Repetitive transcranial magnetic stimulation (rTMS) is a therapeutic reality in post-stroke rehabilitation. It has a neuroprotective effect on the modulation of neuroplasticity, improving the brain's capacity to retrain neural circuits and promoting restoration and acquisition of new compensatory skills.
DevelopmentWe conducted a literature search on PubMed and also gathered the latest books, clinical practice guidelines, and recommendations published by the most prominent scientific societies concerning the therapeutic use of rTMS in the rehabilitation of stroke patients. The criteria of the International Federation of Clinical Neurophysiology (2014) were followed regarding the inclusion of all evidence and recommendations.
ConclusionsIdentifying stroke patients who are eligible for rTMS is essential to accelerate their recovery. rTMS has proven to be safe and effective for treating stroke complications. Functional brain activity can be optimised by applying excitatory or inhibitory electromagnetic pulses to the hemisphere ipsilateral or contralateral to the lesion, respectively, as well as at the level of the transcallosal pathway to regulate interhemispheric communication. Different studies of rTMS in these patients have resulted in improvements in motor disorders, aphasia, dysarthria, oropharyngeal dysphagia, depression, and perceptual-cognitive deficits. However, further well-designed randomised controlled clinical trials with larger sample size are needed to recommend with a higher level of evidence, proper implementation of rTMS use in stroke subjects on a widespread basis.
La estimulación magnética transcraneal repetitiva (EMTr) constituye una realidad terapéutica en la rehabilitación postictus, ya que confiere efectos neuroprotectores incidiendo favorablemente en la modulación de la neuroplasticidad (NP), ayudando así al cerebro en su capacidad para readaptar circuitos neuronales y, con ello, la restauración y adquisición de nuevas habilidades compensatorias.
DesarrolloBúsqueda de artículos en PubMed, últimos libros y recomendaciones de las guías de práctica clínica y sociedades científicas publicadas más relevantes, referentes al uso terapéutico de la EMTr en la rehabilitación de pacientes con ictus. Se incluyen las evidencias y recomendaciones según los criterios de la International Federation of Clinical Neurophysiology (2014) al respecto.
ConclusionesLa identificación de pacientes con ictus subsidiarios de recibir EMTr es importante para acelerar la fase de recuperación. La EMTr ha demostrado ser segura y efectiva para tratar los déficits que aparecen tras un ictus. Los pulsos electromagnéticos excitatorios e inhibitorios aplicados en el hemisferio cerebral ipsolateral o contralateral a la lesión, respectivamente, así como a nivel transcalloso para regular la comunicación interhemisférica cerebral, nos brindan la posibilidad de optimizar la actividad cerebral funcional. Los diferentes estudios realizados sobre EMTr han demostrado la mejoría de los trastornos motores, la afasia, la disartria, la disfagia orofaríngea, la depresión y las dificultades perceptivo-cognitivas que aparecen en estos pacientes. Sin embargo, se necesitan ensayos clínicos controlados, aleatorizados, bien diseñados, que incluyan a un mayor número de pacientes, para poder recomendar con un mayor nivel de evidencia y de forma generalizada, la utilización adecuada de la EMTr en los enfermos afectados por un ictus.
Stroke patients should receive early neurorehabilitation after convalescence. For many years, researchers have aimed to identify new therapeutic targets to hasten recovery from stroke. However, we continue to lack a universally accepted, approved pharmacological therapy for these patients.1–5 After stroke, organisational changes in brain interneuronal activity in the affected area and the surrounding healthy tissue may on occasion promote functional recovery. Neurorehabilitation may help achieve this aim. Unfortunately, there are also occasions when neural reorganisation is suboptimal; in these cases, the problem persists and becomes chronic. In this context, transcranial magnetic stimulation (TMS) emerged as a tool for studying the brain and has been used since the mid-1980s to treat certain neuropsychiatric disorders. Neurorehabilitation is based on the idea that the brain is a dynamic entity able to adapt to internal and external homeostatic changes. This adaptive capacity, called neuroplasticity, is also present in patients with acquired brain injuries. The degree of recovery and the functional prognosis of these patients depend on the extent of neuroplastic changes.1–6 When performed by experienced physicians, TMS is a safe, non-invasive technique which enables the organisation of these neural changes (Fig. 1). The technique's applications are expanding rapidly.1–9
We present the results of a literature review of the most relevant articles, manuals, and clinical practice guidelines addressing TMS (background information, diagnostic and therapeutic uses, and especially its usefulness for stroke neurorehabilitation) and published between 1985 (when the technique was first used) and 2015.
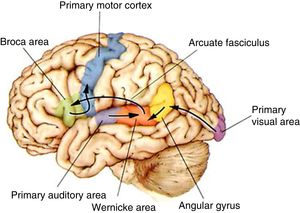
DevelopmentThe organisation of language in the brainThe left hemisphere of the brain is the anatomo-functional seat of language in 96% of right-handed and 70% of left-handed individuals. Language processing in the left hemisphere involves certain anatomical pathways for language comprehension, repetition, and production (Fig. 2). Positron emission tomography and functional magnetic resonance imaging (fMRI) studies conducted during multiple language tasks have shown brain activation not only in the main language centres (lesions to these areas may cause Broca aphasia, Wernicke aphasia, etc.) (Fig. 3) but also in many other locations, such as the thalamus (alertness), the basal ganglia (motor modulation), and the limbic system (affect and memory). Language is the perfect model for understanding how the central nervous system works as a whole.10,11
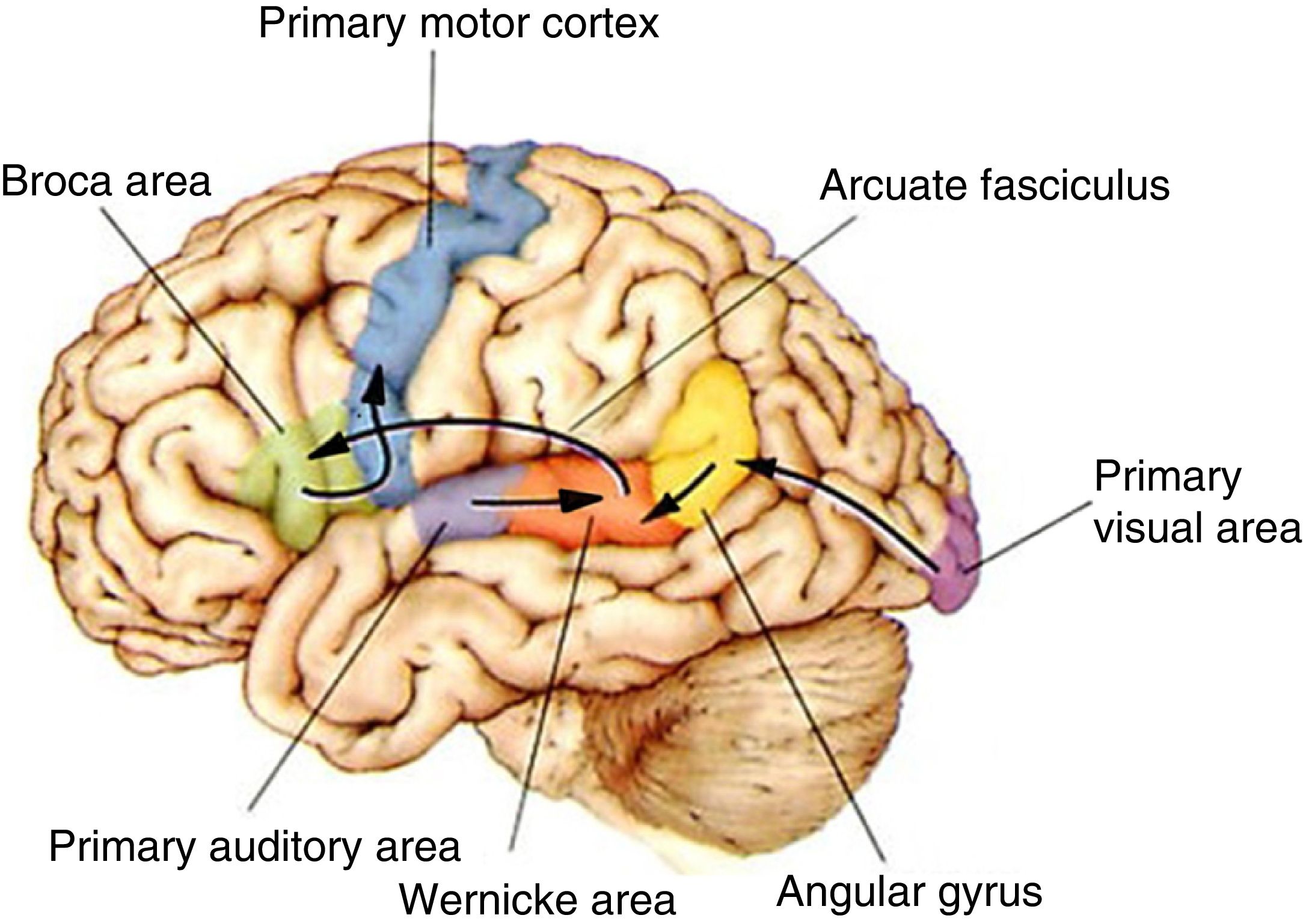
The functional pathways involved in comprehension, repetition, and production of written, gesture, and spoken language, according to the Wernicke-Geschwind model. Within the left hemisphere, language organisation follows certain anatomical pathways for language comprehension, repetition, and production. Sounds are processed by the bilateral auditory cortex, in the superior temporal gyrus (primary auditory area), and decoded in the posterior area of the left temporal cortex (Wernicke area); the latter is connected to other cortical areas or networks which assign meaning to words. During reading, output from the primary visual area (bilaterally) travels to other parieto-occipital association areas for word and phrase recognition (especially the left fusiform gyrus, located in the inferior surface of the temporal lobe, where there is a key word recognition centre) and reaches the angular gyrus, which processes language-related visual and auditory information. In spontaneous language repetition and production, auditory information must travel through the arcuate fasciculus towards the left inferior frontal region (Broca area), which is responsible for language production; this area is also known to be involved in such other functions as action comprehension (mirror neurons). To produce written or spoken language, output from the Wernicke area, the Broca area, and nearby association areas must reach the primary motor cortex.10,11
Adapted with permission from Bear et al.10
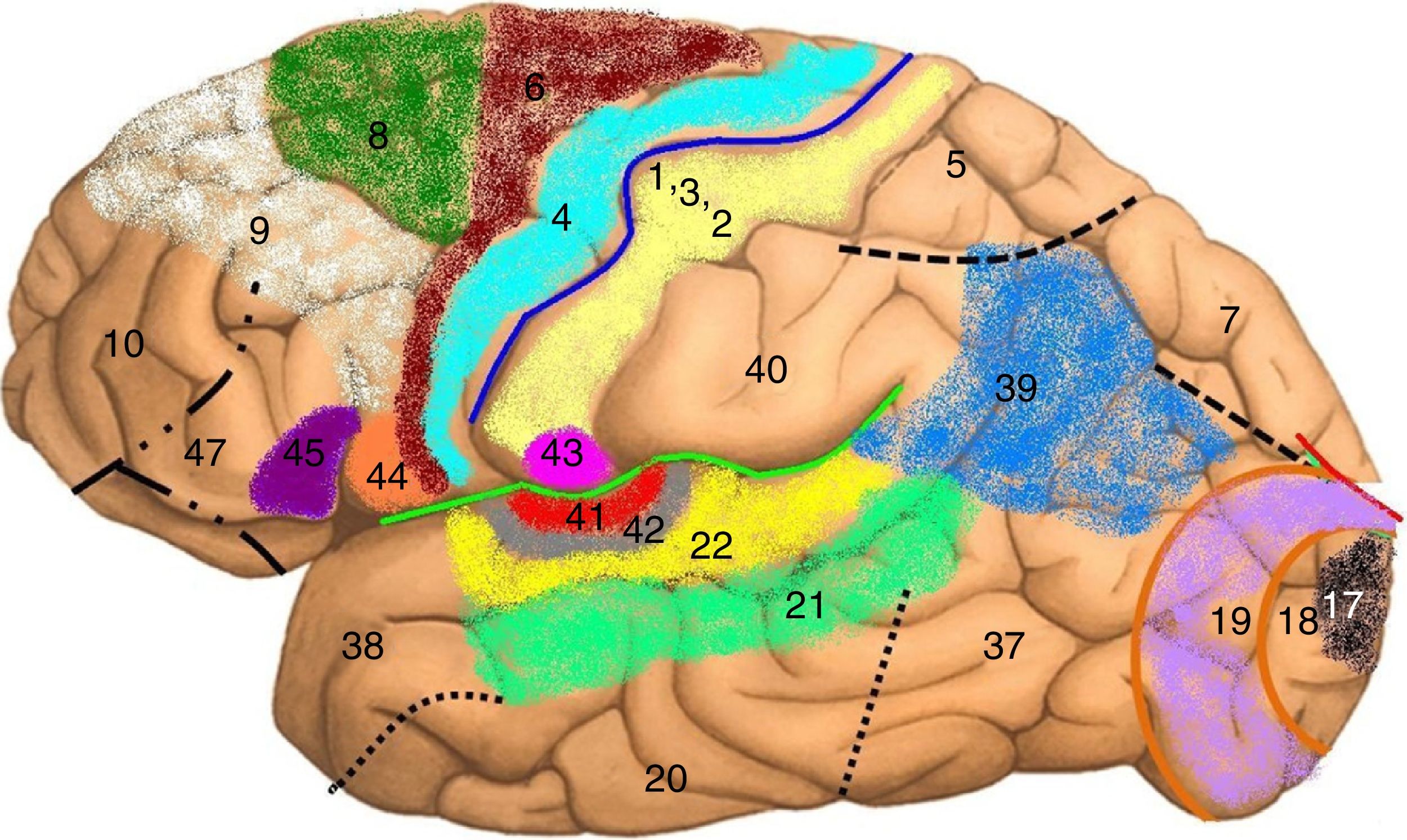
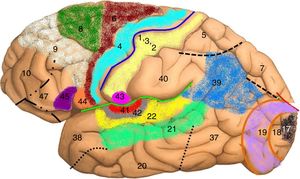
Map of Brodmann areas in the Homo sapiens brain. In 1909, Korbinian Brodmann used Nissl staining to divide the cerebral cortex into 52 areas (Brodmann areas 1-52). A Brodmann area is a region of the cerebral cortex with a distinct cytoarchitecture.
Brodmann area 4: primary motor cortex; Brodmann area 17: primary visual cortex; Brodmann area 22: Wernicke area; Brodmann area 41: primary auditory cortex; Brodmann areas 44 and 45: Broca area.
Aphasia is an acquired language disorder resulting in the inability to produce, repeat, and/or understand language (spoken/written language and/or gestures) despite the integrity of the neuromuscular structures involved in language processing. The disorder reveals the presence of a cortical lesion to the perisylvian region of the dominant hemisphere (normally the left hemisphere), although the literature also includes cases of crossed aphasia and aphasia secondary to basal ganglia lesions (striatum and thalamus).1,10,11 In most cases, aphasia involves the loss of writing (agraphia) and reading ability (alexia), regardless of the writing system used by the patient (whether alphabet- or logogram-based). Aphasia affects linguistic communication skills in general, including gestures, and results in inability to use other non-verbal communication systems (Morse code, Braille, sign language, etc.). Symptoms vary depending on lesion size and location and the brain's ability to produce neuroplastic changes. Aphasia has multiple aetiologies, including stroke (the most frequent), head trauma, encephalitis (especially in cases of frontal and/or temporal lobe involvement, as in herpes simplex encephalitis), brain tumours, and dementia (especially Alzheimer disease and frontotemporal dementia, in which aphasia may be the first manifestation of the condition).6,10–14
What is transcranial magnetic stimulation?ConceptTMS is a non-invasive cortical stimulation technique offering numerous opportunities for neuroscience research and for the treatment of various neuropsychiatric disorders. The technique involves safe, painless, non-invasive stimulation of the nervous tissue (cerebral cortex, spinal cord, central motor pathways, and peripheral nerves) and regulates brain activity (Fig. 4). The interaction between TMS and neurons may produce a wide range of changes, including electrophysiological (membrane potentials), biochemical and molecular (signalling, neurotransmitters, genes, etc.), and cellular changes (growth, differentiation, etc.). The technique also affects behaviour, mood, memory, myelination, and neuroplasticity.6,15,16
HistoryIn 1821, Hans Christian Ørsted laid the grounds for the theory of electromagnetism after discovering a relationship between electricity and magnetism. In 1830-1832, Michael Faraday demonstrated the relationship between magnetic and electric fields (Fig. 5); this was subsequently formulated by James Clerk Maxwell. In 1959, Kolin and colleagues demonstrated that a fluctuating magnetic field could stimulate a peripheral frog muscle preparation. In 1980, Patrick Merton and Bert Morton of the National Hospital for Neurology and Neurosurgery, in London, were the first to use TMS to stimulate the motor cortex, recording a motor action potential. However, it was Barker and colleagues, of the University of Sheffield, who in 1985 developed a device capable of depolarising cortical neurons and evoking movement in one side of the body by activating corticospinal pathways, in order to evaluate the integrity of central motor pathways.15 In 1987, the researchers used this device in patients with multiple sclerosis, demonstrating slowed conduction velocity in motor pathways and showing the superiority of TMS over transcranial electrical stimulation. Since then, the use of TMS both for research and for clinical purposes has increased exponentially.6,15–19
Faraday discovered electromagnetic induction after wrapping 2 copper wire solenoids around an iron cylinder; when electric current started to flow in one solenoid, an electric current was temporarily induced in the other. This phenomenon is known as mutual induction. The original apparatus is displayed in the Faraday Museum at the Royal Institution, London.
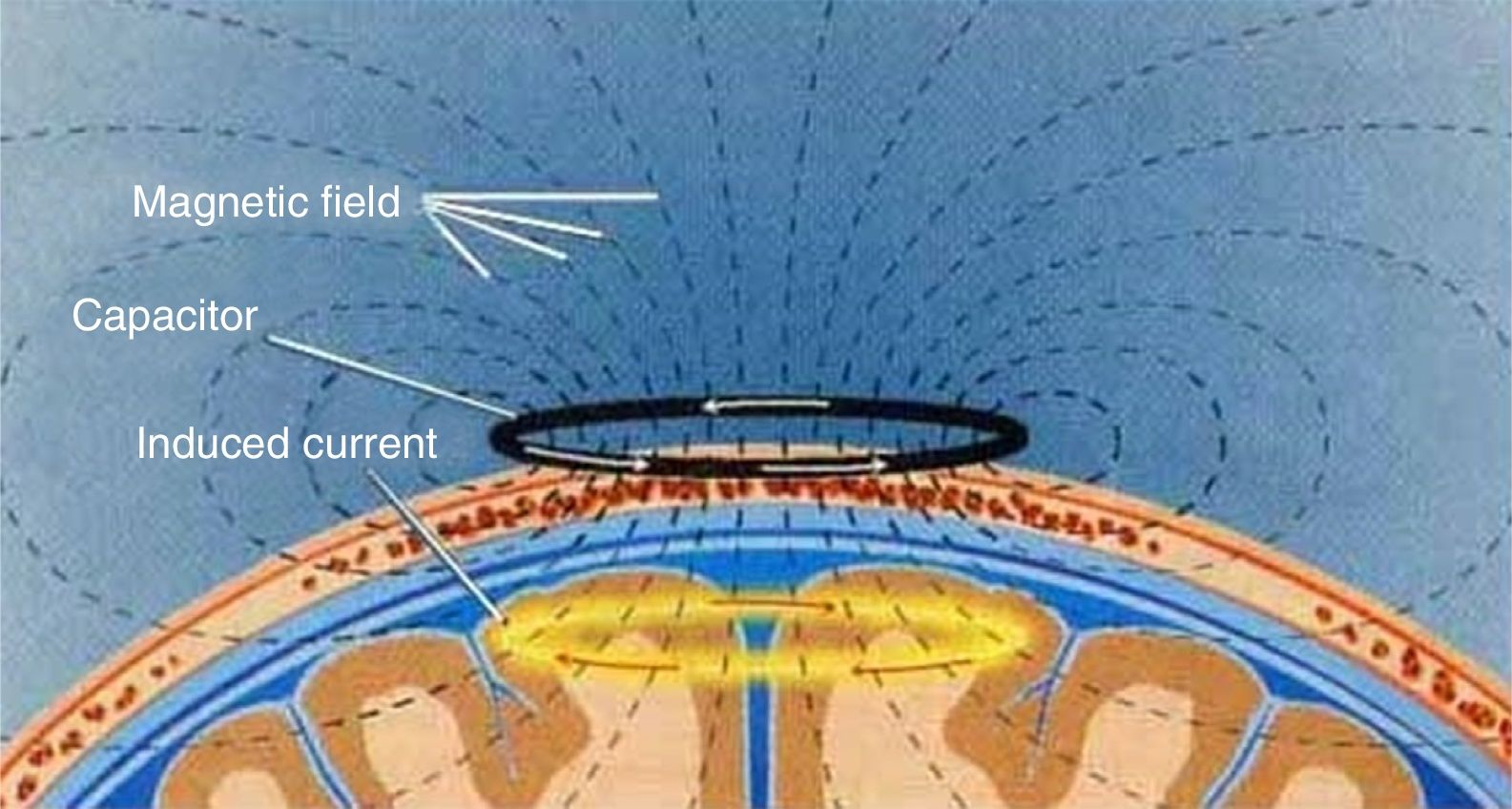
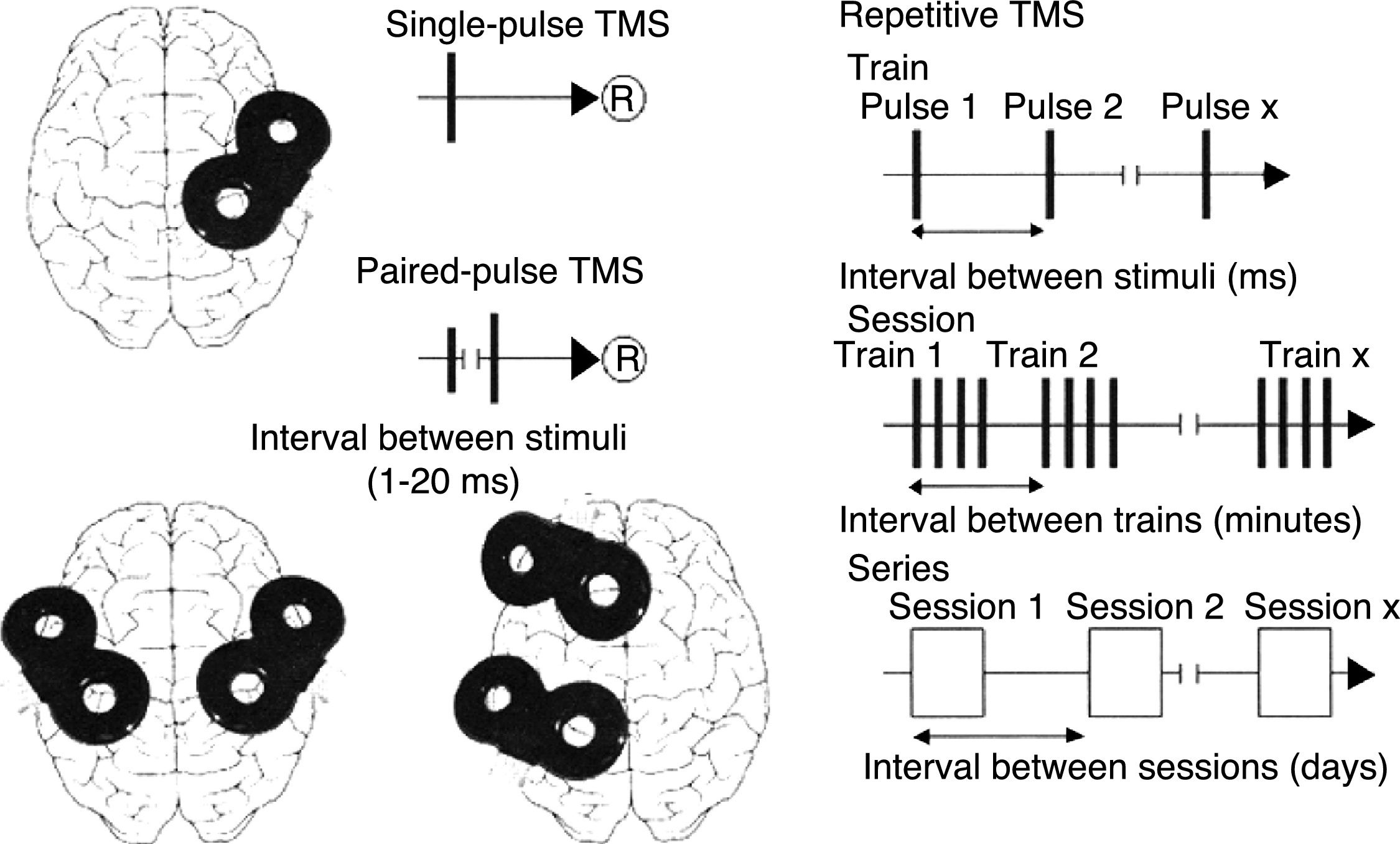
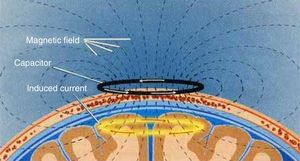
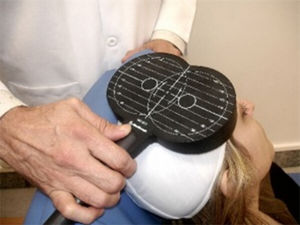
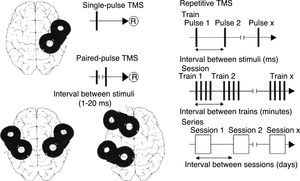
TMS is based on the principle of electromagnetic induction, formulated by Michael Faraday in 1831. According to this principle, an electric field produces a perpendicular magnetic field, and vice versa (Fig. 4). In TMS, a capacitor discharges an electric pulse through a copper-wire coil embedded in a plastic case. The coil is placed on the patient's head, generating a magnetic field perpendicular to the head (Fig. 6). This changing magnetic field induces an electric current in any nearby conductive material.6,15–20 When an electric pulse is sent through the coil, a magnetic field travels through the patient's scalp and skull without attenuating (it only decays with the square of the distance). The most widely used stimulation coils are circular coils and figure-8 coils. Circular coils produce a wider electric field, stimulating both hemispheres simultaneously. Figure-8 coils, in contrast, focus on a more specific area (Fig. 7).6,15–22 These magnetic pulses selectively depolarise cortical neurons, located 1.5-2cm below the scalp. Electric pulses may either inhibit or stimulate neurons, modulating the energy obtained from their mitochondria and affecting electrical signal transmission and cell survival. This depends on the shape, size, type, and position of the coil; the intensity of the magnetic field; and the frequency and duration of magnetic pulses (Fig. 8)6,15–25:
- –
Single-pulse TMS applies a single stimulus to a specific brain region, depolarising cortical neurons and triggering a motor evoked potential (MEP) in a muscle area of the contralateral half of the body.
- –
Paired-pulse TMS generates 2 paired stimuli with identical or different intensity, separated by an interval of several milliseconds; these pulses are applied to a single cortical region or to different areas. This technique explores intra- and intercortical excitability, the integrity of interhemispheric connectivity, and transcallosal conduction time.
- –
Repetitive TMS (rTMS) generates a train of low-frequency (≤1Hz; range, 0.5-1Hz) or high-frequency (≥5Hz; range, 5-20Hz) pulses during very short intervals (ms), inducing long-lasting changes in corticospinal excitability. These properties have made rTMS the most frequently used type of TMS for therapeutic purposes.
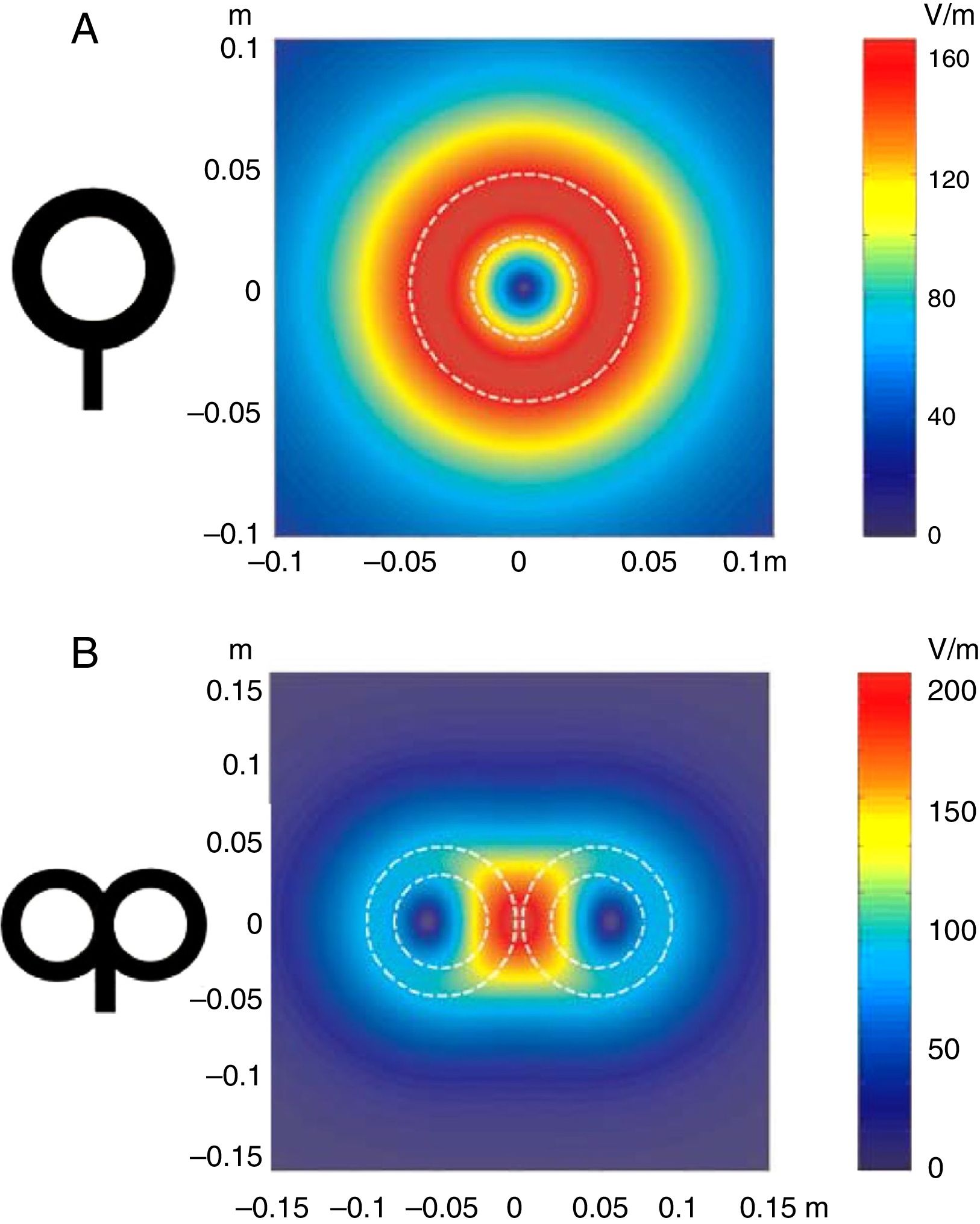
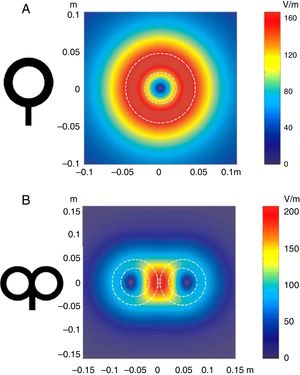
Distribution of electric fields induced by a circular coil (A) and a figure-8 coil (B). The circular coil has an inside turn diameter of 41.5mm, an outside turn diameter of 91.5mm (mean, 66.5mm), and 15 turns of copper wire. The figure-8 coil has an inside turn diameter of 56mm, an outside turn diameter of 90mm (mean, 73mm), and 9 turns of copper wire on each wing. The outer shape of each coil is shown with white dashed lines on the representation of the induced fields. The amplitude of the electric field is calculated for a plane 20mm below a realistic model of the coil (di/dt=A/μs). di/dt represents the derivative of current intensity with respect to time (rate of current change) and is expressed in amperes per microsecond (A/μs). If current intensity changes from 0 to 40A in 2μs, we obtain a di/dt=40A/2μs, that is 20A/μs.
m: metres; V/m: volts per metre.
Adapted with permission from Pascual-Leone et al.15
Schematic representation of the different types of TMS: single-pulse TMS, paired-pulse TMS of one or 2 different brain regions, and repetitive TMS (low-frequency: ≤1Hz; high-frequency: ≥5Hz).
®: response.
Adapted with permission from Pascual-Leone et al.15
Advances are being made in our understanding of the most intricate biochemical, molecular, and cellular mechanisms underlying the therapeutic effects of rTMS. One of these mechanisms is the technique's ability to modulate the expression of certain immediate early genes, such as c-Fos and c-Jun, which are involved in neuroplasticity, neurodegeneration, and early response to brain damage. These genes regulate the expression of multiple growth factors, such as the brain-derived neurotrophic factor, which plays a role in neuroplasticity. Furthermore, rTMS interferes in apoptotic processes and promotes mitochondrial energy production and oxidative balance within neurons and brain tissues, modifying the regulation and activity of certain transcription factors associated with apoptosis (nuclear factor kappa B), oxidative stress (nuclear factor erythroid 2-related factor 2 [NRF2]), and proinflammatory cytokine production. From a functional viewpoint, rTMS regulates the production and release of neurotransmitters N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and the neurohormones dopamine, serotonin, gamma-aminobutyric acid, glutamate, and melatonin.6,15–30
Effects on sensorimotor integrationSensorimotor integration is the brain's ability to process sensory stimuli in multiple areas of the cerebral cortex (association areas) and transform them into motor activity. Following a brain injury, remote cortical areas can reorganise themselves to increase motor learning and performance. Neural reorganisation may be achieved by regulating interactions between the ipsilesional and contralesional primary motor cortex. This is an interesting scenario, considering that complex motor tasks require interhemispheric communication. The application of rTMS to the ipsilesional and contralesional areas may produce a wide range of motor patterns, depending on the frequency of stimulation.6,15–30
Applications and current use of transcranial magnetic stimulation- 1.
Neurophysiology. The physiological mechanisms of TMS are based on the fact that a single stimulus with a specific intensity and orientation causes neuronal depolarisation, followed by an action potential which produces an excitatory postsynaptic response of 1ms, which is in turn followed by an inhibitory postsynaptic potential of 100ms. TMS therefore has a local effect, interrupting normal neural activity, increasing the refractory period, and regulating the discharge pattern. In neurophysiology, TMS is mainly used to explore motor cortical areas and corticospinal tract conduction.6,15–42
- 2.
Neural networks. Neuropsychological, neurophysiological, and neuroimaging studies in animals and humans have shown that cognitive function and behaviour result from interaction between distant brain regions via functional neural networks. According to the principle of diaschisis, the effects of TMS on a specific area extend to other cortical and subcortical areas of both hemispheres; due to brain connectivity, the effects may even reach deep brain areas.36,37 Activation or inhibition of a specific area affects distant areas; the effects depend on whether the stimulus is excitatory or inhibitory.6,8,15–55
- 3.
Treatment. We now know that rTMS has a positive impact on neuroplasticity; its neuroprotective effects may be beneficial, at least temporarily, for patients with a wide range of neuropsychiatric disorders. This has led researchers to investigate the use of rTMS as an adjuvant treatment for a number of disorders. However, further studies are necessary before rTMS may be recommended for treating these conditions with a higher level of evidence. Disorders potentially benefiting from rTMS include6,8,15–71:
- –
Psychiatric disorders: mood disorders (drug-resistant major depression, postpartum depression, dysthymia, mania, bipolar disorder, etc.), schizophrenia, anxiety disorders (obsessive-compulsive disorder, post-traumatic stress disorder, etc.), autism, attention-deficit/hyperactivity disorder, dysphemia, substance use disorders, monosymptomatic nocturnal enuresis, etc.
- –
Neurological disorders: stroke, drug-resistant epilepsy, Parkinson's disease, essential tremor, Huntington disease, Alzheimer disease, tinnitus, focal dystonia, head trauma, gait disorders, migraine with aura, trigeminal neuralgia, multiple sclerosis, amyotrophic lateral sclerosis, etc.
- –
Other conditions: chronic pain in phantom limb syndrome, neuropathic pain, fibromyalgia, visceral pain, complex regional pain syndrome type I (formerly known as reflex sympathetic dystrophy), atypical facial pain, etc.
- –
In Western countries, stroke is the leading cause of death in women and the second most common cause in men (after coronary artery disease), the most frequent cause of disability in adults, and the second most common cause of dementia, after Alzheimer disease.72,73 Neuroplastic changes occur as early as the acute, post-stroke phase. This phenomenon is influenced by a number of factors, including genetic factors; the patient's age and level of dependence prior to stroke; whether the patient receives early neurorehabilitation; social and family support; intercurrent processes; and the location, severity, nature, and extension of the lesion. Neurorehabilitation aims to increase patients’ functional capacity.2–55 In patients with stroke, TMS may be used as a brain mapping technique to quantify several cortical electrophysiological parameters, and as a regenerative treatment technique. The technique's limitations in brain mapping lie in the difficulty of interpreting the measurements reported by different researchers. In fact, brain mapping techniques are essential for understanding the molecular, cellular, and functional mechanisms of stroke recovery. Combining TMS with neuroimaging techniques may provide insight into the changes that occur in brain circuits after stimulation. Regarding its therapeutic effects, TMS may be used to improve neuroplasticity, which in turn leads to improvements in the signs and symptoms of stroke6,8,15–56:
- 1.
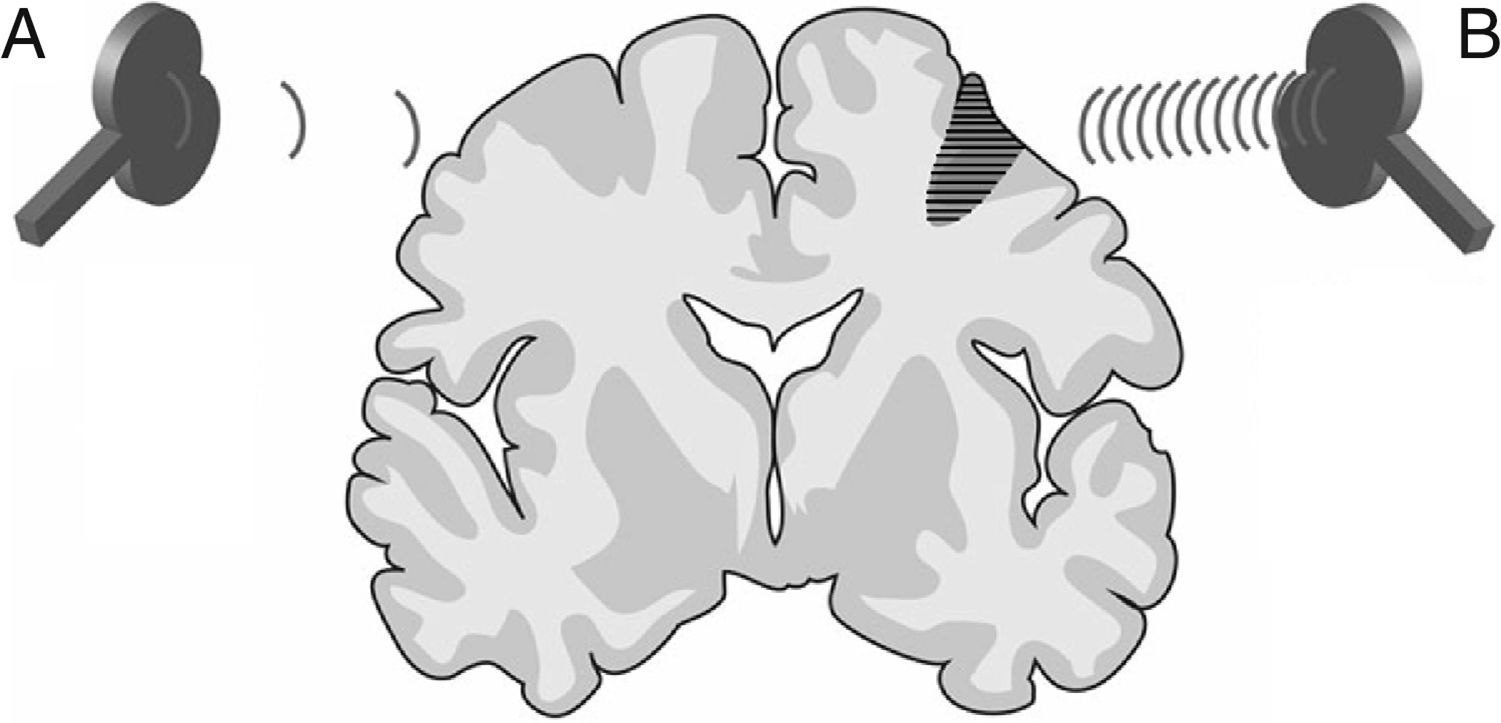
Neuroplasticity. According to the literature, rTMS has a significant effect on neuroplasticity through the stimulation or inhibition of neural synaptic transmission. Most stroke symptoms are not caused by the lesion itself but rather by hyperactivity of the intact hemisphere, which inhibits neural activity in the damaged hemisphere. Low-frequency rTMS (≤1Hz) of the intact hemisphere normalises diffuse cortical activation in the primary and supplementary motor areas of both hemispheres, reactivating the damaged cortical area whose activity was inhibited and promoting excitability and motor recovery. High-frequency rTMS (≥5Hz), in contrast, increases cortical excitability and may be applied to stimulate the cortical neurons of the damaged hemisphere. In this way, rTMS accelerates neuroplasticity, reorganising brain networks and thereby increasing interneuronal connectivity in the damaged area (Fig. 9).6,8,15–56
Figure 9.Representation of a coronal section of the human brain. Types of rTMS that promote neuroplasticity and, consequently, early neurorehabilitation of patients with stroke: (A) Low-frequency rTMS (≤1Hz) of the unaffected hemisphere in the area contralateral to a cortico-subcortical vascular lesion (dark grey area) reduces inhibition in the damaged hemisphere. (B) High-frequency rTMS (≥5Hz) of the ipsilesional hemisphere stimulates neural activity and promotes the reorganisation of interneuronal networks.Adapted with permission from Edwardson et al.7
(0.07MB). - 2.
Motor recovery. Treatment with rTMS and task-oriented motor rehabilitation induce neuroplastic changes by activating NMDA receptors and inhibiting the GABAergic system.6,8,15–56 In a study of 15 stroke patients with chronic hemiparesis, 10Hz rTMS was found to increase MEP amplitude in the treated group. This increase was associated with improved motor performance, assessed with a sequential finger motor task.6,74,75 In another study, treatment with high-frequency rTMS on the lesioned hemisphere plus neurorehabilitation improved upper-limb function except when patients were forced to use the paretic hand (constraint-induced movement therapy).76 It has also been suggested that the effectiveness of rTMS depends on the type of stroke; the technique achieves better results for subcortical stroke.6,77 Furthermore, rTMS of the unaffected hemisphere seems to improve motor performance. Inhibitory rTMS (≤1Hz) of the unaffected hemisphere combined with motor rehabilitation has been found to improve motor deficits since the intact area inhibits the lesioned area via the transcallosal pathway. The technique reduces MEP amplitude in the contralesional primary motor cortex and transcallosal inhibition duration, increasing pinch force in the affected hand.6,78,79 According to the literature, low-frequency rTMS of the intact hemisphere is more effective.80–83 Theta burst stimulation (TBS), a type of TMS consisting of repetitive, high-frequency, low-intensity magnetic pulses (350-Hz pulses every 200ms), has yielded contrasting results. When applied to the unaffected motor cortex of patients with stroke, TBS increases MEP, decreases cortical excitability in the intact hemisphere, stimulates neuroplasticity, and results in better functional recovery at 6 months after stroke.84 There are 2 types of TBS: intermittent TBS (2 pulses applied 10s apart), which has an excitatory effect on the cortex, and continuous TBS, which inhibits cortical activity. In comparative studies of intermittent TBS of the damaged area vs continuous TBS of the contralesional site, intermittent TBS has been found to be effective for motor regeneration whereas the positive effects of continuous TBS disappeared after treatment.85–87 In a larger study, however, no significant improvements were observed in patients receiving motor rehabilitation followed by continuous/intermittent TBS, compared to patients not receiving this treatment.88 Studies of continuous TBS of 2 different cortical areas in the contralesional hemisphere (motor and somatosensory) have shown that, although stimulation of both areas improves motor function, the technique is most effective when applied to the somatosensory area.6,89
- 3.
Aphasia and dysarthria. According to the literature, rTMS is most effective in patients with motor aphasia or mixed, predominantly motor aphasia. Post-stroke recovery depends on 3 conditions: 1) recruitment of lesioned or perilesional regions of the left hemisphere for language tasks; 2) acquisition of language skills by the right hemisphere; and 3) dysfunctional activation of the non-dominant hemisphere, which may interfere with language recovery.6,8,15–56 Patients with non-fluent aphasia show greater cortical hyperexcitability in the Broca area homologue in the right hemisphere. In patients with chronic aphasia, rTMS of the right anterior Broca area (the pars triangularis of the inferior frontal gyrus or Brodmann area 45) for 10min at an inhibitory frequency of 1Hz and at an intensity of 90% of MEP threshold led to transient improvements in naming ability and reaction time in naming pictures. However, when applied to the posterior pars triangularis, the technique had the opposite effect. A larger study of the impact of rTMS applied to the right pars triangularis showed that the effects of a 20-min session of 1-Hz pulses applied 5 days per week for 2 weeks persisted for up to 8 months after stimulation.6,90–93 Several subsequent studies have confirmed that inhibitory rTMS of the right pars triangularis, either alone or combined with continuous positive airway pressure (in a patient with chronic aphasia and sleep apnoea), improves language impairment in terms of both picture naming and spontaneous speech. These studies show that improvements in the speech of patients with chronic aphasia depend on the anatomy of the affected area. When the lesion is extensive, involving inferior frontal gyrus and adjacent areas (the middle frontal gyrus), rTMS seems to have no effect. The positive effects of electromagnetic pulses on aphasia are probably not due to interhemispheric reorganisation of neural networks, but rather to the fact that 1-Hz rTMS suppresses the activity of the contralateral cortex, which may delay speech recovery.6,94–97 fMRI-guided rTMS has also been used in areas contralateral to the regions that are most active during speech tasks. A group of researchers applied inhibitory magnetic stimuli to the right frontal lobes of 2 patients and to the left frontal lobes of 2 additional patients (1200 pulses every 20min; 10 sessions over 6 days). All 4 patients achieved moderate improvements in spontaneous speech, repetition, writing, and picture naming; these results lasted at least 4 weeks after treatment. TMS of the Wernicke area in the left hemisphere has been reported to improve speech.6,98 In a recent study, daily sessions of 10-Hz rTMS of the left inferior frontal gyrus for 3weeks decreased the activity of the right inferior frontal gyrus and activated the left, leading to improvements in repetition, naming, and comprehension. The treatment also increased activity in the right supplementary motor area; this suggests that improvements in aphasia may be due to the effects of rTMS on interhemispheric connectivity alterations following stroke.6,99 In a study of patients with subacute stroke and dysarthria, speech therapy was combined with a 2-week course of daily sessions of 1-Hz rTMS of the contralesional motor cortex (the exact area of stimulation was determined by searching for the MEP of the orbicularis oris muscle on the non-affected side). Dysarthria improved considerably in the group of patients receiving rTMS. Furthermore, these patients repeated a sequence of syllables (/p¿/, /t¿/, and /k¿/) significantly more times than those not receiving rTMS.100
- 4.
Oropharyngeal dysphagia. Despite an incidence of 50% in patients with stroke, oropharyngeal dysphagia is underestimated and underdiagnosed. This condition is one of the main causes of malnutrition and aspiration pneumonia, increasing the mortality rate in these patients (it accounts for 20%-30% of post-stroke deaths). While swallowing is involuntary and the swallowing reflex is dependent on the centres located at the level of the brainstem in the dorsolateral medulla oblongata (solitary tract, nucleus ambiguus, and reticular formation), initiation of swallowing is a voluntary action dependent on the integrity of motor areas in the cerebral cortex.6,101–108 Oropharyngeal dysphagia may cause 2 types of complications: alterations in swallowing efficiency (which causes malnutrition and/or dehydration) and unsafe swallowing (which may lead to aspiration pneumonia). Dysphagia after stroke results from damage to the motor cortex of the dominant hemisphere. Unlike aphasia, dysphagia may be caused by lesions to either hemisphere. Excitatory, high-frequency rTMS (5Hz, 10min per day for 2 weeks) of the contralesional motor cortex (which aims to cause neural reorganisation, as occurs spontaneously after stroke) has been found to improve swallowing function and reduce the risk of aspiration.6,101–103 Some studies have attempted to recover swallowing function by inhibiting the transcallosal pathway with rTMS of the unaffected hemisphere (1Hz, 20min daily for 5 days), whereas other studies have stimulated the damaged hemisphere (300 pulses at 120% of motor threshold for 5days).108 Inhibiting the corpus callosum has been found to improve swallowing coordination, with a decrease in reaction time for liquids and paste, although it has no effect on oral or pharyngeal transit time. Aspiration scores for liquids and paste also decreased.109,110 Several studies have found stimulation of the damaged hemisphere to improve dysphagia, with effects lasting 2 months.6,103
- 5.
Perceptual and cognitive disorders. Hemispatial neglect is a disorder suggestive of poor functional prognosis in patients with stroke.111–115 rTMS has been shown to be effective in treating this type of stroke-related disorders. In a study of several patients with hemispatial neglect due to right-hemisphere stroke, a single session of 1-Hz rTMS of the left parietal area followed by 10 days of occupational therapy was found to improve hemispatial neglect.112 Regarding the effects of rTMS on cognitive function in these patients, a prospective randomised clinical trial revealed that rTMS of the prefrontal cortex had no significant effect on executive and cognitive function but did improve mood after high-frequency rTMS (10Hz for 10 days) to the left dorsolateral prefrontal cortex (Brodmann areas 9 and 46).6,116,117
- 6.
Depression. Depression is the most frequently studied condition in the context of TMS, as well as the most prevalent mood disorder among patients with stroke. Although most studies agree that TMS of the left prefrontal area is safe and well tolerated when applied daily for several weeks, others suggest that its antidepressant effects depend on the area where the coil is placed and even vary between patients despite application within the reference limits of the same lobe. In patients with stroke, high-frequency rTMS (10Hz) of the left dorsolateral prefrontal area in 10 sessions over 2 weeks has been found to significantly improve mood alterations as measured with the Beck Depression Inventory.6,116
- 7.
Treatment protocol for aphasia. The treatment protocol for aphasia recently implemented at San Vicente Clinic (Madrid) is based on the protocol developed by the Berenson-Allen Center for Noninvasive Brain Stimulation (BIDMC; Boston), directed by Álvaro Pascual-Leone. The BIDMC protocol is based on the results reported by Naeser et al.118–122 Our protocol consists of 10 to 20-min sessions of rTMS (one session daily, Monday to Friday for 2 weeks), followed by intensive speech therapy (approximately 1h per day). We apply 1-Hz rTMS to the intact, right hemisphere to reduce its potential inhibition of the Broca area of the damaged, left hemisphere. Before the first session of rTMS, we evaluate patients to determine whether they have any contraindications for this technique and that they are eligible for this type of treatment. Patients are also evaluated by a speech therapist before and after treatment, and at several follow-up consultations, to determine the effects of this combined neurorehabilitation. Once we have a large enough sample, it will be possible to determine the safety and effectiveness of this technique for post-stroke aphasia and whether our results replicate those reported in the recent literature.
Although rTMS is a safe technique, some patients may experience adverse effects, such as headache or neck pain. Pain is usually mild and transient; in the unlikely event it persists, it can be managed with conventional analgesics. The risk of epileptic seizure during rTMS is very low; there is no evidence to support the hypothesis that rTMS increases the risk of seizures after the session in epileptic patients receiving this treatment.21,123–127
ContraindicationsThe main relative contraindications for TMS include pregnancy and age below 2 years. TMS is absolutely contraindicated for patients with drug-resistant epilepsy and those wearing electric devices (pacemakers, implantable defibrillators, vagus nerve stimulators, deep brain stimulators, insulin pumps, etc.) or intracranial ferromagnetic devices and/or ferromagnetic devices located within 30cm of the treatment area (plates, screws, ventriculo-peritoneal shunts, stents, jewellery, dental and cochlear implants, etc.). TMS is safe for patients with titanium implants, such as endovascular coils for brain aneurysms.21,123,124
ConclusionsrTMS has been shown to be a safe, effective, cutting-edge technique for treating multiple post-stroke alterations. This technique is especially useful for promoting neuroplasticity and, consequently, brain regeneration. Applying excitatory or inhibitory electromagnetic pulses to the ipsilesional or contralesional hemisphere, respectively, or to the corpus callosum (which modulates interhemispheric communication) optimises functional brain activity and accelerates recovery from brain injury. Multiple studies of rTMS report improvements in motor impairment, aphasia, dysarthria, oropharyngeal dysphagia, depression, and perceptual and cognitive disorders in patients with stroke.1–127 However, the most suitable treatment duration, time of intervention, and treatment protocol remain to be determined. In the coming years, further well-designed prospective studies with larger samples and longer follow-up periods will surely address these gaps and provide a higher level of evidence to recommend rTMS for the neurorehabilitation of patients with acquired brain injury due to stroke. This treatment should always be applied as part of a holistic, interdisciplinary approach, in combination with other techniques of physical and neurocognitive rehabilitation. In the near future, we will very likely achieve more consistent results in support of TMS for the treatment of other neuropsychiatric disorders.
FundingThis study received no public or private funding.
Conflicts of interestAll authors have given their approval for the publication of the manuscript. The authors have no conflicts of interest to declare.
To all the patients and employees of San Vicente Clinic.
Please cite this article as: León Ruiz M, Rodríguez Sarasa ML, Sanjuán Rodríguez L, Benito-León J, García-Albea Ristol E, Arce Arce S. Evidencias actuales sobre la estimulación magnética transcraneal y su utilidad potencial en la neurorrehabilitación postictus: Ampliando horizontes en el tratamiento de la enfermedad cerebrovascular. Neurología. 2018;33:459–472.