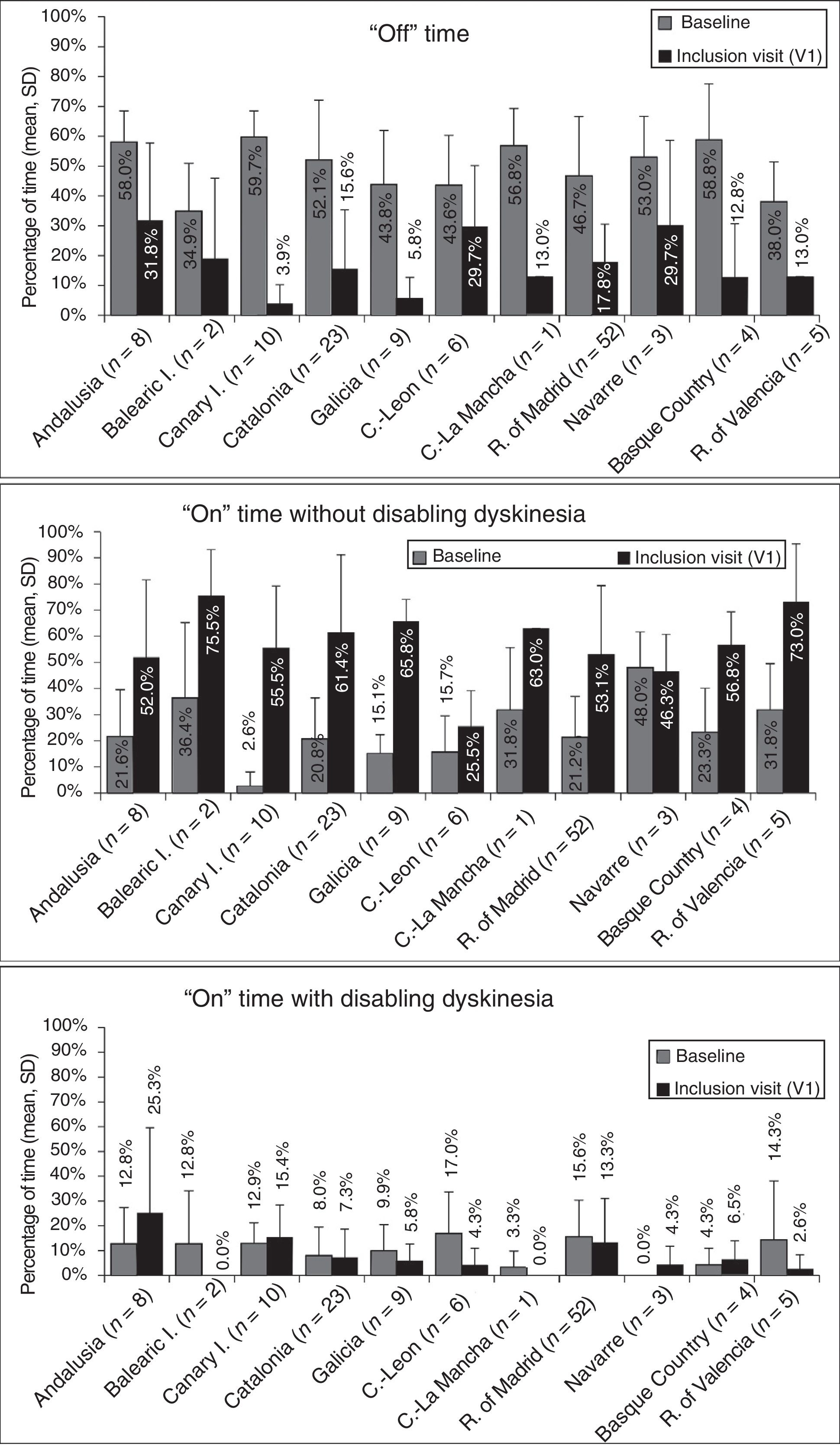
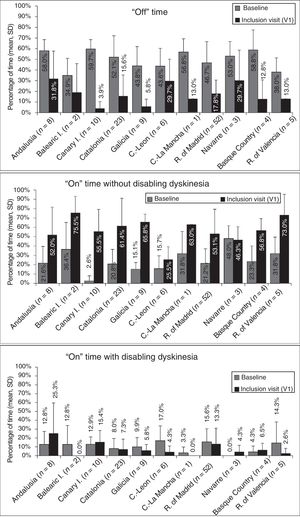
At baseline, the mean percentage of waking day in “off” state was above 33% in all autonomous communities, with significant differences between the autonomous communities showing most divergence (P=.0024; Fig. 1). We also observed significant differences in the mean percentage of waking day in “on” state with and without disabling dyskinesia between regions.
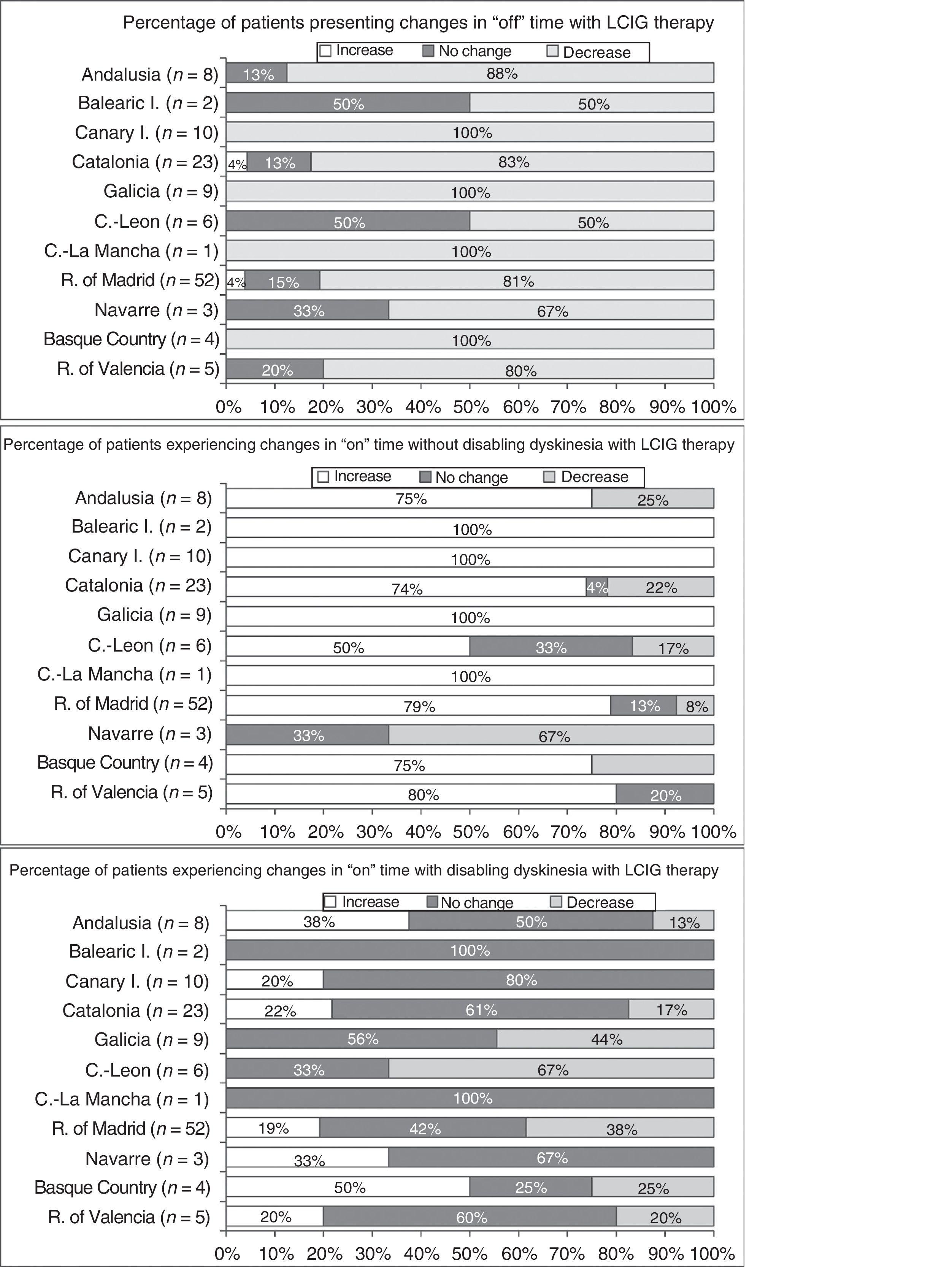
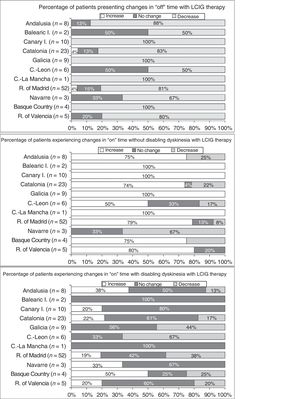
Effectiveness of LCIG therapyThe mean percentage of waking day in “off” state decreased significantly at V1 in all autonomous communities except Navarre; this decrease was accompanied by an increase in the percentage of waking day in “on” state without disabling dyskinesia (Fig. 1). At V1, we observed significant differences between regions in the mean duration of “on” (P=.005) and “off” periods (P=.0068). Most patients showed a decrease in “off” time and an increase in “on” time without disabling dyskinesia (Fig. 2). However, the effect of LCIG therapy on mean “on” time with disabling dyskinesia was more variable (Fig. 2), with decreases in 7 regions and increases in the remaining 4 (Fig. 1).
Regarding H&Y stage (Supplementary Fig. 1), most patients showed no changes in “on” or “off” times in more than half of the autonomous communities (6/11). UPDRS-III scores during “on” periods increased in the majority of patients from 6 regions, whereas scores during “off” periods decreased in most patients from 6 regions (Supplementary Fig. 2). Supplementary Tables 2 and 3 show the percentages of patients in each autonomous community presenting improvements, lack of change, or worsening of the motor and non-motor symptoms analysed in our study.
Patient- and neurologist-rated CGI scores revealed improvements in a majority of patients in all autonomous communities, except for Navarre and the Balearic Islands; neurologists reported improvements in 109 of the 123 patients for whom CGI scores were available, whereas 106 patients perceived improvements in their condition (Supplementary Fig. 3).
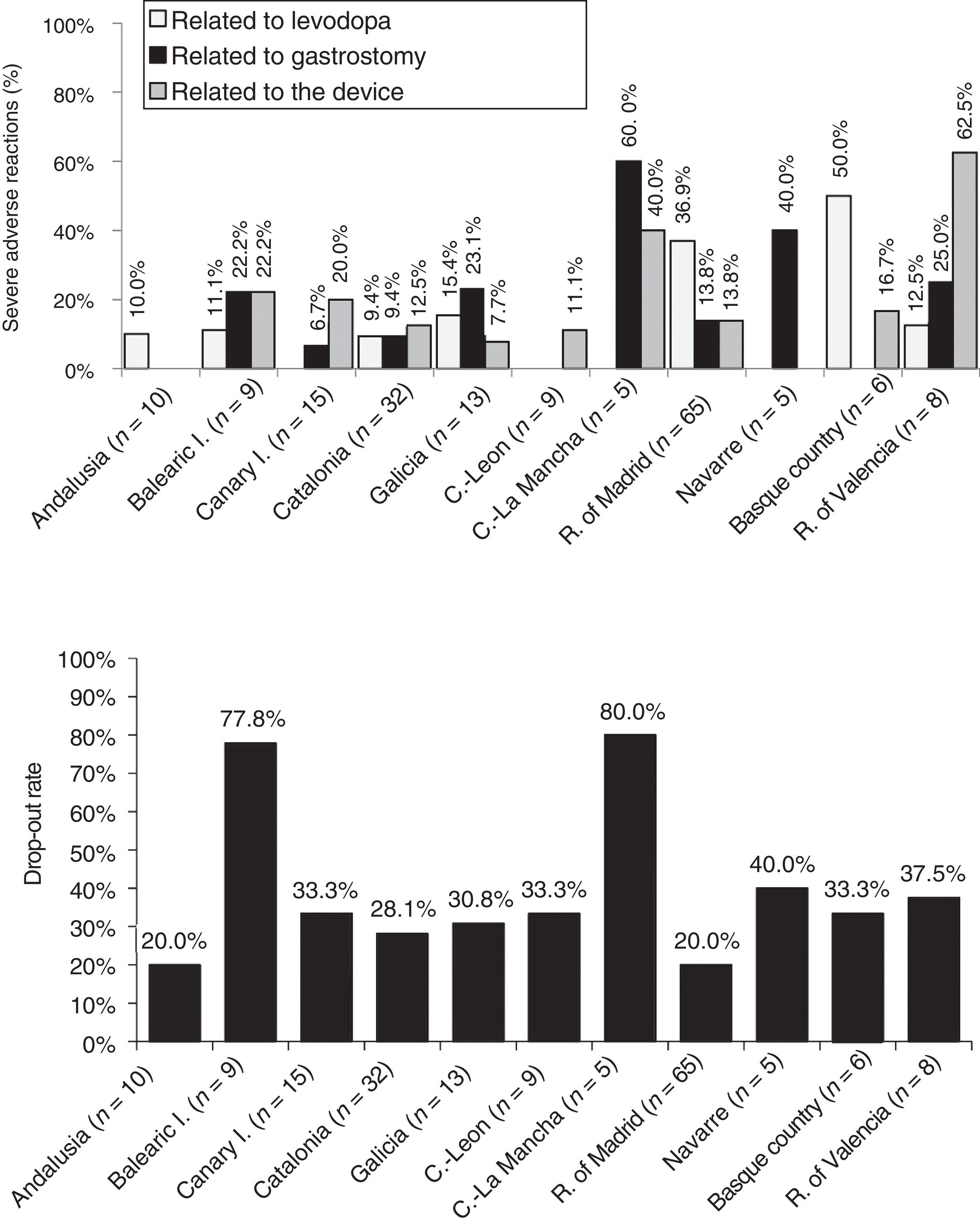
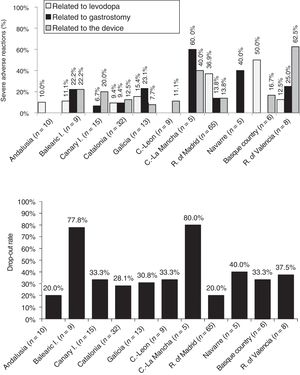
Analysis of safetyThe percentage of patients presenting severe adverse reactions varied between autonomous communities (Fig. 3). Discontinuation rates were similar between regions, except for the Balearic Islands and Castile-La Mancha, where they were considerably higher than the mean (Fig. 3). The reasons for early withdrawal of LCIG therapy are listed in Supplementary Table 4.
DiscussionThis study provides a subanalysis of the data reported by the E-DUO study,14 the largest study of patients treated with LCIG therapy to be conducted in Spain to date. Our results reveal significant differences between autonomous communities in patient selection and management and in the effectiveness and safety of treatment. This is the first study to analyse the management of these patients by autonomous community; comparison between regions provides useful data as it can help determine which management strategy achieves the best results with a view to implementing it in regions with poorer results.
The treatment of advanced PD is complex. Worsening of symptoms and the appearance of complications require thorough assessment and consideration of more invasive treatment alternatives, which may not be suitable for all patients.18–22 The factors involved in treatment success include selecting the most appropriate treatment option for each patient, availability of a multidisciplinary team, the hospital's experience with the procedure, and the learning curve. Other factors include healthcare management, population characteristics, and factors related to clinical practice (e.g., geographical proximity, role of scientific societies). These factors justify the need to compare autonomous communities, as we did in our study.
One major issue in PD management is classifying “advanced PD”20 and determining when patients should be considered eligible for non-conventional treatment (surgery or continuous infusion pump). Progression times in our study are similar to those reported in other studies of patients with PD treated with LCIG. These studies report a mean disease progression time of over 10 years.7–11,23–27 However, very long progression times (median of over 15 years) were observed in the regions of Madrid and Navarre. Age at onset of LCIG therapy is higher in our sample than those reported in the literature,28 particularly in Navarre (n=5; IQR: 78–80 years). A subanalysis of this cohort revealed that patients with a disease progression time below 10 years at treatment onset showed significantly shorter “off” periods and presented fewer adverse reactions.29 Older age has been associated with higher drop-out rates.24,30 Some studies propose earlier onset of LCIG therapy21,24,30–33 in order to maximise its benefits. Indeed, in our study, patients from Navarre (who were older with longer disease progression times) had poorer objective (“on” and “off” times) and subjective outcomes (CGI scores). However, time from diagnosis is not necessarily correlated with age, and disease duration is not always correlated with severity. In a recent open-label phase III study analysing the baseline characteristics of 307 patients,7 improvements were found to be independent of age and disease duration.34 Furthermore, many patients had cognitive impairment, depression, or other neuropsychiatric symptoms, which suggests that LCIG therapy may be indicated for a broader range of patient profiles than other treatments.35,36 In fact, some patients from the various autonomous communities showed improvements in motor and non-motor symptoms; this is consistent with the evidence currently available. This may be due in part to the fact that LCIG is frequently administered in monotherapy (levodopa/carbidopa), as well as the drug's action mechanism in the central nervous system.37–40 Although some patients with contraindications for deep brain stimulation or apomorphine infusion may benefit from LCIG therapy, higher scores on the Neuropsychiatric Inventory have been associated with higher drop-out rates.21 In our study, the regions of Castile-La Mancha and Navarre, where 60% of patients presented neuropsychiatric symptoms and were older than in the remaining regions, showed the highest and the third-highest drop-out rates. Unlike in other clinical practice studies with similar methodologies,24 in our study LCIG was not regarded as the final treatment alternative, behind deep brain stimulation and apomorphine infusion, considering that a sizeable percentage of patients could instead have been given other treatments, according to the neurologist's expert opinion.
Motor complications improved in all autonomous communities except for Navarre (“off” time decreased but “on” time without disabling dyskinesia did not); these findings are consistent with those reported by other studies into the efficacy and/or effectiveness of LCIG therapy.7–12,23–25,27,28 Furthermore, a large percentage of patients and neurologists reported subjective improvements (CGI scale) with LCIG: three-quarters of patients showed moderate or great improvements. This was not true for Navarre, where objective improvements were less marked.
Regarding treatment safety, 16% of patients presented severe adverse reactions, with considerable differences between regions. Other studies have shown good tolerability to levodopa infusion therapy.41 The percentage of adverse reactions to medication (levodopa) was low, except for the Basque Country and the region of Madrid. The other types of complications (those related to the procedure or the device) were more frequent and variable between autonomous communities; this may be due to differences in each hospital's level of experience with the procedure or the availability of a multidisciplinary team. Most autonomous communities showed similar drop-out rates to those reported in the literature (20%–30%).24,30,40,42 The highest rates of severe adverse reactions to gastrostomy were observed in the 2 autonomous communities with the oldest patients (Navarre and Castile-La Mancha).
Our study has several significant limitations due to its methodology, including its retrospective design and the variability in follow-up periods. Although the study includes a large number of patients, the samples from some autonomous communities are small, which may have biased our results. The phenotype was not defined according to the literature,43 but rather to the neurologist's opinion. The study is based on 2011 data, and management of these patients may have changed due to greater familiarity with this treatment in Spain. Some regions included hospitals with differing levels of experience (treatment protocols, availability of a multidisciplinary team, neurologist's and gastroenterologist's experience, etc.) and care (secondary vs tertiary hospitals) or with different characteristics (patient referral protocol, urban vs rural areas, etc.) that may have biased results by autonomous community. Future studies should aim to control for these covariates and to analyse each region's needs to improve healthcare quality.
In conclusion, our study shows great heterogeneity in terms of selection, management, and treatment response of patients with PD treated with LCIG in Spain. In some autonomous communities where experience with LCIG therapy has been less positive, age at treatment onset may have played a pivotal role; prospective analyses with larger patient samples are necessary to confirm this hypothesis.
FundingAbbvie Spain S.L.U. provided funds for the study, was responsible for its design, and coordinated the data collection process. It also provided support for statistical analysis and manuscript drafting.
Author contributionsAll authors had unrestricted access to the data and were equally involved in data analysis and manuscript revision, and all approved the final version of the manuscript.
Conflicts of interestD. Santos-García has received fees from Abbvie, UCB, Zambon, KRKA, and Lundbeck for attending meetings as a speaker; he has also received fees from Abbvie for coordinating clinical trials and for consulting activities. M.J. Catalán has received fees from Abbvie and Merz Pharma for consulting activities, presentations, and research. V. Puente has received fees from Abbvie, UCB, and Lundbeck for teaching courses, and from Abbvie for consulting activities. F. Valldeoriola has received fees from Abbvie, Medtronic, and Boston Scientific for presentations and consulting activities. I. Regidor has received fees and travel expenses from Medtronic, Boston Scientific, and Abbvie to attend scientific meetings. P. Mir has received fees from Abbvie, UCB, Zambon, Allergan, and Merz Pharma for consulting activities and lectures. J.M. Arbelo has received fees from Abbvie and UCB for presentations and consulting activities. J.C. Parra works for Abbvie and has shares or options in Abbvie. F. Grandas has received fees from Merz Pharma, Abbvie, UCB, and GE Healthcare for lectures and consulting activities.
The authors would like to thank Dr Blanca Piedrafita of Medical Statistics Consulting for her assistance in manuscript drafting.
Please cite this article as: Santos-García D, Catalán MJ, Puente V, Valldeoriola F, Regidor I, Mir P, et al. Uso de la infusión intestinal continua de levodopa-carbidopa en pacientes con enfermedad de Parkinson avanzada en España. Subanálisis por comunidades autónomas. Neurología. 2021;36:101–111.
Part of this study was presented in poster format at the 66th Annual Meeting of the Spanish Society of Neurology (2014).