Since its description five decades ago, the pathophysiology of idiopathic chronic adult hydrocephalus (iCAH) has been traditionally related to the effect that ventricular dilatation exerts on the structures surrounding the ventricular system. However, altered cerebral blood flow, especially a reduction in the CSF turnover rate, are starting to be considered the main pathophysiological elements of this disease.
DevelopmentCompression of the pyramidal tract, the frontostriatal and frontoreticular circuits, and the paraventricular fibres of the superior longitudinal fasciculus have all been reported in iCAH. At the level of the corpus callosum, gliosis replaces a number of commissural tracts. Cerebral blood flow is also altered, showing a periventricular watershed region limited by the subependymal arteries and the perforating branches of the major arteries of the anterior cerebral circulation. The CSF turnover rate is decreased by 75%, leading to the reduced clearance of neurotoxins and the interruption of neuroendocrine and paracrine signalling in the CSF.
ConclusionsiCAH presents as a complex nosological entity, in which the effects of subcortical microangiopathy and reduced CSF turnover play a key role. According to its pathophysiology, it is simpler to think of iCAH more as a neurodegenerative disease, such as Alzheimer disease or Binswanger disease than as the classical concept of hydrocephalus.
Desde la descripción hace 5 décadas de la hidrocefalia crónica del adulto idiopática (HCAi), su fisiopatología ha sido considerada básicamente relacionada con el efecto que la dilatación ventricular ejerce sobre las estructuras adyacentes al sistema ventricular. Sin embargo, las alteraciones en el flujo sanguíneo cerebral (FSC) y, sobre todo, la reducción en el recambio licuoral parecen emerger como componentes fisiopatológicos principales de esta enfermedad.
DesarrolloEn la HCAi se observa una compresión del tracto piramidal, de los circuitos cortico-subcorticales fronto-estriatales y fronto-reticulares, y de las fibras profundas del fascículo longitudinal superior. En el cuerpo calloso se objetiva un descenso en el número de fibras comisurales, que son reemplazadas por gliosis. El FSC se encuentra alterado, con un patrón de última pradera en la región subcortical adyacente a los ventrículos, correspondiente a la intersección entre las arterias subependimarias y las arterias perforantes dependientes de los grandes troncos arteriales de la circulación anterior. El recambio diario del LCR se ve disminuido en un 75%, lo que conlleva una reducción del aclaramiento de neurotóxicos y la interrupción de las señalizaciones neuroendocrinas y paracrinas que ocurren a través del LCR.
ConclusionesLa HCAi emerge como una entidad nosológica compleja, en la que los efectos de la microangiopatía subcortical y la disminución del recambio de LCR desempeñan un papel fundamental. Esta base fisiopatológica aleja la HCAi del concepto clásico de hidrocefalia y la acerca al perfil de otras enfermedades neurodegenerativas, como la enfermedad de Alzheimer o la enfermedad de Binswanger.
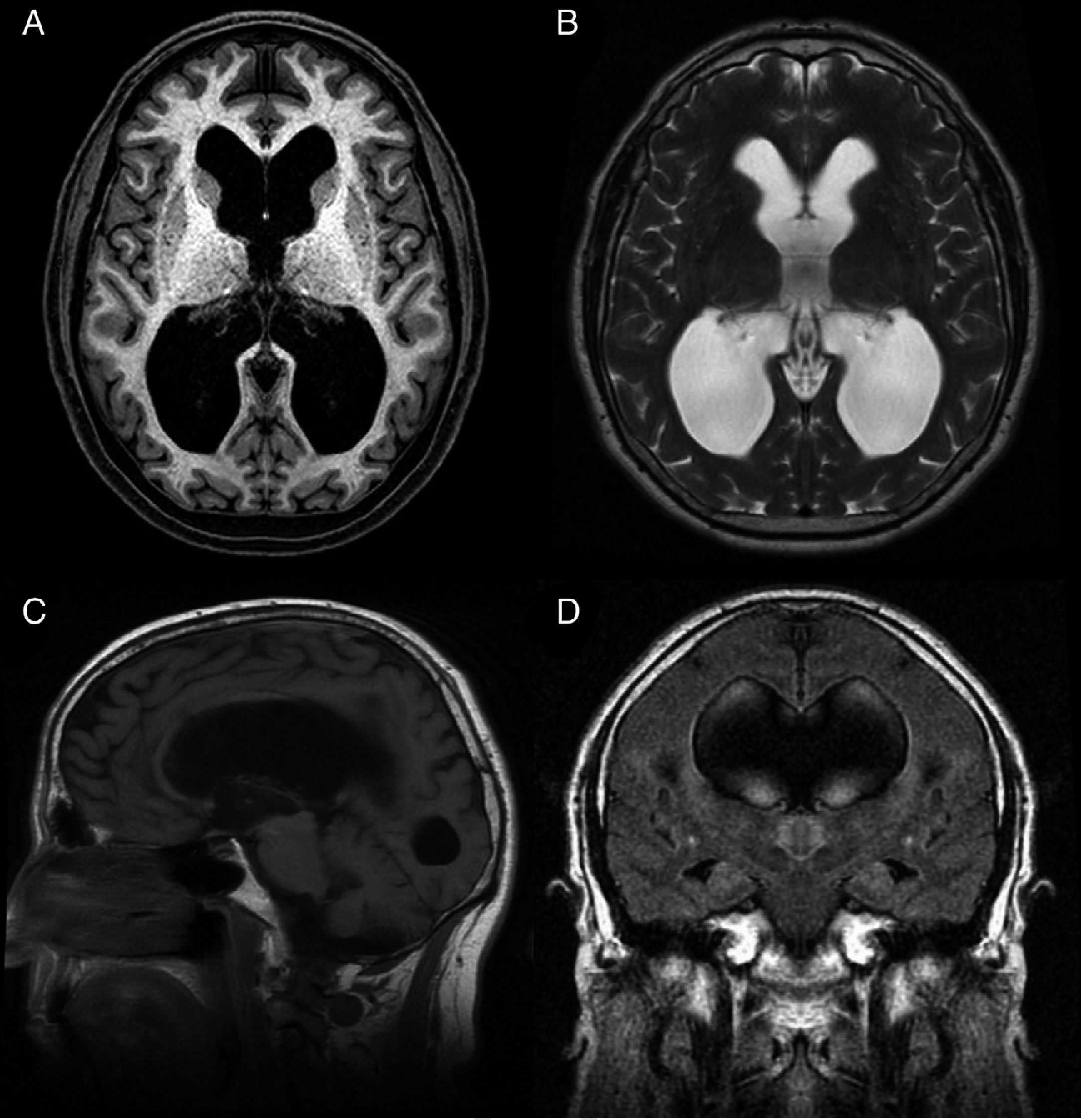
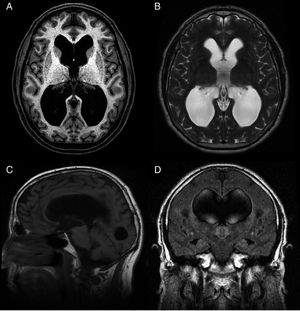
Idiopathic normal-pressure hydrocephalus (iNPH) is a nosological entity characterised by the clinical triad of gait disturbance, cognitive impairment, and urinary incontinence, with neuroimaging findings of ventricular dilatation (Fig. 1) and in the absence of any other cause that may explain clinical findings.
Preoperative MR image of a patient with idiopathic normal-pressure hydrocephalus who responded well to ventriculoperitoneal shunting. (A) Axial T1-weighted sequence. Moderate dilatation of the lateral ventricles and the third ventricle. (B) Axial T2-weighted sequence. CSF flow artefacts in the third ventricle; absence of hyperintensity at the periventricular and subcortical levels. (C) Sagittal T1-weighted sequence. Descent of the third ventricle floor, rounding of the third ventricle, and decreased mamillopontine distance. The image shows no obstruction in the aqueduct of Sylvius that may explain ventricular dilatation. (D) Coronal FLAIR sequence. Typical pattern of effacement of convexity sulci, especially in the midline. The CSF flow signal void on the T2-weighted sequence extends towards both Monro foramina, reaching the lower and middle portions of the ventricular cavities. Absence of periventricular and subcortical hyperintensities.
Although this clinical syndrome had previously been described in the literature1–5 (particular emphasis should be placed on the descriptions made by French neurologist Etienne Mouline6 in 1819 and German pathologist Friedrich Dörner7 in 1826), it was the late Colombian neurosurgeon Salomón Hakim Dow who provided a systematic description of the clinical and radiological features of iNPH in his doctoral thesis, written over 50 years ago.8 Hakim, together with 2 renowned neurologists from the Massachusetts General Hospital, Raymond D. Adams and Charles M. Fisher, disclosed his findings in 2 articles, which were published simultaneously in the New England Journal of Medicine9 and the Journal of Neurological Sciences.10
The classic triad of symptoms has traditionally been thought to be caused by the effect of ventricular dilatation on periventricular nerves11–17 and vessels.18–25 However, recent studies also suggest an inability of the CSF to remove waste products from the extracellular fluid as a causal factor for iNPH.26–29
We provide updated information on the pathophysiology of the disease, placing special emphasis on decreased CSF turnover, a novel factor which may have an impact on long-term prognosis. These findings challenge the classic concept of hydrocephalus, suggesting that iNPH is a neurodegenerative disease.
DevelopmentCompression of periventricular subcortical fibresCompression of the frontal projections descending close to the frontal horns of the lateral ventricles alters the function of the projections. These alterations may be completely reversible when dysfunction is caused by slowing or interruption of axonal transport at that level, or permanent in the case of demyelination or loss of axonal integrity.16,17 Different MRI techniques have increased our knowledge of the fascicles affected in patients with iNPH, including the cortical and subcortical projections of these fascicles, and help determine whether lesions are reversible. Diffusion-weighted imaging and diffusion tensor imaging (DTI) are the forms of MRI most frequently used to assess these patients. The former evaluates the presence of free interstitial water by calculating apparent diffusion coefficient (ADC) values (these values are higher in the extracellular oedema). However, axon regeneration by gliosis, which invariably occurs in the chronic phase of axonal damage, may cause an even greater increase in ADC values. DTI, including fibre tractography, is much more sensitive than ADCs for evaluating the integrity, density, and potential displacement of nerve fascicles, as it detects anisotropic changes in water molecules due to the unidirectional propagation of action potentials. Mean diffusivity (MD) may be analogous to ADC values, whereas fractional anisotropy (FA) decreases significantly when axonal disruption occurs (even in the areas where ADC maps show no significant increase of ADC coefficients or where these are normal due to the artefact generated by residual gliosis), and increases with axon density, for example as a consequence of axon compaction due to the effect of a force perpendicular to the axons’ trajectory. Consequently, an increase in ADC or MD values in the presence of normal or increased FA values suggests interstitial oedema, whereas a decrease in FA values is suggestive of axonal damage regardless of MD or ADC values.30
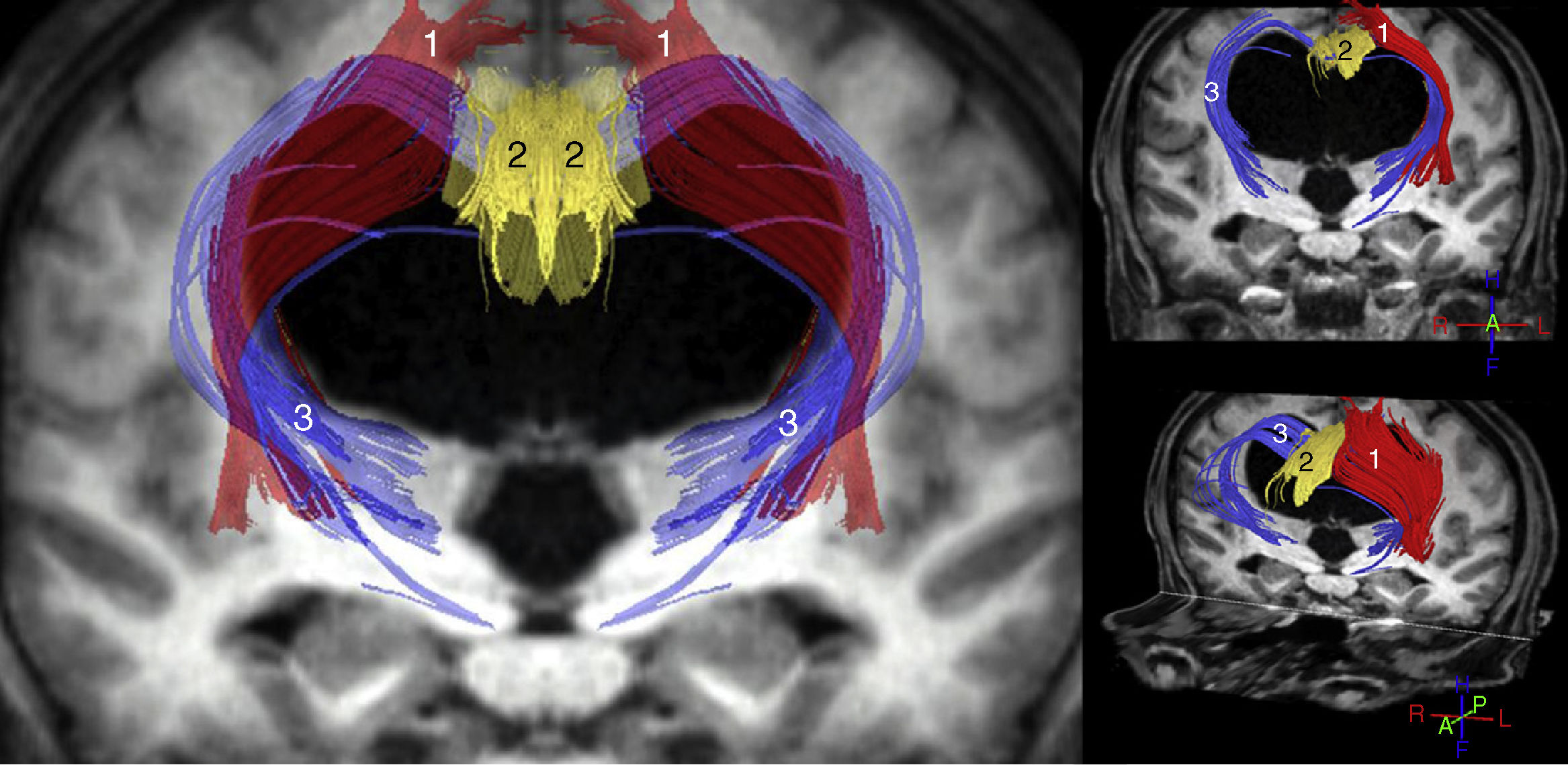
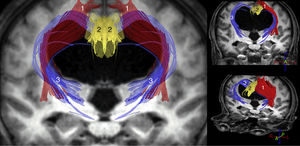
The most important studies conducted to date using these techniques are consistent in that increased ADC and/or MD values are observed both in the corpus callosum and in the internal capsule. FA, however, increases in the internal capsule, especially in its anterior limb, and decreases in the corpus callosum, especially at the level of the genu.11–14,31 Other findings reported in the literature include increases in FA in the caudate nucleus,11,31 increases in MD with no FA changes in the white matter associated with the precentral cortex,15 and increased axonal density in the corticospinal tract at the paraventricular level, as a consequence of compaction in the areas adjacent to the ventricle.32 In the light of these findings, we may conclude that iNPH involves compression of the pyramidal tract and the frontostriatal and frontoreticular cortico-subcortico-cortical circuits (Fig. 2), in addition to dysfunction of the deep fibres of the superior longitudinal fascicle. In the corpus callosum, decreased FA and increased ADC and/or MD values are suggestive of a reduced number of commissural fibres, which would have been replaced by gliosis.
Tractography reconstruction of DTI sequences of the patient shown in the previous figure. The image shows the distortion generated by ventricular dilatation along the pyramidal tract (1) and in the corticostriatal and corticoreticular connections (3). The corticostriatal and corticoreticular tracts are considerably thinner than normal; thinning is also observed in the cingulate fasciculus (2) and, to a lesser extent, in the pyramidal tract (1).
Multiple studies have shown reduced cerebral blood flow (CBF) in patients with iNPH, although global involvement is rather discreet, with CBF decreases ranging from 20% to 30%.18–25,33 A recent study conducted at Osaka University found patients with confirmed iNPH and those with ventricular dilatation displaying the radiological signs but no symptoms of iNPH to have lower CBF than do healthy controls.25 The literature reports conflicting results regarding the severity of clinical symptoms and its association with CBF; most studies do not show a direct relationship,18,22,25 whereas some do suggest a correlation between clinical severity and a progressive decrease in CBF.19,21,34
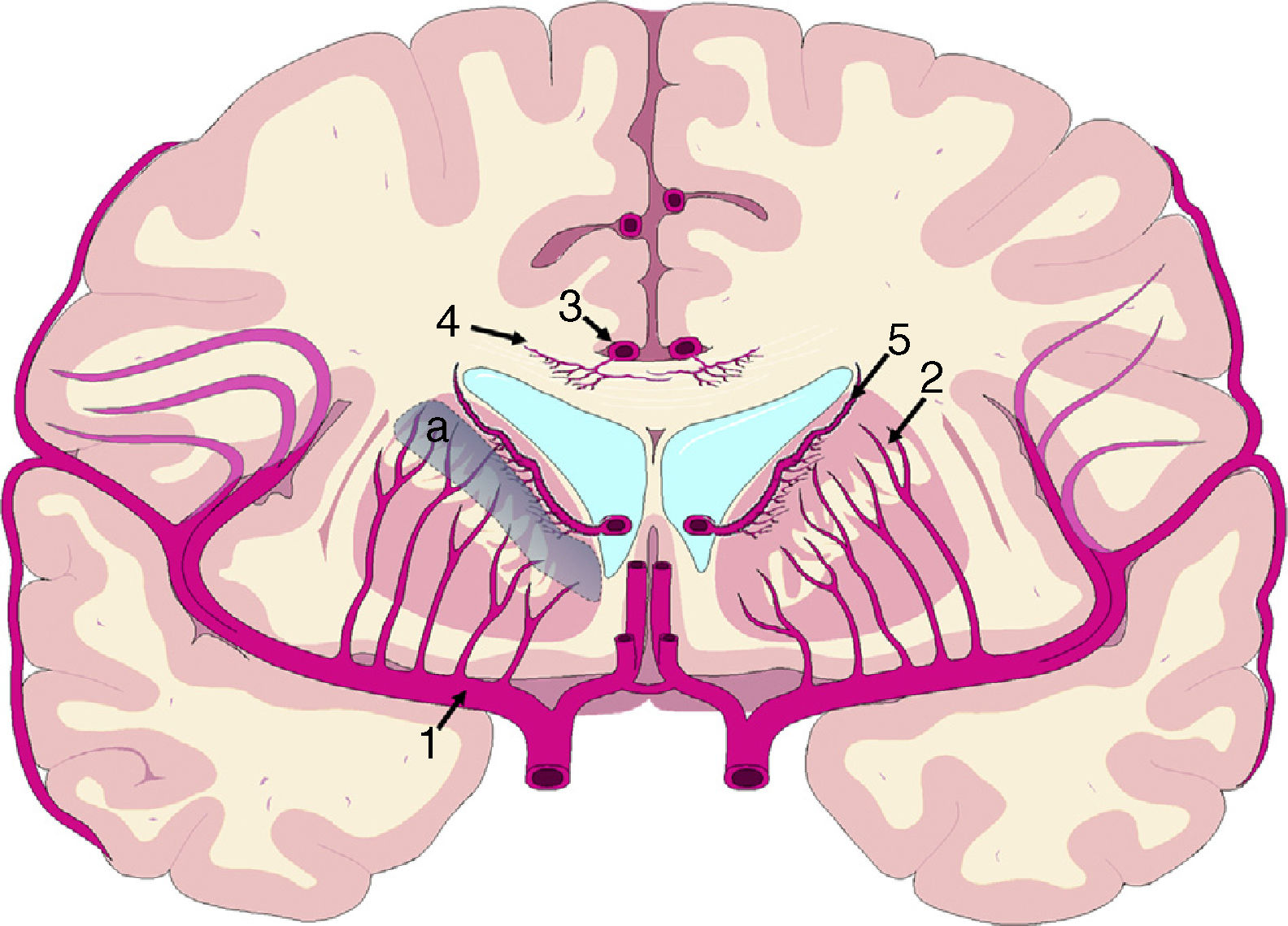
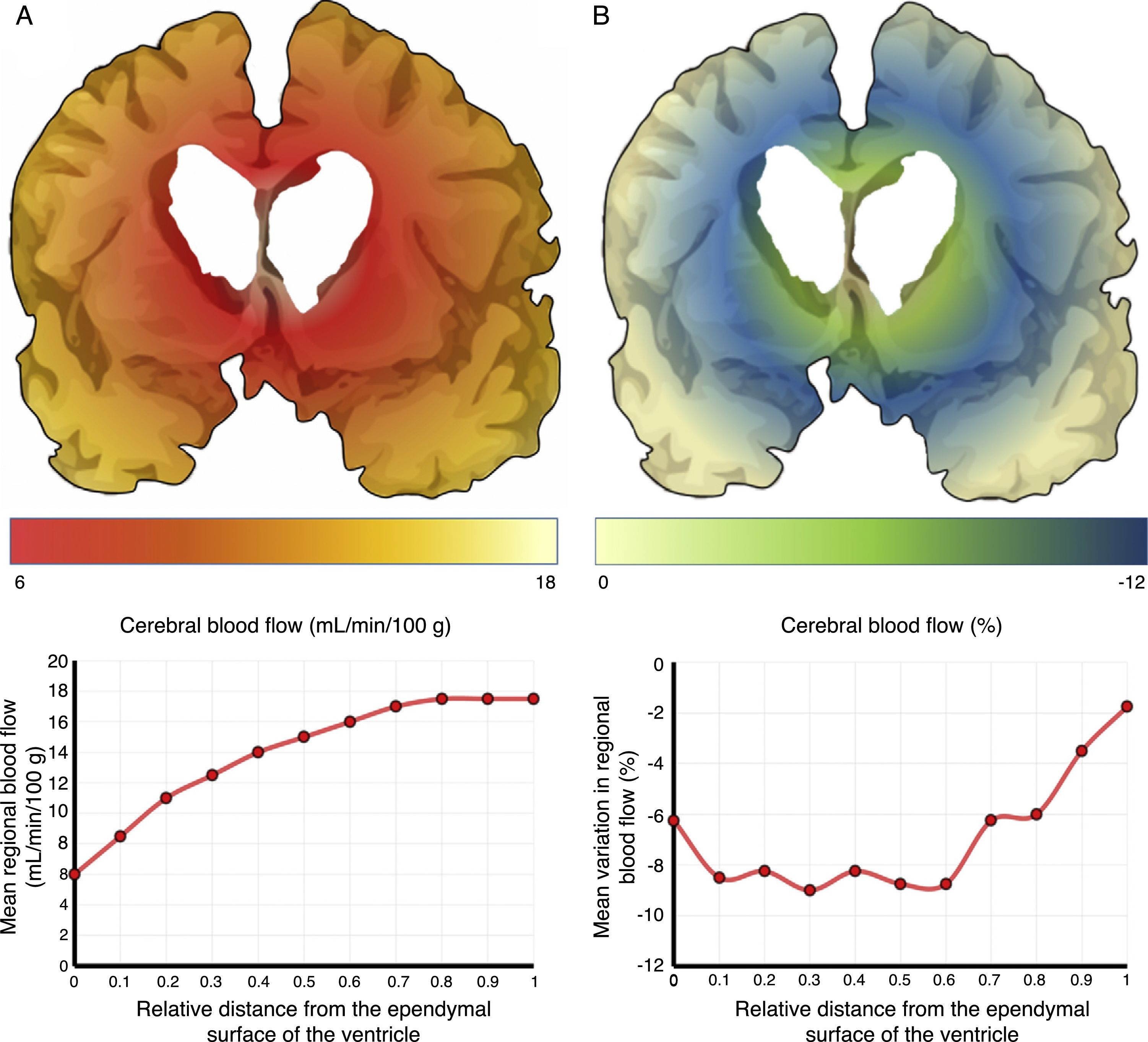
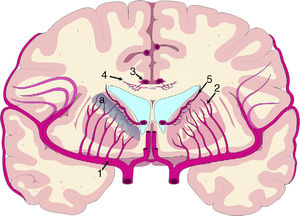
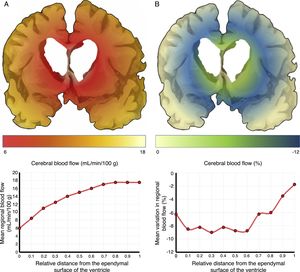
The mechanisms underlying CBF alterations are yet to be understood. If decreases in CBF were due only to the distortion caused by ventricular dilatation and the forces exerted on cerebral microcirculation, we may expect to observe a negative correlation between decreases in CBF and the distance to the ventricular system, with greater CBF decreases in the most internal and inferior portion of the centrum semiovale, in the thalamus, and in the head and tail of the caudate nucleus. Some recent studies have overcome the limitations of poor spatial resolution of traditional techniques for measuring CBF by co-registering H215O-PET images onto MRI images, demonstrating a CBF gradient from the periventricular region to the cortex; the relationship between changes in CBF and distance is not proportional, however.18,19 The study by Momjian et al.18 is particularly interesting: the researchers located the area of maximal CBF decrease in the subcortical white matter, 1cm from the ventricular wall, observing a 50% decrease in the cerebrovascular reserve (CVR) in that location (Fig. 3). These findings are compatible with the concept of last meadow in the subcortical region adjacent to the ventricles. This phenomenon has a coherent microanatomical basis, since the tissue closest to the ependyma is irrigated by subependymal arteries and the tissue farthest from the ependyma by perforating branches of the major arteries of the anterior circulation (Fig. 4).35,36 The area of greatest sensitivity to ischaemia is the area where both vascular territories intersect and where CBF and CVR alterations are most marked. In any case, basal ganglia alterations seem to be a constant in most clinical studies of iNPH.19,34,37
Vascularisation of periventricular structures. The basal ganglia and internal capsule are located in a last meadow area (a) between the territory of the perforating branches (2) of the middle cerebral artery (1) and the branches of the subependymal arteries (5). The corpus callosum is irrigated mainly by the short callosal arteries (4) from the pericallosal artery (3), which belong to the terminal circulation.
Cerebral blood flow (CBF) in the periventricular region in patients with idiopathic normal-pressure hydrocephalus. (A) The relationship between CBF and distance to the ependymal surface of the ventricle follows a logarithmic curve.18 (B) When patients experience fluid overload, CBF drops in the subependymal last meadow area, located 5-15mm from the ventricular surface.
Other studies have also detected cortical alterations which may not be explained by a merely mechanical phenomenon, given the relative distance from the ventricular system. Hypoperfusion is more marked in the anterior and inferior mesial regions of the frontal lobe than in other structures.22,34 Several studies have also described alterations in such areas as the left anterior temporal cortex,22 the hippocampus and parahippocampus,37 the frontal lobe white matter corresponding to the superior longitudinal fasciculus,34 and parietal association areas.34
Cerebrovascular involvement associated with iNPH is considerable, also affecting cerebral vasoreactivity, which is reduced as a result of exhaustion of the CVR. To evaluate the CVR, Chang et al.21 studied CBF in 167 patients diagnosed with iNPH using 99mTc-HMPAO SPECT before and after administering 1g acetazolamide. The researchers found that the CBF increased by 50%-80% less in patients with iNPH than in healthy individuals after the infusion of acetazolamide; this may reflect decreased vasoreactivity in response to increased partial pressure of CO2 in arterial blood or a significant decrease in the CVR. Fortunately, these changes are not accompanied by alterations in the metabolic coupling of the areas involved, since the cerebral metabolic rate decreases in line with decreases in CBF, and the oxygen extraction fraction remains within normal limits.38,39
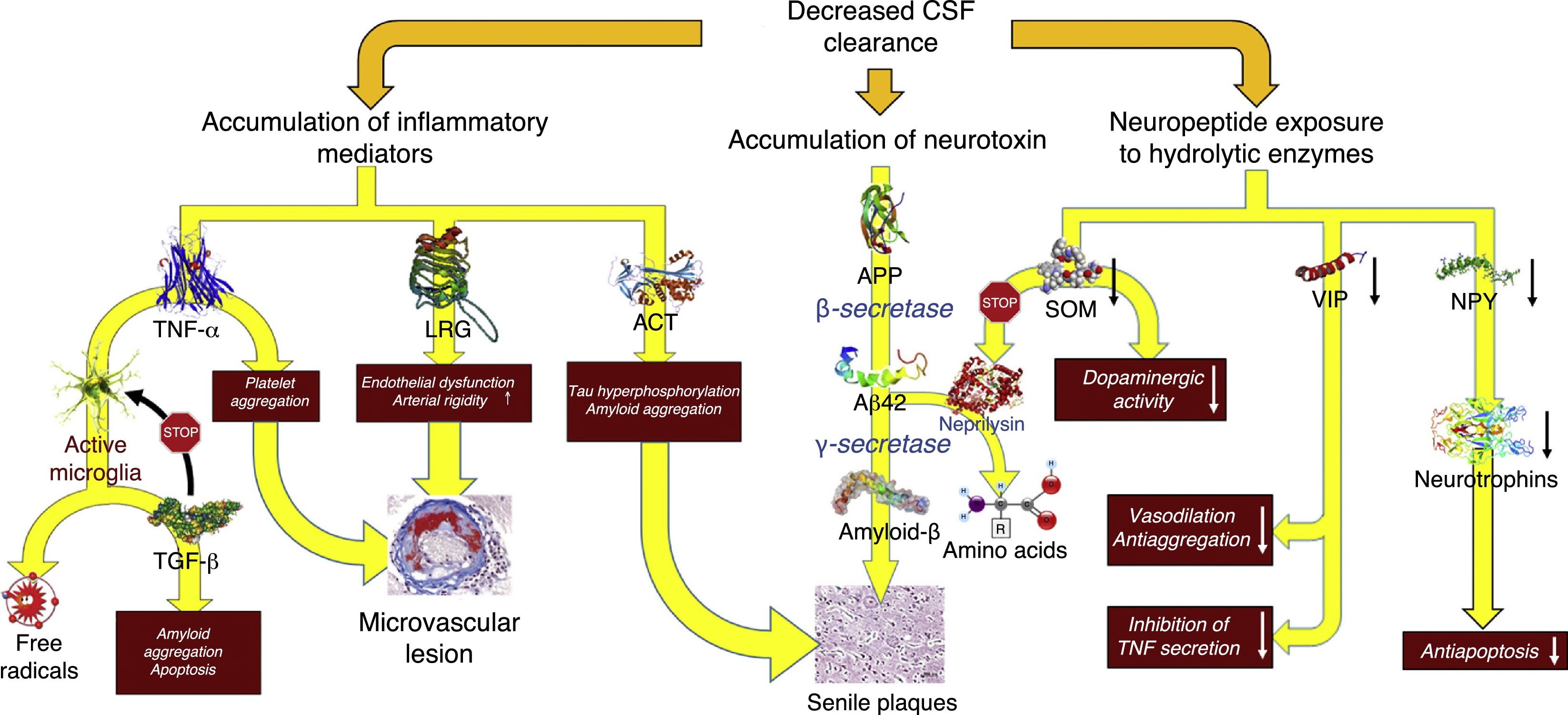
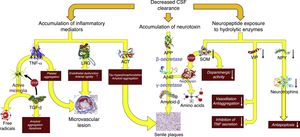
Decreased CSF turnoverAs occurs with the lymph in the rest of the body, CSF excretes the macromolecules present in the interstitial fluid that cannot be reabsorbed by venous capillaries. Under normal circumstances, CSF contains over 2000 different proteins, which amount to less than 4‰ of its weight.40 Reabsorption of CSF water may be unrelated to macromolecule excretion: the former takes place in the venous ends of the capillaries, whereas the latter occurs in arachnoid granulations or in extracranial lymphatic vessels. Alterations in macromolecule excretion may persist even in the case of volumetric balance in the production and absorption of CSF water (Fig. 5).
Pathophysiological consequences of decreased CSF clearance: accumulation of neurotoxins and inflammatory mediators, and exposure of the neuropeptides responsible for neuroendocrine signalling to the activity of hydrolytic enzymes.
Aβ-42: 42-residue fragment of amyloid-β peptide; ACT: antichymotrypsin; APP: amyloid precursor protein; LRG: leucine-rich glycoprotein; NPY: neuropeptide Y; TGF: transforming growth factor; TNF: tumour necrosis factor; VIP: vasoactive intestinal peptide.
In patients with iNPH, the CSF production rate may drop to 0.25±0.08mL/min, which represents a decrease of over 30%.41 Furthermore, increased ventricle size results in a 30% increase in distribution volume (an increase of over 200mL in most patients). This, combined with impaired CSF reabsorption, results in a 75% decrease in the CSF turnover rate. Adaptive changes in the choroid plexus and the vascular feet of the astrocytes prevent CSF production-absorption imbalances. Aquaporin-1 expression decreases in the choroid plexus42–44 as a result of the activation of the natriuretic peptide system of the circumventricular organs and hypothalamic nuclei45,46; this results in decreased water transport in the apical membrane of the choroid epithelium and, consequently, reduced CSF production. On the other hand, aquaporin-4 expression increases in white matter astrocytes42,43,47–49 probably in order to increase CSF water reabsorption into the venous capillaries.42,50 However, increased absorption into venous capillaries may reduce periventricular interstitial fluid pressure, promoting the appearance of a pressure gradient between the interstitium and the ventricle and maintaining ventricular dilatation.51,52 There is insufficient evidence for conclusions to be drawn on how this situation affects protein clearance in patients with iNPH, although it may be hypothesised that CSF turnover involves at least 2 processes: the reduction of neurotoxin clearance and the interruption of neuroendocrine and paracrine signalling in the CSF.53
Although there is no direct evidence of decreased neurotoxin clearance in patients with iNPH, this may be observed indirectly in the results from biomarker studies and CSF proteomic analysis. Most studies report decreased concentration of the majority of metabolites of the amyloid proteolytic processing pathway, including amyloid precursor protein (APP) and amyloid-β–42 peptide (Aβ-42).54–58 Total and phosphorylated tau protein levels remain within normal ranges or are lower than normal.54,57,59 Decreased CSF turnover results in deficient APP clearance from the interstitial space: APP would therefore be processed by β-secretase and then by γ-secretase, resulting in Aβ-42 aggregation into amyloid plaques, and decreasing the concentration of these 2 components in the CSF.26–29 A study by Fagan et al.,60 including patients without dementia, provides evidence in support of this hypothesis. The researchers found that patients with cerebral amyloid deposition in 11C-PiB PET images had lower CSF Aβ-42 levels. Pyykö et al.61 confirmed these results in patients with iNPH, observing a linear, inversely proportional relationship between APP levels in brain biopsy samples and Aβ-42 concentrations in both ventricular and lumbar intrathecal CSF. Moriya et al.62 and Jeppsson et al.58 report increased Aβ-42 levels in the CSF of patients with iNPH after shunt implantation; this increase was correlated with the patients’ neurological improvements. These findings suggest that normalisation of CSF flow dynamics after shunting promotes a shift from oligomeric to monomeric Aβ as a result of increased concentration of Aβ-38, an isomer with low aggregability.62
The inflammatory profile provides additional data: an excess of acute-phase reactants in the CSF (in the absence of abnormal cellularity according to biochemical analysis or of microglial activation in the periventricular region, which may point to an inflammatory process as the cause of these findings) suggests that decreased CSF clearance is responsible for the accumulation of astrocytic proinflammatory mediators. Several studies have reported increased levels of leucine-rich α-2-glycoprotein,63,64 α-1-antichymotrypsin,63,65 haptoglobin,63 transferrin,66 α-1-β glycoprotein,65 and tumour necrosis factor α.67 Other researchers have also observed an increase in free-radical peroxidation products, which may also be associated with decreased CSF turnover.68
Li et al.69 described an increase in TGF-β–dependent signalling. Although excessive TGF-β may be partially due to deficient TGF-β clearance, this mechanism does not explain TGF-β type II receptor upregulation, another finding reported by these researchers. These findings may be explained by the presence of an adaptive mechanism in response to the increasing levels of inflammatory mediators and acute-phase reactants, since this cytokine has a protective effect, blocking inflammatory response in glial and endothelial cells.70–72
The potential impact of abnormal accumulation of these proteins is evident:
- –
Reduced APP clearance promotes amyloid deposition in blood vessels and tissues, promoting the development of intercurrent neurodegenerative processes or accelerating their progression (e.g. Alzheimer disease).53
- –
The diffusion of proinflammatory proteins, especially TNF-α (which promotes aggregation and cell adhesion in capillaries), in the periventricular region may alter microvascular dynamics, compounding the mechanical effect of ventricular dilatation in the periventricular region.18
- –
Free radicals damage neurons, glial cells, and endothelial cells, and their effects at the molecular level have been associated with the pathophysiology of a wide range of neurological diseases; it is therefore very likely that they play a role in tissue damage in iNPH.68
- –
TGF-β induces neuronal and oligodendrocytic apoptosis.73,74 Delayed programmed cell death may explain the progressive clinical deterioration frequently seen in these patients despite initially successful shunting.
Alterations in neuroendocrine signalling in the CSF constitute another possible mechanism, despite the lack of objective evidence of the existence of this neuroendocrine signalling pathway. Over 100 neuropeptides are described in the literature as potentially using CSF circulation to reach distant regions of the CNS; these neuropeptides are similar in terms of their synthesis, release, and regulation, and their functions are radically different from those of conventional neurotransmitters.75
Patients with iNPH have been found to have low CSF levels of somatostatin (SOM),76–80 vasoactive intestinal peptide (VIP),59,79,81 neuropeptide Y (NPY),59,79,80,82 cholecystokinin,83 δ sleep-inducing peptide,79,84 and corticotropin-releasing hormone.80 The interpretation of these findings is not straightforward: they may result from a nearly global dysfunction of peptidergic neurotransmission secondary to iNPH, or may reflect the action of an active mechanism by which deficient CSF turnover would result in the exposure of peptides to the action of neuropeptidases, decreasing CSF peptide levels and interrupting neurotransmission in the CSF.85,86
SOM is probably the peptide that has been studied most extensively in this context. This peptide is diffusely located throughout the brain, although it is mainly produced in the preoptic, paraventricular, arcuate, and ventromedial nuclei of the hypothalamus. In addition to its involvement in regulating the endocrine system, acting as a growth hormone–releasing hormone antagonist, SOM promotes dopaminergic transmission in the striatum and contributes to normal neuronal function in ageing.87 The latter function is linked to the proteolytic activity of neprilysin; this protease is involved in Aβ-42 catabolism and its activity induces SOM expression.88 Reduced SOM expression secondary to impaired CSF turnover may promote Aβ-42 accumulation, worsening clinical symptoms. NPY is also diffusely distributed throughout the brain, including in such areas as the amygdala, hippocampus, basal ganglia, and of course the hypothalamus, where NPY colocalises with SOM-producing neurons in the paraventricular and arcuate nuclei.87 A recent article shows that NPY balances the toxic effects of Aβ, promoting neurotrophin synthesis in cells.89 Decreased NPY levels in patients with iNPH may therefore promote neuronal loss, especially due to the synergistic effect of SOM. VIP has a similar effect: in addition to its function in vasodilation and the synthesis of neurotrophin-3 and activity-dependent neurotrophic factor in glial cells, the peptide inhibits the inflammatory response in the neuroglia, mainly by blocking the production of TNF-α and free radicals in the microglia.90
ConclusionsCompression of the periventricular subcortical fibres is not the only pathophysiological mechanism in iNPH. The characteristic, long-term clinical progression of this type of hydrocephalus may also be explained by CBF alterations in the last meadow areas between the perforating branches of the major arteries of the anterior circulation and the subependymal arteries, and by reduced CSF turnover, which leads to reduced neurotoxin clearance and altered neuroendocrine signalling in the CSF.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martín-Láez R, Valle-San Román N, Rodríguez-Rodríguez EM, Marco-de Lucas E, Berciano Blanco JA, Vázquez-Barquero A. Actualización en la fisiopatología de la hidrocefalia crónica del adulto idiopática: ¿nos enfrentamos a otra enfermedad neurodegenerativa? Neurología. 2018;33:449–458.