Multiple sclerosis (MS) is a chronic disease affecting the central nervous system and is characterised by inflammation, demyelination, gliosis, and axonal damage. The introduction of dimethyl fumarate and teriflunomide has led to an increase in the number of alternative first-line therapies for MS. The objective of this study was to evaluate the economic impact of the incorporation of new oral therapies at the reference unit (CSUR) at Hospital Universitario Puerta de Hierro Majadahonda.
Materials and methodsWe performed a retrospective observational study including patients diagnosed with MS, who underwent treatment with disease-modifying drugs in 2015 and were followed up for a minimum mean time of one year. Data were collected from patients’ electronic clinical histories and the pharmacy service's programme for dispensing drugs to outpatients.
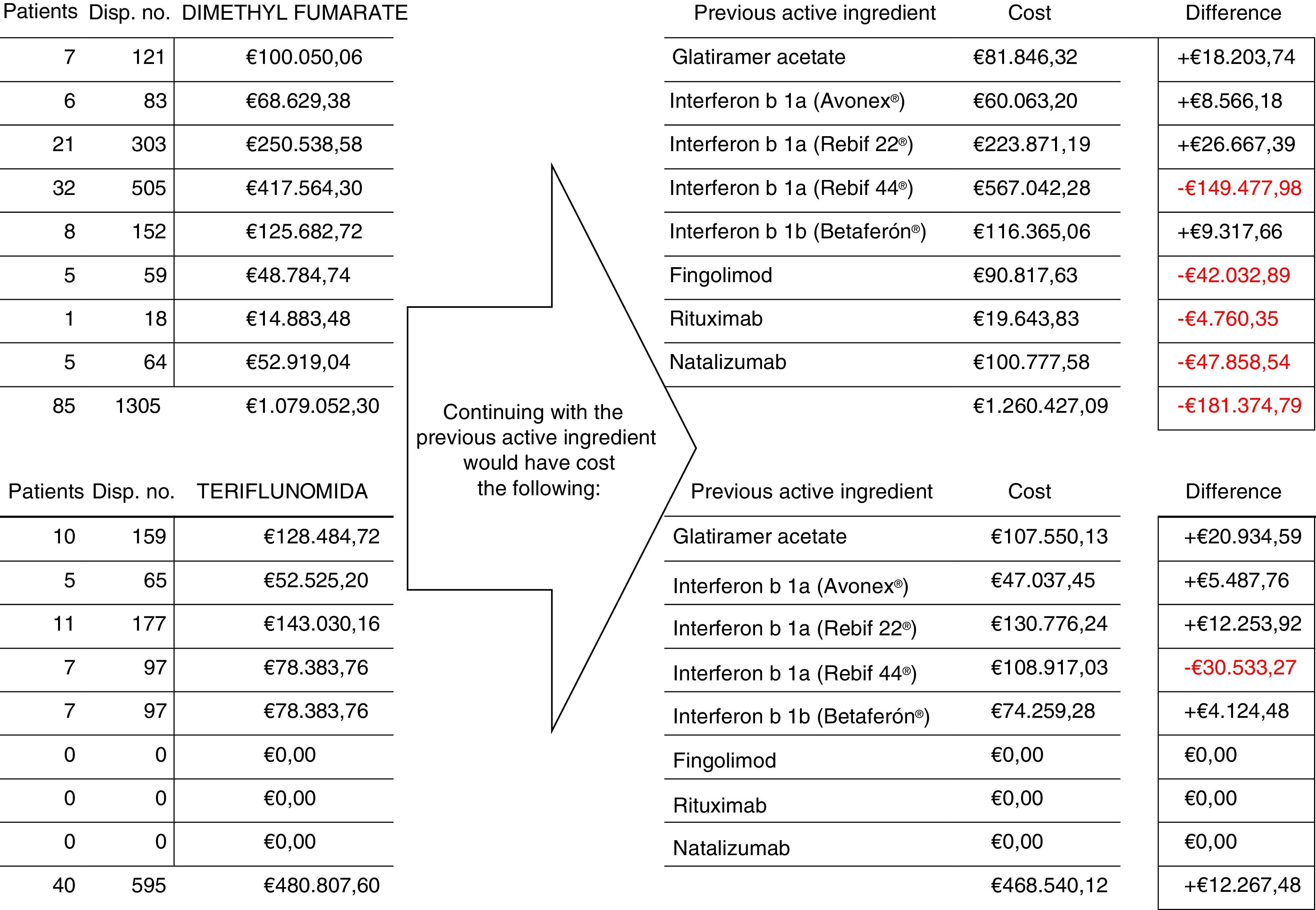
ResultsEvaluating the cost of changing 125 patients’ treatment from other drugs to dimethyl fumarate and teriflunomide, and comparing this with the cost that would have resulted from maintaining their previous treatment, demonstrated a total saving of €169107.31 over the study period.
ConclusionsIn addition to contributing new therapeutic alternatives, dimethyl fumarate and teriflunomide produced an economic saving in MS treatment at our hospital.
La esclerosis múltiple (EM) es una enfermedad crónica del sistema nervioso central que se caracteriza por la existencia de inflamación, desmielinización, gliosis y daño axonal. La introducción de dimetilfumarato y teriflunomida ha supuesto un aumento de las alternativas terapéuticas en la primera línea de tratamiento de la EM. El objetivo de este estudio fue evaluar el impacto económico de la incorporación de estas nuevas terapias orales en la Unidad de Referencia (CSUR) del Hospital Universitario Puerta de Hierro Majadahonda.
Material y métodosSe realizó un estudio observacional retrospectivo en la población de pacientes diagnosticados de EM, en tratamiento con fármacos modificadores de la enfermedad durante el año 2015, y su seguimiento se prolongó hasta obtener un seguimiento medio superior a un año de tratamiento. Los datos se recogieron de la historia clínica electrónica y del programa de dispensación de medicamentos a pacientes externos y ambulantes del Servicio de Farmacia.
ResultadosEvaluando el coste del cambio del tratamiento en 125 pacientes desde otros fármacos a dimetilfumarato o teriflunomida y comparando con el coste que habría supuesto el mantenimiento de los tratamientos previos, el ahorro total durante el periodo de observación fue de 169.107,31€.
ConclusionesDimetilfumarato y teriflunomida, además de aportar nuevas alternativas terapéuticas, no solo no han supuesto un incremento sino, por el contrario, una disminución en los costes del tratamiento de la EM en nuestro hospital.
Multiple sclerosis (MS) is a chronic disease of the central nervous system characterised by inflammation, demyelination, gliosis, and axonal damage leading to varying degrees of persistent neurological impairment. It usually develops in early adulthood and is 2-3 times more frequent in women than in men. In the early stages of the disease especially, 90% of patients present episodes of neurological dysfunction, named relapses, that last days or weeks and usually fully or partially resolve. As the disease progresses, a significant number of patients develop secondary-progressive MS, in which neurological impairment progresses with no evidence of relapses. Furthermore, approximately 10% of patients present primary-progressive MS, which is characterised by a progressive neurological impairment with no relapses.1
The personal and socio-economic impact of MS is considerable, given its frequency (in Spain, prevalence is estimated at 100-125 cases per 100000 population), the associated disability it causes in young adults, its interference with productivity at work, the associated need for care, and high treatment costs.1
Over the last 20 years, the European Union has successively approved 11 drugs for treating MS, of which some are for parenteral administration and some are administered orally. The first to be marketed were interferon beta 1b (Betaferón®), intramuscular interferon beta 1a (Avonex®), subcutaneous interferon beta 1a (Rebif®), glatiramer acetate (Copaxone®), mitoxantrone (Novantrone®), natalizumab (Tysabri®), and fingolimod (Gilenya®, the first to be approved for oral administration). In 2014, another 4 drugs were approved: alemtuzumab (Lemtrada®), PEGylated interferon beta 1a (Plegridy®) and 2 other oral drugs, dimethyl fumarate (Tecfidera®) and teriflunomide (Aubagio®).1 The latter 2 are the first-line oral drugs.
Each of these drugs was approved based on the results of clinical trials, and indications are established according to the clinical form of the disease and the population in which the trial was performed.1
Dimethyl fumarate and teriflunomide have been approved as first-line treatments for relapsing–remitting MS.1–8 Their introduction has increased the number of treatment options and represents an opportunity to improve patients’ quality of life.
However, we should note that approving new drugs usually increases the cost of treatment. The aim of our study is to assess the economic impact of the introduction of the new first-line oral treatments for MS in the reference centres, services, and units of Hospital Universitario Puerta del Hierro in Majadahonda (Spain).
Material and methodsWe conducted a retrospective observational study including patients diagnosed with MS, treated with disease-modifying drugs during 2015, and followed up for a mean period of over one year.
The study was granted ethical and methodological approval by the hospital's Drug Research Ethics Committee.
Data were gathered from patients’ electronic clinical histories and the hospital pharmacy's dispensing programme for outpatients. Data were managed in accordance with the applicable legislation on personal data protection (Spanish Organic Law 15/1999 of 13 December). Anonymised patient data were included in the database.
The variables included were age at the time of the study, sex, duration of follow-up, active ingredient used, changes of active ingredient during follow-up, and treatment adherence.
Treatment adherence was measured by monitoring the drugs dispensed with the following formula: % adherence=no. of days’ supply of drug dispensed/no. of days between dispensing events×100. We excluded patients treated with the same drug for less than 120 consecutive days.
In those patients whose previous treatment was changed to dimethyl fumarate or teriflunomide, we compared the cost of these treatments with that of maintaining the previous prescription (net price×no. of dispensing events during the follow-up period). We did not account for other direct costs (associated with the preparation and administration of drugs at the day centre, biochemical analysis, imaging studies, etc.) or indirect and intangible costs (quality of life, productive capacity, etc.), as this study exclusively assessed the economic impact of the introduction of these drugs for the hospital pharmacy.
We performed a descriptive analysis of the variables to determine the general characteristics of the study population. Qualitative variables were presented as absolute frequencies and percentages. Quantitative variables were expressed as means and standard deviations. We calculated the number of patients treated with each drug and the duration of treatment with each drug. We analysed the association between qualitative variables with the chi-square test, with a significance level of P<.05. Data were processed using the STATA software.
ResultsDuring the period between January and December 2015, 501 patients diagnosed with MS were treated with disease-modifying drugs.
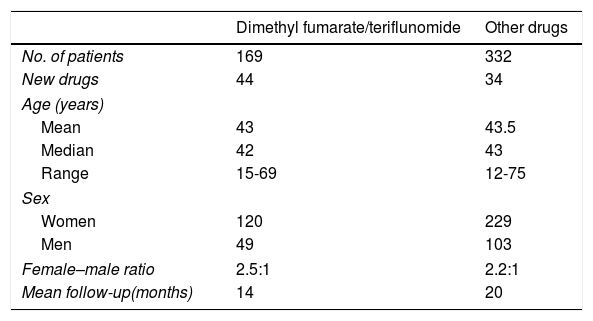
The mean age was 43.2 years (median, 43 years; range, 12-75 years); 349 were women and 152 were men (female–male ratio of 2.3:1). Patients were followed up for a mean of 20 months.
During the study period, 169 patients (34%) were treated with the new first-line oral drugs (dimethyl fumarate in 113 patients and teriflunomide in 56); these drugs were the first treatment option in 44 cases (56% of the patients newly diagnosed that year), whereas in the remaining 125 cases, they replaced another treatment (Table 1).
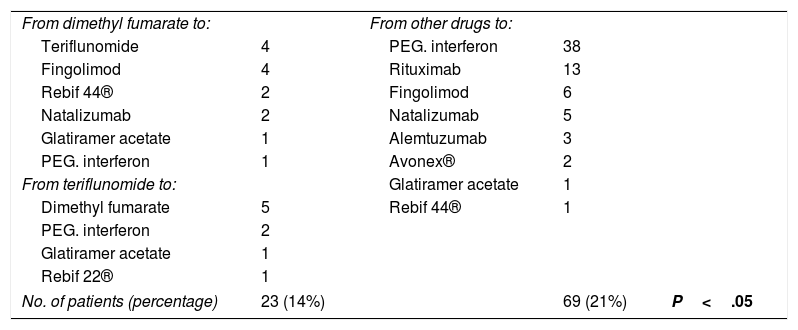
We recorded treatment changes during follow-up (Table 2).
Changes in medication.
| From dimethyl fumarate to: | From other drugs to: | |||
| Teriflunomide | 4 | PEG. interferon | 38 | |
| Fingolimod | 4 | Rituximab | 13 | |
| Rebif 44® | 2 | Fingolimod | 6 | |
| Natalizumab | 2 | Natalizumab | 5 | |
| Glatiramer acetate | 1 | Alemtuzumab | 3 | |
| PEG. interferon | 1 | Avonex® | 2 | |
| From teriflunomide to: | Glatiramer acetate | 1 | ||
| Dimethyl fumarate | 5 | Rebif 44® | 1 | |
| PEG. interferon | 2 | |||
| Glatiramer acetate | 1 | |||
| Rebif 22® | 1 | |||
| No. of patients (percentage) | 23 (14%) | 69 (21%) | P<.05 | |
Of the 113 patients treated with dimethyl fumarate, treatment was changed to another drug in 14 (12.5%); changes were also made in the treatment of 9 of the 56 patients (16%) receiving teriflunomide. Therefore, changes were made in a total of 23 patients (14%) receiving the new drugs, and were motivated by the appearance of adverse effects or the need to use a second-line drug due to increased disease activity.
Of the 332 patients treated with other drugs, changes were made in 69 cases (21%), for the same reasons.
The proportion of treatment changes among patients treated with dimethyl fumarate and teriflunomide was significantly lower than in those treated with other drugs (P<.05).
Table 3 shows treatment adherence for the different drugs: oral drug adherence was above 99% (dimethyl fumarate, teriflunomide, and fingolimod), slightly higher than the figure obtained for injectable drugs. However, intergroup differences were not statistically significant.
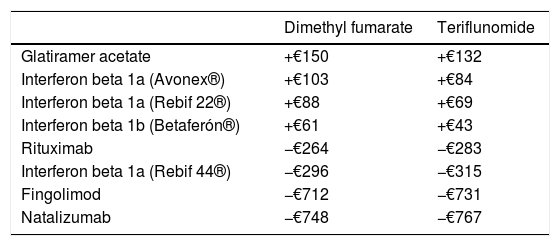
The differences in net price/dispensing event between the different active ingredients or products are summarised in Table 4. Dispensing dimethyl fumarate and teriflunomide is more expensive than dispensing glatiramer acetate, interferon beta 1a (Avonex® and Rebif 22®), or interferon beta 1b, but cheaper than dispensing rituximab, interferon beta 1a (Rebif 44®), fingolimod, or natalizumab.
Differences in net price/dispensing event (€).
| Dimethyl fumarate | Teriflunomide | |
|---|---|---|
| Glatiramer acetate | +€150 | +€132 |
| Interferon beta 1a (Avonex®) | +€103 | +€84 |
| Interferon beta 1a (Rebif 22®) | +€88 | +€69 |
| Interferon beta 1b (Betaferón®) | +€61 | +€43 |
| Rituximab | −€264 | −€283 |
| Interferon beta 1a (Rebif 44®) | −€296 | −€315 |
| Fingolimod | −€712 | −€731 |
| Natalizumab | −€748 | −€767 |
Dimethyl fumarate/teriflunomide – rest of active ingredients.
Fig. 1 shows the number of patients, number of dispensing events, and the cost of treatment with dimethyl fumarate and teriflunomide for 125 patients who switched from treatment with another drug, and compared to the cost of continuing with the previous treatment. Total savings during the follow-up period amounted to €169107.31.
DiscussionWe present a retrospective study finding that the use of the new oral drugs (dimethyl fumarate and teriflunomide) has reduced the cost of MS treatment; this is contrary to the usual situation when new treatment alternatives are introduced.
These drugs were introduced in our hospital in 2015. They were used as the first-line treatment in newly diagnosed patients and as alternative to other drugs used previously.
In addition to the prescription recommendations included in international guidelines,1–5 reasons for selecting or replacing the drugs included each patient's individual characteristics, especially: age of disease onset; clinical, radiological, and biological prognostic markers; sex; intention to become pregnant; other comorbidities; history and/or presence of neoplasms; and adverse drug reactions.
By analysing all changes in treatment during follow-up, we observed a greater number of changes among patients treated with drugs other than dimethyl fumarate and teriflunomide. On the one hand, we consider this to be due to the fact that patients treated with injectable drugs usually present higher rates of adverse reactions related to long-term administration and require other alternatives; on the other hand, patients receiving second-line treatments usually present more aggressive forms of the disease and are potentially more prone to require changes.
We also analysed treatment adherence, defined as the degree to which the patient's behaviour corresponds with his/her doctor's recommendations, which is essential for obtaining the maximum benefit from a treatment.9,10 Adherence rate is defined as the percentage of the prescribed dose that the patient actually takes over a given period; it can be measured by direct or indirect means, which include monitoring the amounts of the drug dispensed, as in our study. We assessed the concordance of time between the schedule prescribed and the collection of the medication, although this does not guarantee that the patient is taking the medication as prescribed. However, the results are considered reasonably objective in systems using electronic medical records and dispensing the analysed drugs exclusively at the hospital,9 as in our study.
Adherence rates among our patients were higher for first- and second-line oral treatments than for injectable treatments, although differences between groups were not statistically significant.
Adherence rates in our sample were also higher than those published by the Global Adherence Project.10 Differences with this study may be explained by the fact that we used a smaller patient population and shorter follow-up time, although we consider that our hospital's system of coordination between the neuroimmunology department and the pharmacy may have played a significant role. After every prescription by the neuroimmunology department, which educates and periodically follows up patients, the pharmacy conducts a first interview to inform them about the treatment and monitors every dispensing event; an electronic alert system is in place to inform the neurologist if the patient does not collect the treatment when he/she should. When this happens, the patient is contacted to identify the problem, attempt to solve it, and to achieve the highest possible adherence in every case.
Regarding costs, while switching to dimethyl fumarate and teriflunomide from fingolimod and natalizumab accounted for a greater difference in price/dispensing event, the number of patients switching from interferon beta 1a (Rebif 44®) to the first-line oral drugs, as well as changes from second-line treatment to dimethyl fumarate, represented the main source of cost savings, which in our study exceeded €169000 in 20 months. To this number we should add the potential cost of changes to second-line treatments (fingolimod, natalizumab), which were delayed and/or reduced thanks to the availability of these new treatments.
Despite the net price of the new oral drugs being slightly higher than that of other established first-line therapies, our study shows that the introduction of new treatment alternatives is not always associated with cost increases.
In addition to representing therapeutic alternatives and offering the patient improved quality of life in many cases, dimethyl fumarate and teriflunomide not only do not involve higher costs, but in fact decrease the cost of MS treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Álvarez Ayuso L, Rodríguez Marrodán B, Blasco Quílez MR, García-Merino JA, Sánchez Guerrero A. Impacto económico de las nuevas terapias orales en esclerosis múltiple. Neurología. 2021;36:95–100.