Parkinson's disease (PD) is characterised by motor alterations, which are commonly treated with l-DOPA. However, long-term l-DOPA use may cause dyskinesia. Although the pathogenic mechanism of l-DOPA-induced dyskinesia is unclear, the condition has been associated with alterations in dopamine receptors, among which D2 receptors (D2R) have received little attention. This study aims to: (i) develop and standardise an experimental model of l-DOPA-induced dyskinesia in rats with hemiparkinsonism; and (ii) evaluate the correlation between D2R expression and presence of abnormal involuntary movements (AIM). We allocated 21 male Wistar rats into 3 groups: intact controls, lesioned rats (with neurotoxin 6-hydroxydopamine (6-OHDA)), and dyskinetic rats (injected with l-DOPA for 19 days). Sensorimotor impairment was assessed with behavioural tests. Dyskinetic rats gradually developed AIMs during the treatment period; front leg AIMs were more severe and locomotor AIMs less severe (P<.05). All AIMs were significantly evident from day 5 and persisted until the last day of injection. D2R density was greater in the striatum and the medial anterior brain of the lesioned and dyskinetic rats than in those of controls. Our results suggest an association between D2R expression and locomotor AIMs. We conclude that RD2 is involved in l-DOPA-induced dyskinesia.
La enfermedad de Parkinson (EP) se caracteriza por una serie de deficiencias motoras que son tratadas comúnmente con L-DOPA; sin embargo, tras un uso crónico se desarrollan disquinesias inducidas por L-DOPA (DIL). Por otra parte, el origen de las DIL no está del todo claro, pero se asocia con alteración en receptores dopaminérgicos, donde los receptores D2 (RD2) han sido poco estudiados. El presente trabajo buscó: 1)desarrollar y estandarizar un modelo experimental de disquinesia con L-DOPA en ratas hemiparkinsonizadas, y 2)evaluar la correlación entre la expresión del RD2 y la manifestación de movimientos involuntarios anormales (MIA). Se utilizaron 21 ratas Wistar macho asignadas a 3 grupos: control intacto, lesionados (con la neurotoxina 6-OHDA) y lesionados disquinéticos (inyectados con L-DOPA durante 19días). Los reactivos biológicos se sometieron a pruebas comportamentales para evaluar el deterioro sensoriomotor. Los animales del grupo disquinético desarrollaron de forma gradual MIA durante el tratamiento, siendo mayores los MIA de miembro anterior y menores los de tipo locomotor (p<0,05). Todos los MIA fueron significativamente evidentes a partir del día 5 y se mantuvieron hasta el último día de inyección. Además, se pudo evidenciar incremento en la densidad del RD2 en el estriado y el cerebro anterior medial en los grupos lesionados con respecto al control, así como también una posible asociación entre la expresión del RD2 y MIA de tipo locomotor. Por lo que concluimos que el RD2 está implicado en el fenómeno disquinético generado con la L-DOPA.
PD is the second most prevalent neurodegenerative disease.1 Levodopa is the most effective symptomatic treatment, although long-term use is associated with AIM known as levodopa-induced dyskinesia (LID).2 Research into LID requires the use of experimental models. In the case of PD, models must show nigrostriatal dopamine loss, which is induced with such neurotoxins as 6-OHDA and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which have frequently been used in rodents3 and non-human primates, respectively. LID is induced with levodopa, mimicking the human version of the condition.4–6
It has been suggested that LID is caused by the sensitisation of dopamine receptors D1 and D2 (DRD1 and DRD2) secondary to PD; however, the association between LID and DRD1 is reported to be stronger than the association with DRD2.7 It has been proposed that only co-activation of both receptors produces normal movement.8 Due to its presynaptic location, DRD2 may be involved in more complex mechanisms; this has therapeutic implications for dyskinesia.9,10 In theory, improved DRD2 signalling may reduce LID.11
In view of the potential association between DRD2 and dyskinesia, and considering the limited research addressing DRD2 in the context of levodopa treatment, we aimed to evaluate the role of levodopa in DRD2 expression in an animal model of dyskinesia, and to analyse the correlation between DRD2 density and AIMs.
Material and methodsAnimalsWe included 21 male Wistar rats with a body weight of 250-350g and age 12-16 weeks. Animals were kept in the vivarium at Universidad del Tolima, in Colombia. The study was approved by the university's research ethics committee and observed the ethical standards established in the NIH Guide for the care and use of laboratory animals (NIH Publications No. 80-23).
Study designRats were randomly assigned to one of 3 different experimental groups: intact control group, 6-OHDA-induced lesion group, and dyskinesia group (rats with 6-OHDA-induced lesions and treated with levodopa/benserazide [Sigma-Aldrich®]). Each group included 7 rats. Lesions were induced by injecting 6-OHDA into the substantia nigra pars compacta (SNpc) of the right hemisphere. One month after lesions were induced, animals received subcutaneous injections of apomorphine dosed at 0.5mg/kg (Sigma-Aldrich®).
Dyskinetic rats then received daily intraperitoneal injections of 6mg/kg levodopa (Sigma-Aldrich®) plus 12mg/kg peripheral decarboxylase inhibitor benserazide12 (Sigma-Aldrich®), dissolved in physiological saline solution. All behavioural tests were performed in the morning (09.00–11.00). The research team was divided into 3 groups: the first group performed surgeries and randomly assigned rats to the 3 experimental groups, the second performed behavioural testing, and the third performed immunofluorescence studies. Results were analysed by experimental group.
SurgeryRats were anaesthetised with intraperitoneal injections of ketamine (90mg/kg) plus xylazine (10mg/kg) and placed in a stereotactic device. A Hamilton syringe was used to administer 3μL of 6-OHDA per animal at a rate of 1μL/min. The stereotactic coordinates for 6-OHDA lesions were: AP≥−4.4, ML≥1.2, DV=−7.8.13
Behavioural testsApomorphine-induced spinning behaviourPresence of spinning behaviour induced by dopamine agonists is essential to evaluate the effectiveness of the lesion and is observed in few models of neurodegenerative diseases.14 One month after inducing lesions with 6-OHDA, we evaluated apomorphine-induced spinning behaviour to determine the degree of the lesion. After apomorphine injection, rats were placed in a circular pool of water for 20min; we counted the number of spins to the side contralateral to the lesion. Rats had to spin 140 times, which indicates 80% neuron loss in the lesioned hemisphere according to the literature.15
Neurological examination and pole test6-OHDA-induced sensorimotor alterations were evaluated by analysing postural reflexes, limb flexion and extension reflexes, support reflex, and visual location, as well as with the pole test, which evaluates posture, limb use, and time taken to walk down a pole measuring 60cm long and 3cm in diameter.16 Quantitative scales were used to analyse the results of all tests.
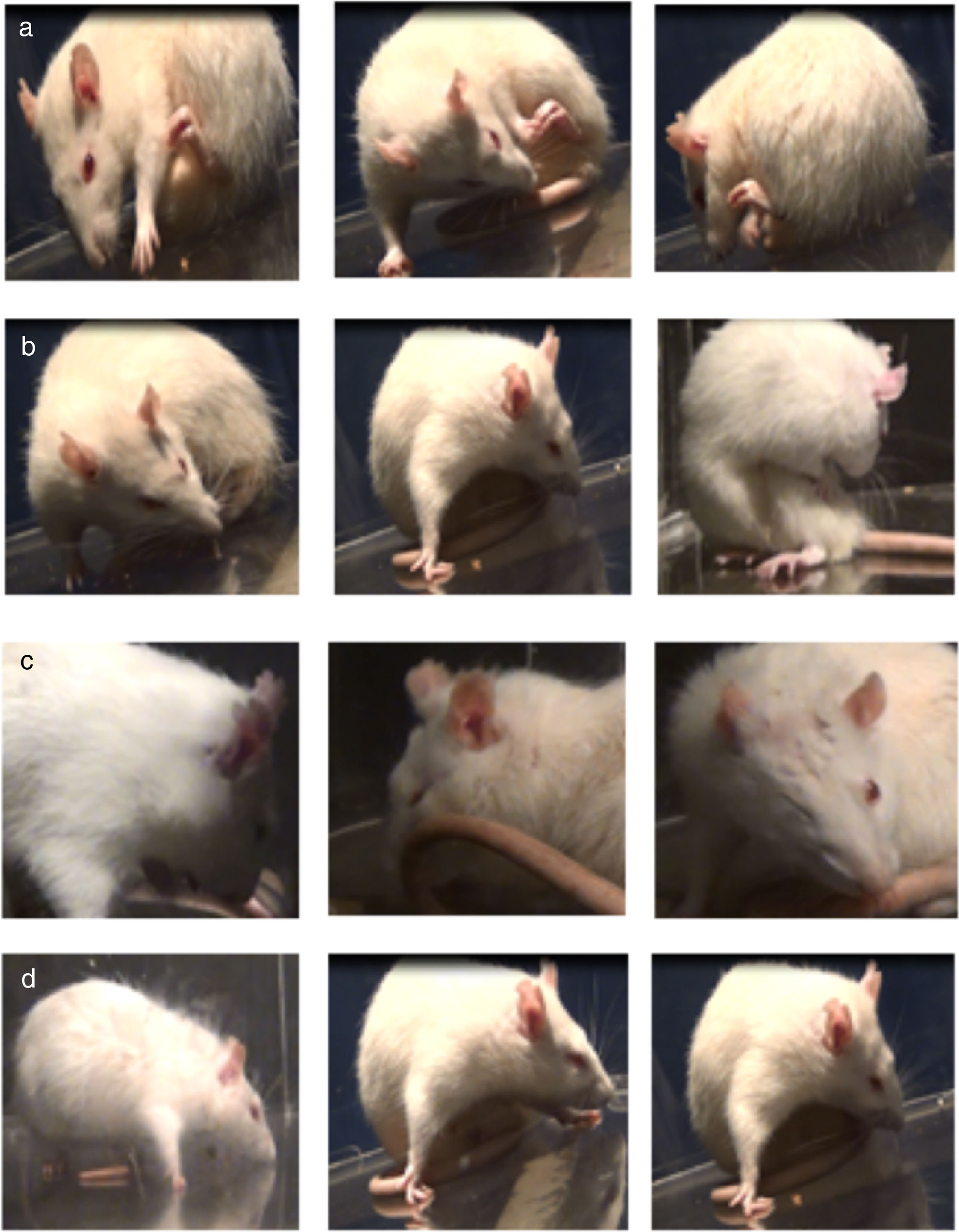
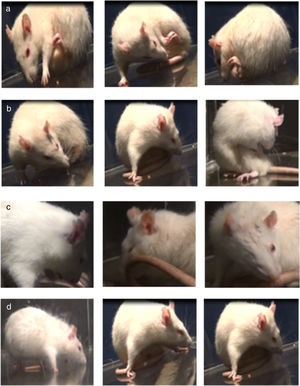
Assessment of abnormal involuntary movementsDyskinesia was evaluated with the scale proposed by Cenci et al.4 Rats were placed in a circular, transparent acrylic cage (50cm×30cm) and filmed for one hour. We evaluated the following AIMs: (1) orofacial dyskinesia: jaw movements on the vertical axis (opening and closing), with slight deviations to the side contralateral to the lesion, empty jaw movements, and tongue protrusion; (2) forelimb dyskinesia: jerking and choreic or ballistic movements in the forelimb contralateral to the lesion; (3) axial dyskinesia: lateral deviation of the head, neck, and trunk to the side contralateral to the lesion, resulting in a twisted posture of the trunk and limbs; and (4) locomotor dyskinesia: walking in circles towards the side contralateral to the lesion.7 Severity of each AIM subtype was rated 0 to 4 according to the following scale: 0, absent; 1, occasional (less than 50% of the time); 2, frequent (over 50% of the time); 3, continuous but interrupted by strong sensory stimuli; and 4, continuous and not interrupted by strong sensory stimuli.17–19
Preparation of brain tissue for immunofluorescenceAfter completing all behavioural tests, rats were anaesthetised with an overdose of pentobarbital (50mg/kg, intraperitoneal administration) and subsequently administered 4% paraformaldehyde in 0.9% saline solution by gravity-driven perfusion. Brains were dehydrated with sucrose and sliced into 50-μm sections at −0.96, −1.8, −3.72, and −4.68 from bregma13 using a microtome (KD-3358, KEDEE).
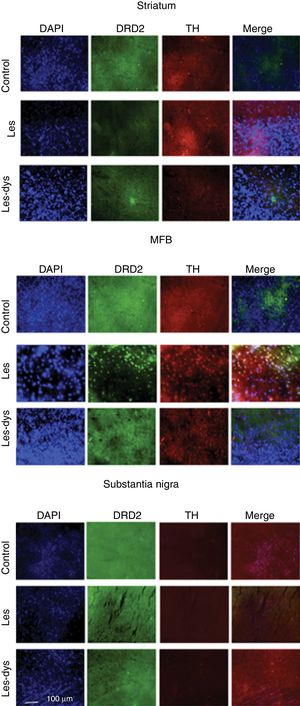
We analysed the striatum, medial forebrain bundle (MFB), and SNpc to detect differences in immunoreactivity between groups. Brain tissue was analysed by immunofluorescence microscopy. Four slices from the same animal were placed on each plate, corresponding to the 4 regions analysed. We analysed 8 slices from each rat. Sections were incubated with anti-tyrosine hydroxylase (1:500; Sigma-Aldrich) and anti-DRD2 primary antibodies (1:800; Abcam), and subsequently with Alexa Fluor 488 anti-rabbit polyclonal (1:500; Invitrogen) and Alexa Fluor 594 anti-mouse secondary antibodies (1:500; Invitrogen), respectively. Sections were incubated with DAPI (1:2000; Invitrogen); one drop of Fluoromount® was applied to the plates before viewing with a FLoid Cell Imaging Station. Cell counts were performed using the Fiji-ImageJ software (version 2017).
Statistical analysisData normality and homogeneity of variances were tested with the Kolmogorov-Smirnov and Bartlett tests, respectively. We compared spinning behaviour, density of neurons expressing tyrosine hydroxylase (TH) and DRD2, and pole test results between groups using ANOVA and the Tukey post hoc test. AIMs were analysed with the non-parametric Kruskal-Wallis test. Correlations between the location of AIMs and DRD2 expression were analysed using linear correlation and the Pearson correlation coefficient. Statistical significance was set at P<.05; data were analysed with the InfoStat statistical software (2015 version). Data are presented as means±SEM.
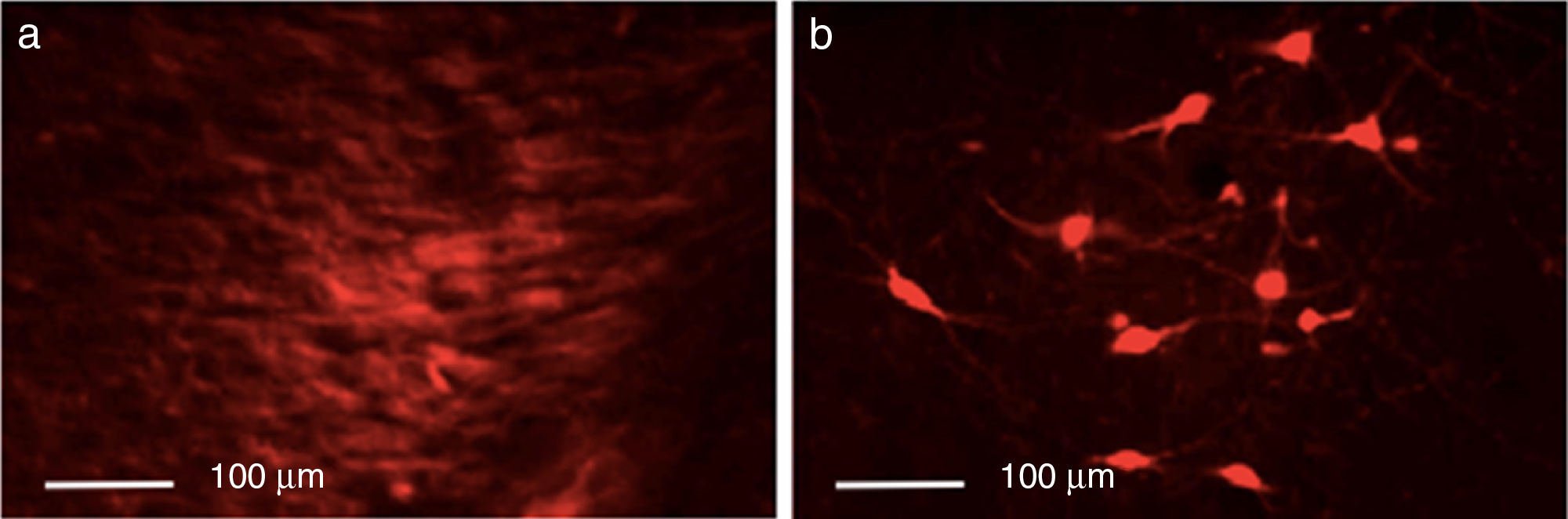
ResultsEfficacy of 6-OHDA-induced lesionsInjection of 6-OHDA into the SNpc resulted in a dramatic loss of dopaminergic neurons in the lesioned hemisphere, as shown by TH immunoreactivity. Control rats showed greater numbers of TH+ cells in all areas analysed than animals from the other 2 groups (P<.05). Differences were especially marked in the SNpc, with controls displaying a mean of 133.25 TH+ cells vs. 29.25 in the lesion group and 23.25 in the dyskinesia group (Fig. 1).
Behavioural testsApomorphine-induced spinning behaviourANOVA showed a spin rate of over 7 spins/min in all rats with 6-OHDA-induced lesions (F=7.69; P<.05), although the post hoc analysis revealed significant differences between the lesion group and the dyskinesia group (7.57 vs. 16.46spins/min; P<.05).
Neurological examination and pole testSensorimotor alterations were more marked on the side contralateral to the lesion, and in the rear limbs. Dyskinetic rats showed more marked alterations in the limbs contralateral to the lesion. In the pole test, rats in both the lesion and the dyskinesia groups took significantly longer to walk down the pole than control rats (P<.05); the dyskinesia group also showed great variability between individuals. Likewise, dyskinetic rats made a greater number of errors (F=14.33; P<.05) due to not using their tails or rear limbs, or using them incorrectly.
Assessment of abnormal involuntary movementsLesioned rats receiving levodopa gradually developed AIMs secondary to long-term treatment. All dyskinetic rats presented all 4 types of AIMs, although severity varied considerably. Fig. 2 shows the AIMs affecting different topographical locations.
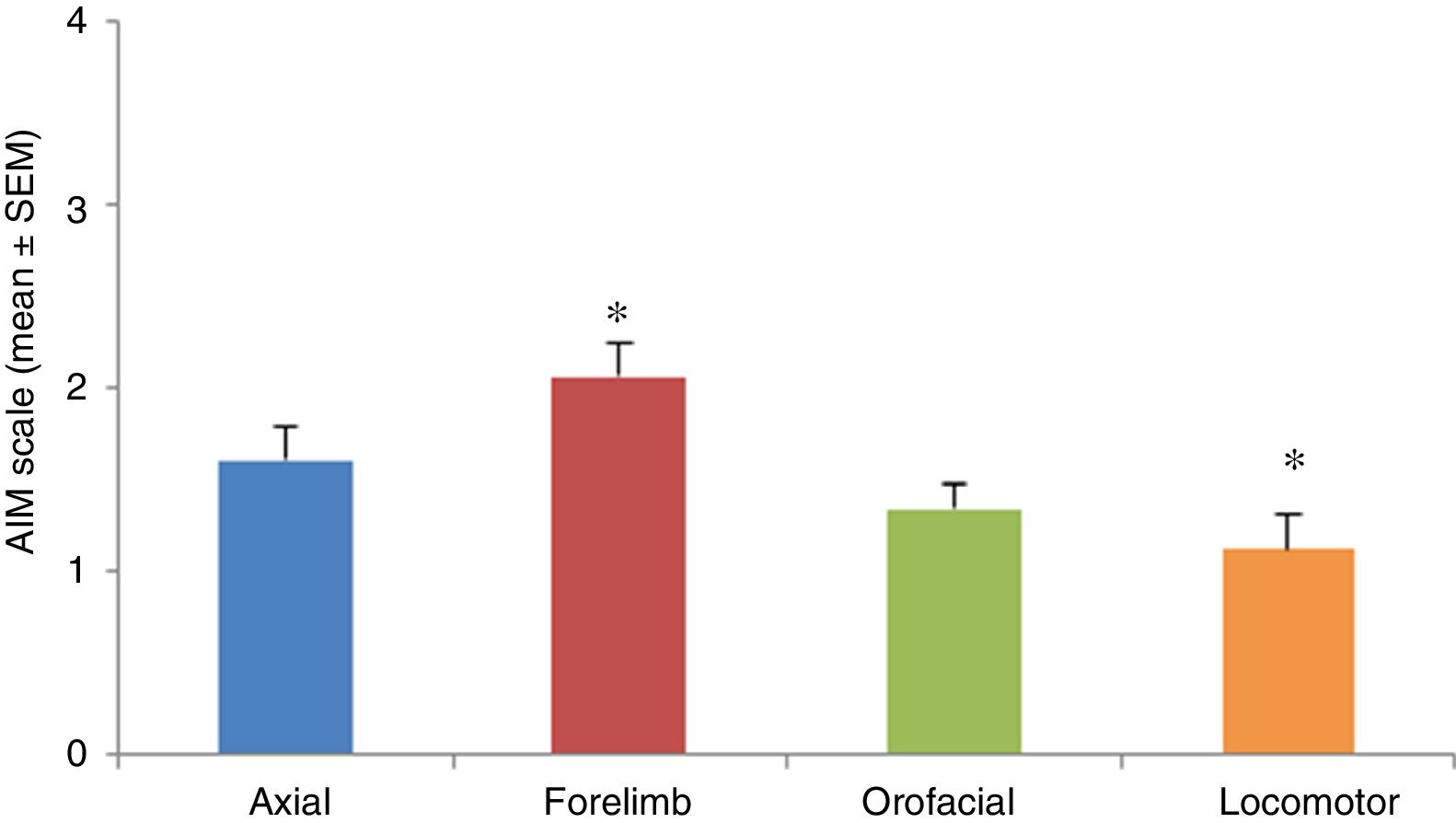
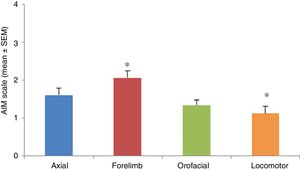
AIMs were most pronounced at 20 to 60minutes after injection of levodopa. All AIMs were significantly noticeable from day 5, stabilising around day 11. Once AIMs appeared, severity remained stable until the time of the last injection. The Kruskal-Wallis test only detected significant differences in forelimb and locomotor dyskinesia (H=23.82; P=.0001). Severity was rated a mean of 1.12 for locomotor AIMs and 2.06 for forelimb AIMs. Orofacial and axial AIMs were also frequent (mean severity: 1.43 and 1.60, respectively), although no significant differences were observed with the other types of AIMs (Fig. 3).
Immunofluorescence studyTyrosine hydroxylase expressionControl rats showed greater numbers of TH+ cells in all regions (P<.05). TH expression in the striatum varied between groups (P<.05; F=39.18); it was most marked in the control group, with a mean of 55.25 TH+ cells, compared to 7.75 in the lesion group and 24.00 in the dyskinesia group. Controls displayed significant differences in TH expression in the MFB and SNpc compared to the lesion and dyskinesia groups (P<.05; F=71.82 and F=143.11, respectively). In the MFB, controls showed a mean of 62.50 TH+ cells, vs. 11.75 in lesioned rats and 20.00 in dyskinetic rats. The highest numbers of TH+ cells were found in the SNpc in all groups: 133.25 in the control group, 23.25 in the lesion group, and 29.25 in the dyskinesia group (Fig. 4).
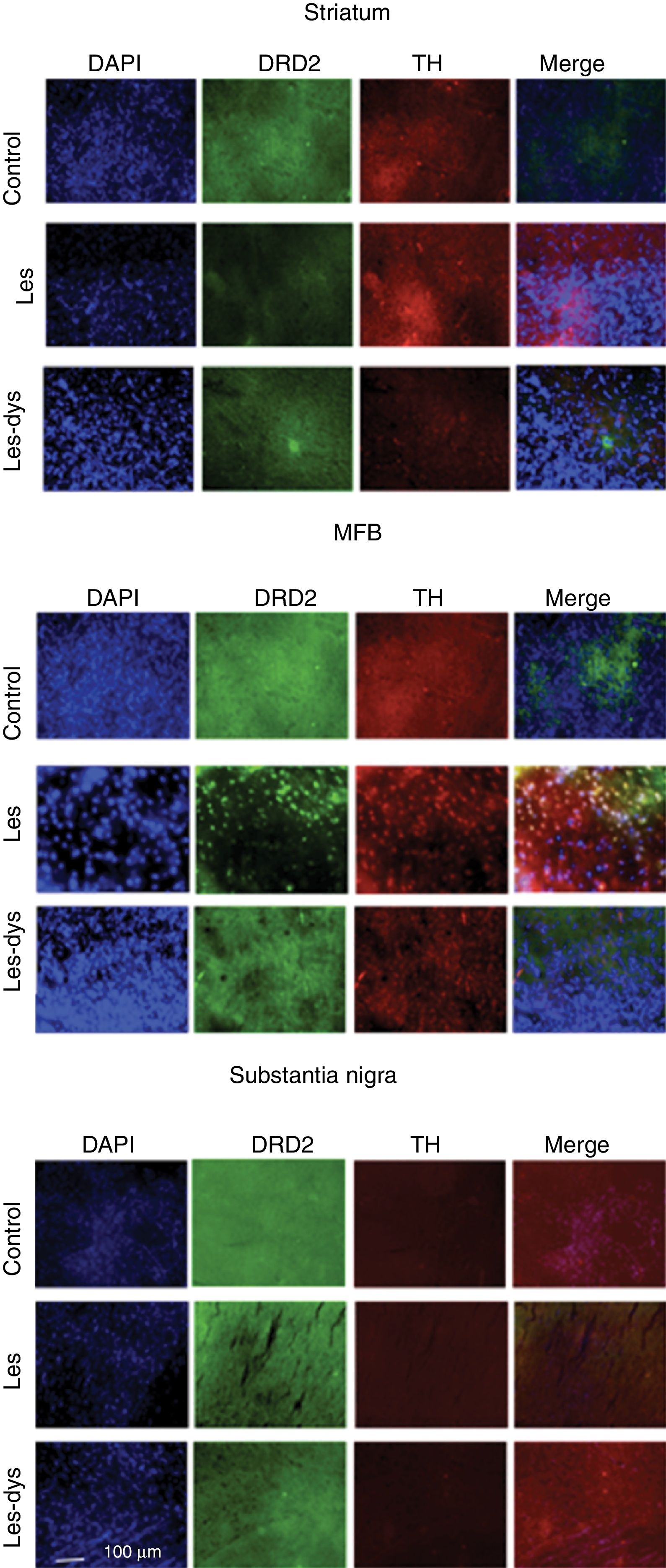
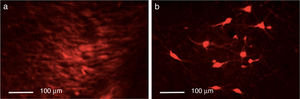
Triple immunofluorescence staining with DAPI, tyrosine hydroxylase (TH), and DRD2 in the striatum (a), medial forebrain bundle (b), and substantia nigra (c) in the groups receiving treatment. Greater cell density is observed in the striatum and medial forebrain bundle. White arrows indicate reactivity to all 3 stains (DAPI+, TH+, D2+). Scale bar: 1:100μm.
DRD2 expression was different in the striatum and MFB in all groups. The Tukey post hoc test revealed significant differences between the 3 groups. In the striatum, controls showed fewer DRD2+ cells (mean of 110) than lesioned rats (139); dyskinetic rats presented the highest number of DRD2+ cells (241). In the MFB, the mean number of DRD2+ cells in the control, lesion, and dyskinesia groups was 269, 116, and 128, respectively; only the dyskinesia group showed significant differences with the other groups. DRD2+ cell density was lower in the SNpc than in the remaining areas; the control group showed the most marked immunoreactivity to DRD2 (48.5 DRD2+ cells), followed by the dyskinetic group (34.5) and the lesion group (21.5). However, differences between groups were not significant (P>.05) (Fig. 4).
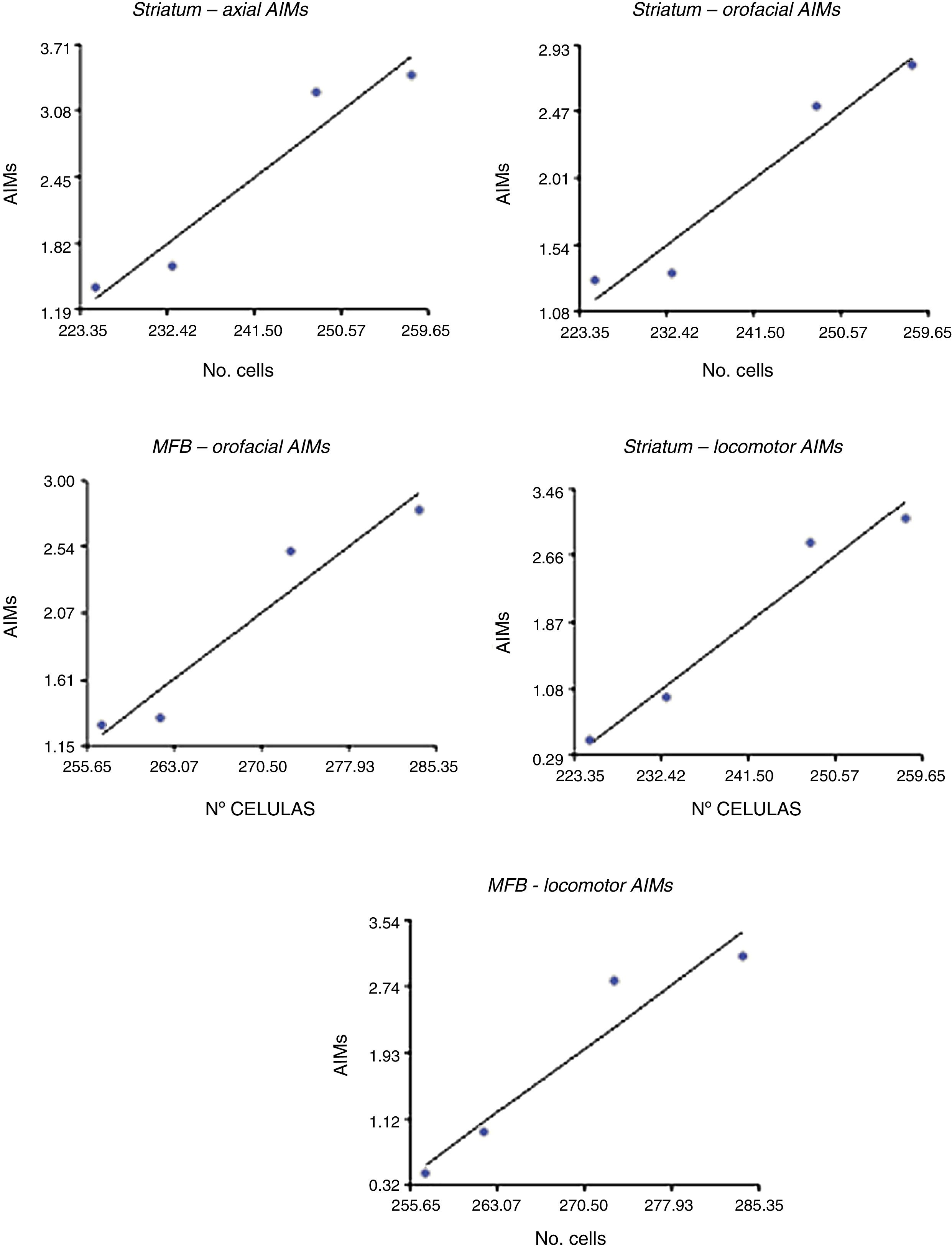
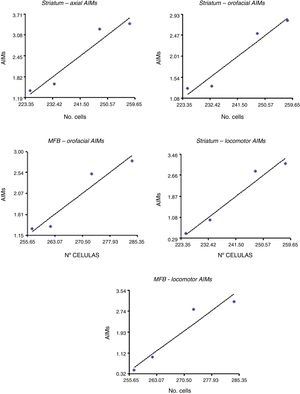
Correlation between dopamine receptor D2 expression and presence of abnormal involuntary movementsDRD2 expression was correlated with AIMs in the striatum and MFB (P<.05), but not in the SNpc (Fig. 5). The correlation was stronger in the case of orofacial (R2=0.95, F=34.42 in the striatum and R2=0.93, F=25.10 in the MFB) and locomotor AIMs (R2=0.96, F=49.04 in the striatum and R2=0.92, F=22.36 in the MFB), whereas axial AIMs were correlated with DRD2 expression in the striatum only (R2=0.93, F=27.70). Forelimb AIMs were not correlated with DRD2 expression in any of the areas studied (P>.05, F=16.83).
DiscussionPD is a neurodegenerative disease characterised by degeneration of dopaminergic neurons in the SNpc, which results in motor impairment. Levodopa, the most effective treatment available to date, significantly improves symptoms. However, approximately 40% of patients develop motor fluctuations, known as LID, after 5 years of treatment.20 Levodopa's role in the pathogenesis of dyskinesia is poorly understood, which underscores the need for experimental models that can accurately reproduce the main features of PD and LID.21 To this end, we created a rat model using 6-OHDA and long-term treatment with levodopa. Dopaminergic lesions were verified by assessing apomorphine-induced spinning behaviour. Apomorphine is a better predictor of 6-OHDA-induced lesions; previous studies have shown that dopaminergic neuron loss of at least 90% is necessary for animals to display spinning behaviour with apomorphine. Apomorphine has 100% capacity to predict dopaminergic neuron depletion of over 90%.14,22 Dyskinetic rats showed a higher number of spins than lesioned rats; this may be due to levodopa-induced changes in the intensity of the motor response, which is known to increase with long-term levodopa treatment. This phenomenon is known as sensitisation,7 and involves motor changes in response to exposure to exogenous dopamine, given that it induces a non-physiological state.23
Quantifying spinning behaviour is not sufficient to measure the level of motor impairment in lesioned animals.24 Neurological examination and pole test results revealed poorer motor skills in the lesion and dyskinesia groups than in controls. Errors involved poor integration of the movements needed to complete the task, including head position, gaze direction, body symmetry, and limb and tail use.16 These activities depend on motor association areas.2 This impairment in test performance was more marked in the limbs contralateral to the lesion and in the rear limbs; levodopa administration may have led to dopamine receptor supersensitivity in those areas.25 We may conclude that both the lesions and levodopa treatment reduce the rats’ ability to establish a plan for motor execution requiring high levels of planning and control.26
In addition to sensorimotor alterations, AIMs constitute one of the main adverse reactions to levodopa.27 Our findings show that prolonged levodopa treatment gradually induces movement alterations affecting the head, trunk, and limbs. In our animal model, dyskinesia manifested as abnormal movements appearing at the time of maximum treatment effectiveness (peak dose)28; these appeared in all rats receiving treatment, despite variable severity and duration.
It therefore seems clear that long-term treatment with levodopa causes a series of functional changes in the form of AIMs. However, the mechanism underlying LID is yet to be understood. Researchers largely agree that dyskinesia is associated with abnormal stimulation of chronically denervated dopamine receptors.29 Dyskinesia had traditionally been associated with DRD1; following PD-associated dopamine loss, DRD1 is the first type of dopamine receptor affected, whereas DRD2 is affected at later stages, resulting in difficulty executing automatised movements.30 Although little is known about DRD2 function, there is evidence that it is involved in movement alterations. According to Fabbrini et al.,10 DRD2 agonists cause fewer dyskinesias since DRD2 helps maintain adequate dopaminergic tone. However, as degeneration progresses, the numbers of residual dopaminergic terminals are insufficient to maintain adequate dopamine levels. Dyskinesia may therefore be caused by impaired DRD2 activation, as previous studies have suggested.31
Gay32 observed greater locomotor activity in DRD2 knock-out mice than in controls. DRD2 knock-out mice release greater quantities of dopamine due to alterations in neurotransmitter synthesis.33 Our animal model probably presents massive destruction of DRD2 following stereotactic injection of 6-OHDA into the SNpc, where DRD2 is widely expressed at the presynaptic level; DRD2 density was significantly greater in controls than in lesioned and dyskinetic rats. Massive destruction of DRD2 receptors in the SNpc probably leads to increased striatal dopamine release by the surviving neurons; dopamine levels would increase with exposure to exogenous dopamine, such as levodopa, contributing to the appearance of AIMs in animals receiving the treatment. This suggests that DRD2 stimulation may be responsible for decreasing locomotor activity, probably due to reduced firing of dopaminergic neurons and dopamine release. Another hypothesis suggests that DRD2 modulates dopamine synthesis by acting on TH.34 In view of the above, current research is focused on developing a treatment for PD that modulates dopaminergic transmission by indirectly acting on DRD2.35
Our study evaluated DRD2 expression in projection areas of the nigrostriatal pathway, revealing a marked increase in DRD2 density in the striatum and MFB in lesioned and dyskinetic rats. DRD2 is markedly expressed in those regions.36 However, increased DRD2 density may be explained by sensitisation to dopamine secondary to nigrostriatal pathway degeneration; afferent striatal neurons are sensitised due to lack of dopamine, and increase the externalisation of dopamine receptors. Therefore, AIMs increase with higher DRD2 concentrations. This is explained by the greater amount of receptors in the striatum and MFB in the dyskinesia group as compared to the other 2 groups; differences were statistically significant in the striatum only.
Our findings partially coincide with those reported by Pavón et al.7 in that DRD2 expression seems to be involved in orofacial AIMs. According to these researchers, DRD2 knock-out mice present orofacial AIMs but no locomotor, axial, or forelimb AIMs. DRD2 probably allows normal mobility of the mouth region, although our results showed that locomotor AIMs also were positively correlated with DRD2 expression.
Our results provide a deeper insight into the role of DRD2 in the pathogenesis of dyskinesia; further research into the topic is needed.
ConclusionsDyskinesia can be replicated in rats with hemiparkinsonism through continuous levodopa treatment, as occurs in humans. This animal model is useful for the study of the pathophysiological mechanisms underlying motor alterations. Animals receiving levodopa developed behavioural sensitisation, displaying locomotor alterations on the side contralateral to the lesion following apomorphine administration.
Long-term treatment with levodopa alters DRD2 expression, which was found to be more strongly correlated with orofacial and locomotor AIMs. This suggests that DRD2 plays a pivotal role in the development of these AIMs. We may conclude that DRD2 is involved in the pathogenesis of dyskinesia; the study of dopamine receptors is therefore essential to gain a deeper understanding of a wide range of functional alterations affecting the basal ganglia and to explore new therapeutic targets.
FundingThis study was funded by the research and scientific development office at Universidad del Tolima (project no. 250113).
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to Universidad del Tolima's research and scientific development office and to the Experimental Models for Zoo-Human Sciences Study Group.
Please cite this article as: Caro Aponte PA, Otálora CA, Guzmán JC, Turner LF, Alcázar JP, Mayorga EL. Correlación entre la expresión del receptor dopaminérgico D2 y presencia de movimientos involuntarios anormales (MIA) en un modelo de disquinesia en ratas Wistar hemiparkinsonizadas. Neurología. 2021;36:191–200.