A growing number of studies have evaluated the effects of transcranial magnetic stimulation (TMS) for the symptomatic treatment of multiple sclerosis (MS).
MethodsWe performed a PubMed search for articles, recent books, and recommendations from the most relevant clinical practice guidelines and scientific societies regarding the use of TMS as symptomatic treatment in MS.
ConclusionsExcitatory electromagnetic pulses applied to the affected cerebral hemisphere allow us to optimise functional brain activity, including the transmission of nerve impulses through the demyelinated corticospinal pathway. Various studies into TMS have safely shown statistically significant improvements in spasticity, fatigue, lower urinary tract dysfunction, manual dexterity, gait, and cognitive deficits related to working memory in patients with MS; however, the exact level of evidence has not been defined as the results have not been replicated in a sufficient number of controlled studies. Further well-designed, randomised, controlled clinical trials involving a greater number of patients are warranted to attain a higher level of evidence in order to recommend the appropriate use of TMS in MS patients across the board. TMS acts as an adjuvant with other symptomatic and immunomodulatory treatments. Additional studies should specifically investigate the effect of conventional repetitive TMS on fatigue in these patients, something that has yet to see the light of day.
Existe un creciente número de estudios en esclerosis múltiple (EM) que abordan el uso terapéutico sintomático de la estimulación magnética transcraneal (EMT) en estos pacientes.
MétodosBúsqueda de artículos en PubMed, últimos libros y recomendaciones de las guías de práctica clínica y sociedades científicas publicadas más relevantes referentes al empleo de la EMT como terapia sintomática en la EM.
ConclusionesLos pulsos electromagnéticos excitatorios aplicados en el hemisferio cerebral afecto permiten optimizar la actividad cerebral funcional, incluyendo la transmisión de los impulsos nerviosos a través de la vía corticoespinal desmielinizada. Los diferentes estudios realizados sobre EMT han demostrado, sin poder definir nivel exacto de evidencia dada la ausencia de resultados replicados en un número suficiente de estudios controlados, de forma segura, la mejoría estadísticamente significativa de la espasticidad, la fatiga, la disfunción del tracto urinario inferior, la destreza manual, las alteraciones de la deambulación y los trastornos cognitivos relativos a la memoria de trabajo que aparecen en estos pacientes. Se precisa la realización de más ensayos clínicos controlados, aleatorizados, bien diseñados, que incluyan un mayor número de pacientes, para poder recomendar con un mayor nivel de evidencia y de forma generalizada la utilización adecuada de la EMT en los enfermos afectados por EM, la cual aporta su capacidad de adyuvancia sobre la acción de los otros tratamientos sintomáticos y fármacos inmunomoduladores. Así como estudios que investiguen específicamente la aplicación de la EMT repetitiva (EMTr) convencional sobre la fatiga, algo que no ha visto la luz hasta el momento.
Given the increasing body of research into the therapeutic potential of transcranial magnetic stimulation (TMS) for multiple sclerosis (MS), we considered there to be a need for further development of this subject, and performed a systematic review of the literature on this promising, cutting-edge diagnostic and treatment technique. There is growing evidence of the beneficial effects of TMS for MS symptoms, which often have a considerable negative effect on patients’ quality of life. We searched for articles on the PubMed database, recent books, and recommendations from the most relevant clinical practice guidelines and scientific societies on the use of TMS for the symptomatic treatment of MS and promotion of remyelination, as well as the effects of the treatment on oxidative stress in the murine model of experimental autoimmune encephalitis (EAE).
DevelopmentWhat is transcranial magnetic stimulation?ConceptTMS is a non-invasive cortical stimulation technique offering numerous opportunities for neuroscience research; it also constitutes a novel treatment option for many neuropsychiatric disorders (Appendix 1). This cutting-edge technique enables safe, painless, non-invasive stimulation of nerve tissue (cerebral cortex, spinal cord, central motor pathways, and peripheral nerves) and controlled regulation of brain activity. The interaction between TMS and neurons, the basic functional unit with electric activity in the brain, may cause a wide range of changes, including electrophysiological (membrane potentials), biochemical and molecular (signalling, neurotransmitters, genes, etc.), and cellular changes (growth, differentiation, etc.). The technique also influences behaviour, mood, memory, myelination, and neuroplasticity.1–4
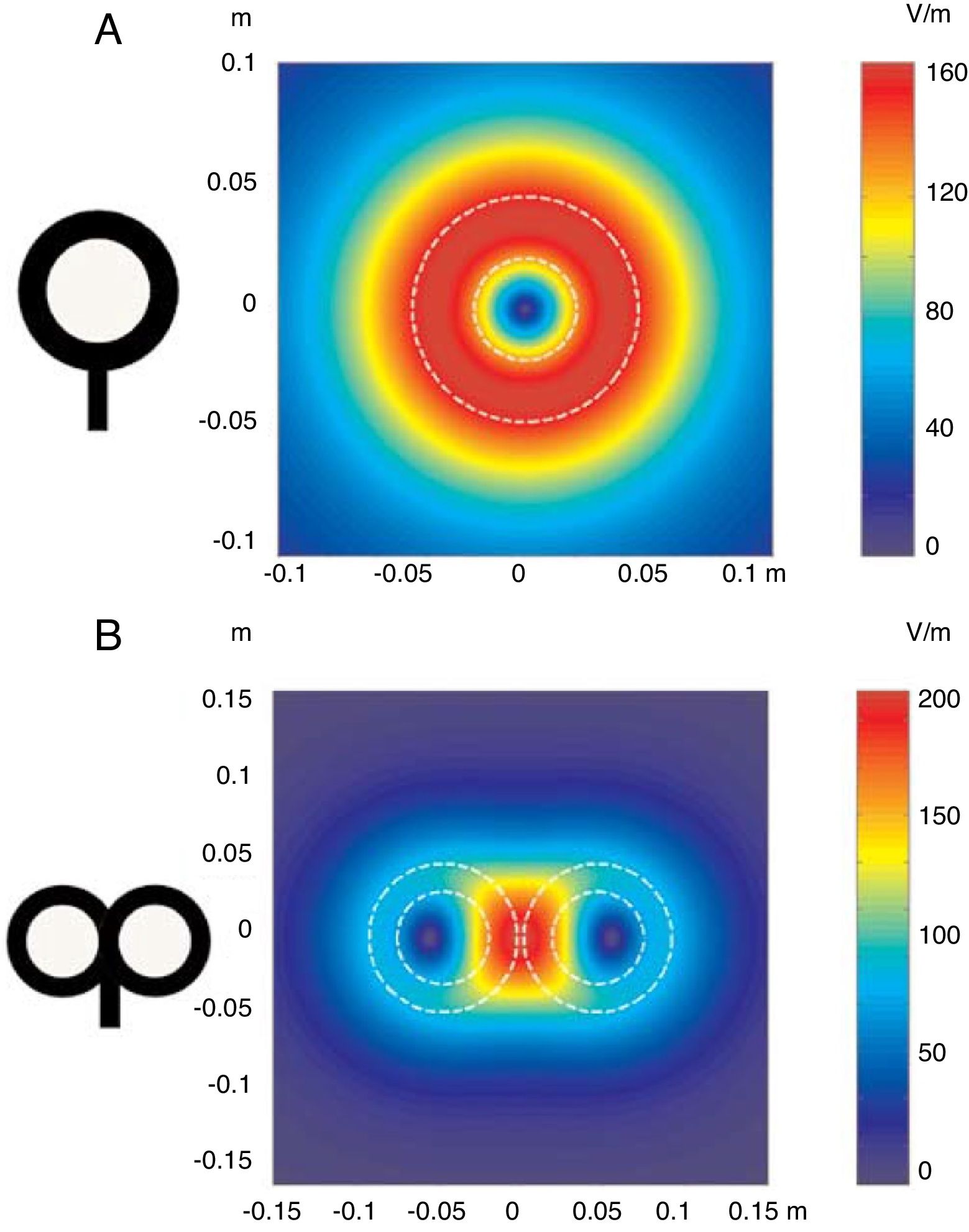
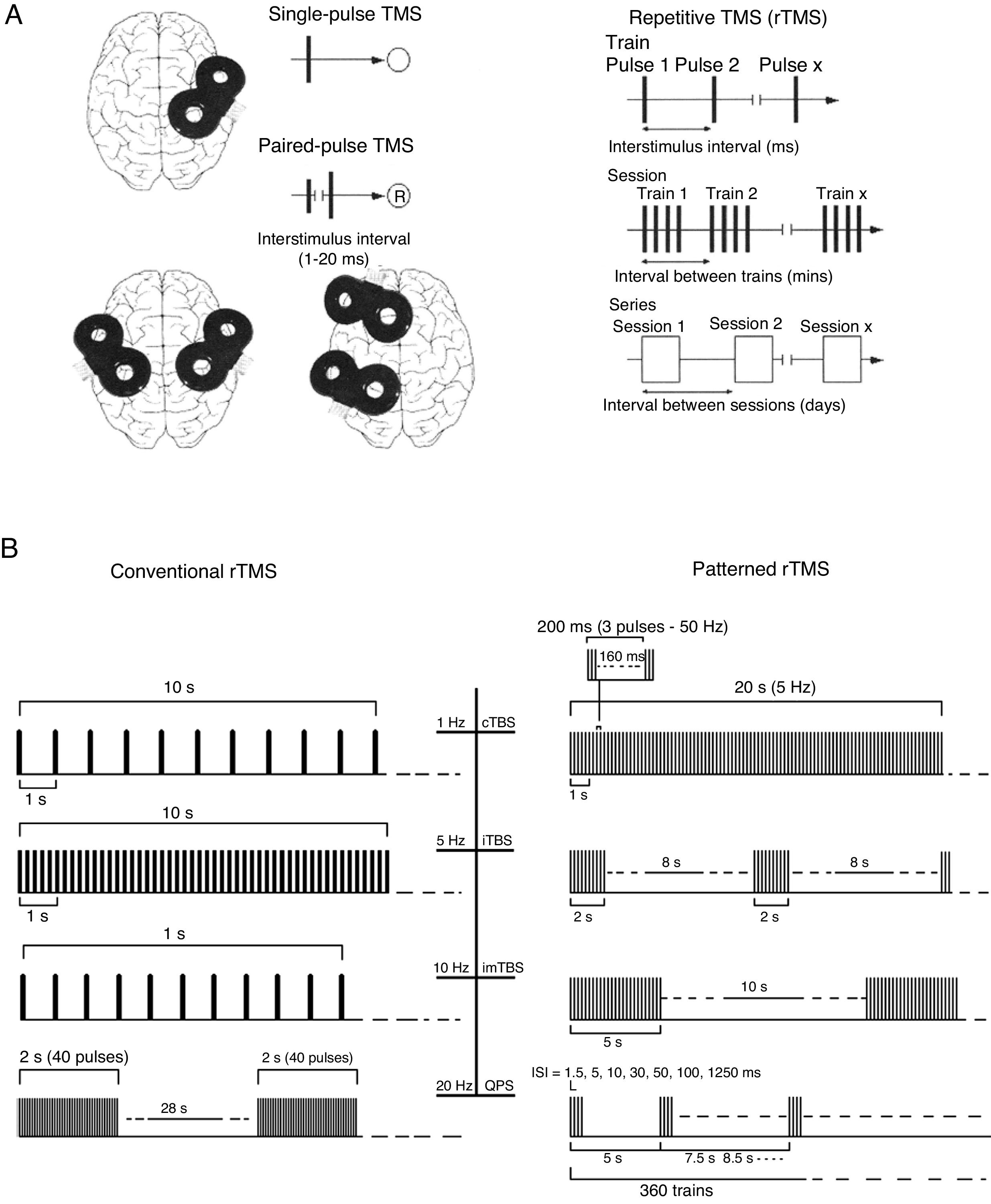
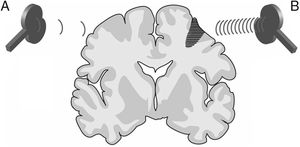
FundamentalsTMS is based on the principle of electromagnetic induction, formulated by Michael Faraday in 1831. According to this principle, an electric field produces a perpendicular magnetic field, and vice versa (Appendix 2). In TMS, a capacitor discharges an electric pulse through a copper wire coil, which is embedded in a plastic case. The coil is placed on the patient's head, generating a magnetic field perpendicular to the plane of the coil. This changing magnetic field induces an electric current in any nearby conductive material. When an electric pulse is sent through the coil, a magnetic field is generated through the patient's scalp and skull, without attenuation (decay is equivalent to the square of the distance). The most widely used stimulation coils are circular or figure-8 coils. Circular coils produce a wider electric field, stimulating both hemispheres simultaneously. Figure-8 coils, in contrast, focus on a more specific area (Fig. 1). These magnetic pulses selectively depolarise cortical neurons, located 1.5-2cm below the scalp. Electric pulses may either inhibit or stimulate neurons, modulating the energy produced by mitochondria and affecting electrical signal transmission and cell survival. The effect of stimulation depends on the shape, size, type, and position of the coil; the intensity of the magnetic field; and the frequency and duration of magnetic pulses (Fig. 2A and B)1–11:
- ∘
In single-pulse TMS, a single stimulus is applied to a specific brain region, depolarising cortical neurons and triggering a motor evoked potential (MEP) in a muscle area of the contralateral half of the body.
- ∘
Paired-pulse TMS generates 2 paired stimuli with identical or different intensities, separated by an interval of several milliseconds; these pulses are applied to one or several cortical areas. This technique enables the study of intra- and intercortical excitability, the integrity of interhemispheric connectivity, and transcallosal conduction time.
- ∘
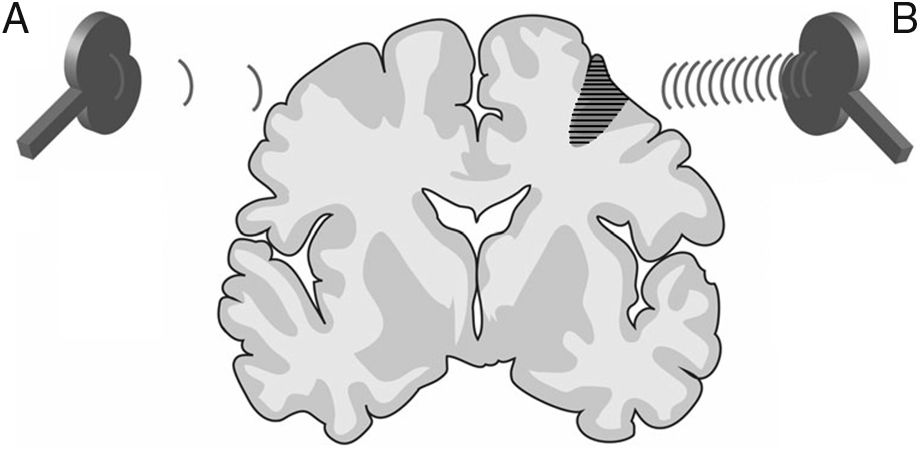
Repetitive TMS (rTMS) generates a train of low-frequency (≤ 1Hz; range, 0.5-1Hz) or high-frequency (≥ 5Hz; range, 5-20Hz) pulses of very short duration (milliseconds), inducing long-lasting changes in corticospinal excitability. These properties have made rTMS the most frequent therapeutic application of TMS (Fig. 3).
Figure 3.Representation of a coronal section of the human brain. Types of rTMS for promoting neuroplasticity. (A) Low-frequency rTMS (≤1Hz) of the unaffected hemisphere, in the area contralateral to a subcortical lesion (darker area with horizontal lines), reduces inhibition in the damaged hemisphere. (B) High-frequency rTMS (≥5Hz) of the affected hemisphere stimulates neural activity and promotes the reorganisation of interneuronal networks. Adapted from Edwardson et al.9
(0.03MB).
Distribution of electric fields induced by a circular coil (A) and a figure-8 coil (B). The circular coil has an inside turn diameter of 41.5mm, an outside turn diameter of 91.5mm (mean, 66.5mm), and 15 turns of copper wire. The figure-8 coil has an inside turn diameter of 56mm, an outside turn diameter of 90mm (mean, 73mm), and 9 turns of copper wire on each wing. The external shape of each device is shown with white dashed lines on the diagram of the induced fields. The amplitude of the electric field is calculated for a plane 20mm below a realistic model of the coil (di/dt=A/μs), where di/dt represents the derivative of current intensity with respect to time (rate of current change) and is expressed in amperes per microsecond (A/μs). If intensity increases from 0 to 40A in 2μs, we obtain the value di/dt=40A/2μs (simplified, 20A/μs). m: metres; V/m: volts/metre. Adapted from Pascual-Leone and Tormos-Muñoz.2
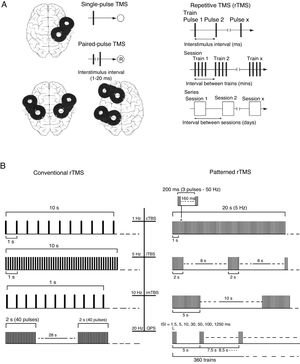
(A) Diagrams of the different types of TMS: single-pulse, paired-pulse in one or 2 different cortical areas, and repetitive TMS (low-frequency, ≤1Hz; high frequency, ≥5Hz). TMS: transcranial magnetic stimulation; rTMS: repetitive TMS; ®: response. Adapted from Pascual-Leone and Tormos-Muñoz.2
(B) Left: conventional rTMS (regularly repeated single pulses of TMS). From top to bottom: 10s of rTMS at 1Hz (first trace) and 5Hz (second trace); 1s of rTMS at 10Hz and a typical example of the therapeutic use of rTMS at 20Hz (2-second trains interleaved with 28-second intervals). Right: patterned rTMS (repeated application of short bursts of rTMS at a high inner frequency interleaved with short intervals of no stimulation). From top to bottom: 20s of continuous TBS (first trace); intermittent TBS (second trace); and intermediate TBS (third trace). The fourth trace shows quadri-pulse stimulation protocols.
cTBS: continuous TBS; imTBS: intermediate TBS; ISI: interstimulus interval; iTBS: intermittent TBS; QPS: quadri-pulse stimulation; TBS: theta burst stimulation. Adapted from Rossi et al.5
Special types of TMS include:
- 1.
Theta burst stimulation (TBS), a different type of rTMS characterised by the application of bursts of 3 pulses of TMS at ≥50Hz every 200ms. Two patterns are available, with a range of different possible protocols, although the majority aim to replicate the 2 original protocols designed by Huang et al.7:
- (a)
Intermittent (excitatory): 600 pulses administered in 20 trains of theta bursts of 2 seconds’ duration (3 pulses every 200ms per burst; each 2-second train is equivalent to 30 pulses) at 10-second intervals (i.e., 2 seconds of bursts followed by 8 seconds’ rest), resulting in long-term potentiation of neuroplasticity.
- (b)
Continuous (inhibitory): trains of theta bursts at 50-Hz frequency, administered at 5Hz for 20 seconds (300 pulses in total) or 40 seconds (600 pulses in total). This reduces cortical excitability, as demonstrated by a long-term reduction in MEP amplitude.
- 2.
Quadripulse stimulation, a type of rTMS administered in trains of 4 monophasic pulses at intervals of 1.5-1250ms, which either promotes or inhibits (for shorter or longer intervals, respectively) cortical activity, probably by acting on excitatory intracortical circuits.8
- 3.
Extremely low-frequency electromagnetic field (ELF-EMF) stimulation, defined as the continuous application of 0.7 milliteslas of stimulation at a frequency of 60Hz; this technique has been used experimentally in animal models, showing truly surprising results in models of Huntington disease, Parkinson's disease, and demyelinating processes.11
- (a)
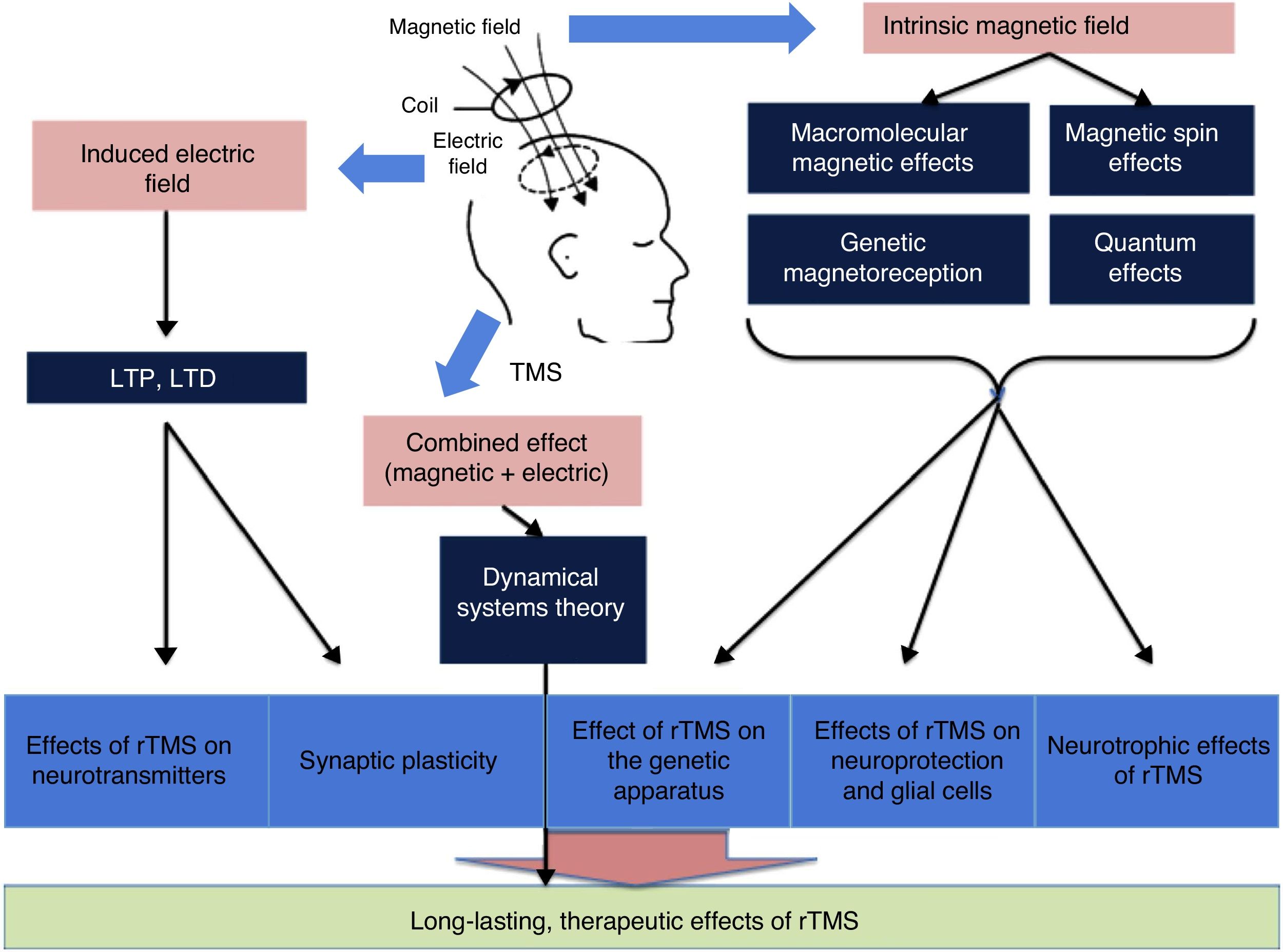
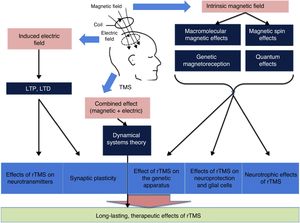
There is a growing body of evidence on the most intricate mechanisms (biochemical, molecular, and cellular) underlying the therapeutic effects of rTMS (Fig. 4).12 Most researchers believe that part of the lasting effect of rTMS is connected with 2 phenomena: long-term potentiation and long-term depression of synaptic plasticity.13 Both processes are caused by neuroplastic changes at the cellular and molecular level triggered by the induction of the electric field. Repetitive TMS promotes these mechanisms by activating N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (both of these glutamate receptors allow the entry of calcium into the neuron, triggering a cascade of signals and the activation of secondary messengers involved in neuroplasticity) and modulating the release of such neurotransmitters as dopamine and serotonin.1 In addition, due to macromolecular effects, magnetic spin, genetic magnetoreception, and quantum effects, the intrinsic magnetic field seems to constitute the basis of the influence of rTMS on the expression of the genes encoding BDNF and nerve growth factor, as well as inhibiting apoptosis and activating neurotrophic mechanisms (dendrite sprouting and growth) in glial cells through BDNF. Finally, according to the dynamical systems theory, the combination of separate electric and magnetic stimulation may explain the lasting therapeutic effects of rTMS.12,13
General overview of the influence of magnetic and electric fields. LTD: long-term depression; LTP: long-term potentiation; rTMS: repetitive TMS; TMS: transcranial magnetic stimulation. Adapted from Chervyakov et al.12
Repetitive TMS is known to have a positive impact on neuroplasticity; its neuroprotective effects may be beneficial for patients with a wide range of neuropsychiatric disorders, at least temporarily. This has led to research into the use of rTMS as an adjuvant treatment for a number of disorders. However, further studies are needed before rTMS can be recommended for treating these conditions with a higher level of evidence. The disorders potentially benefiting from rTMS include1,14–29:
- •
Psychiatric disorders: mood disorders (drug-resistant major depression, postpartum depression, dysthymia, mania, bipolar disorder, etc.), schizophrenia, anxiety disorders (obsessive-compulsive disorder, post-traumatic stress disorder, etc.), autism, attention-deficit/hyperactivity disorder, dysphemia, substance use disorders, monosymptomatic nocturnal enuresis, etc.
- •
Neurological disorders: stroke, drug-resistant epilepsy, Parkinson's disease, essential tremor, Huntington disease, Alzheimer disease, focal dystonia, head trauma, gait disorders, migraine with aura, trigeminal neuralgia, MS, amyotrophic lateral sclerosis, etc.
- •
Other conditions: chronic phantom limb pain, neuropathic pain, fibromyalgia, visceral pain, complex regional pain syndrome type I (formerly known as reflex sympathetic dystrophy), atypical facial pain, tinnitus, etc.
The main relative contraindications for TMS include pregnancy and age younger than 2 years. TMS is also contraindicated for patients with untreated epilepsy and those wearing electric devices (pacemakers, implantable cardioverter defibrillators, vagus nerve stimulators, deep brain stimulators, insulin pumps, etc.) or intracranial ferromagnetic devices and/or ferromagnetic elements located within 30cm of the treatment area (plates, screws, ventriculoperitoneal shunts, stents, jewellery, dental and cochlear implants, etc.). TMS is safe for patients with titanium implants, such as endovascular coils for brain aneurysms.5,30–32
Adverse effectsAlthough TMS is a safe technique,5,33 some patients may experience adverse effects, such as headache or neck pain. Pain is usually mild and transient; in the unlikely event it persists, it can be managed with conventional analgesics. However, a case has been reported of trigeminal autonomic cephalalgia secondary to administration of high-frequency rTMS, resolving after suspension of the stimulation and treatment with indomethacin at 50mg/day.34 The risk of epileptic seizures during rTMS is very low, and the technique has not been shown to increase the risk of seizures after the stimulation session in patients with known, well-controlled epilepsy; these patients show a similar risk of adverse reactions to healthy individuals and patients with other conditions.35 In patients with MS, TMS can increase the risk of epileptic seizures; it should therefore be avoided in patients with history of uncontrolled epilepsy and used with caution in patients with MS presenting cortical and/or extensive juxtacortical lesions.36,37
Therapeutic applications of repetitive transcranial magnetic stimulation in multiple sclerosisMS is a demyelinating disease of the central nervous system; it has no known treatment and currently constitutes the leading cause of non-traumatic neurological disability in young adults.38–41 Many patients present such motor disorders as fatigue, spasticity, and alterations to gait and manual dexterity, which lead to disability and poor quality of life. While some novel treatments have improved the management of MS, the drugs currently available are only partially effective in some patients (particularly those with progressive forms) and do not always modify the course of the disease; therefore, there continues to be a need for new treatment alternatives.36,40 TMS is a non-invasive technique in which a magnetic field is formed around a coil placed on the patient's scalp; this induces small electric currents in the brain region immediately below the coil, which have beneficial effects.42 The most widely used forms are rTMS, TBS, and ELF-EMF stimulation. Other potentially useful applications of this treatment in patients with MS aim to achieve neuromodulation for therapeutic purposes. Over the last 2 decades, numerous studies have evaluated the effect of rTMS in treating various neuropsychiatric conditions (see Global therapeutic applications section); in 2008, it was approved by the United States Food and Drug Administration for treatment of depression showing resistance to at least one antidepressant.14,40 A series of pivotal trials (Table 1) have demonstrated that TMS (most commonly rTMS) improves spasticity43–46; urinary dysfunction47; manual dexterity48,49; gait50; and working memory, brain activation, and functional connectivity in patients with MS.51 These studies are discussed in detail later in the review. Mori et al.45 conducted a study to assess whether intermittent TBS (iTBS) improves the effects of physiotherapy on disability in patients with MS; they report positive effects on fatigue, although these results were not replicated in another study using iTBS. Furthermore, these researchers did not specifically study the effects of conventional rTMS on fatigue in these patients; this issue should be addressed in future studies.52
Pivotal studies on the therapeutic application of repetitive TMS in patients with multiple sclerosis.
| Main clinical domain | Study | Type of stimulation | Sample size and clinical status | Study design | Stimulation protocol/intensity in each session | Duration | Region stimulated | Number of sessions | Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|
| Spasticity | Centonze et al.43 | Conventional rTMS | 19 RRMS (EDSS: 3.0-6.0)12: real rTMS7: sham rTMS | Parallel | 18 trains of 50 stimuli (900 stimuli) at 5Hz at 100% RMT (train duration, 10s; interval, 40s) | 15min | M1 (lower limb hot spot) contralateral to the more affected limb | 5 consecutive daily sessions per week for 2 weeks | Real rTMS>sham rTMS |
| Mori et al.44 | iTBS | 20 RRMS (EDSS: 3.0-6.0)10: real iTBS10: sham iTBS | Parallel | 10 bursts of 3 stimuli at 50Hz repeated at 5Hz every 10s (600 stimuli) at 80% AMT | 200s | M1 (lower limb hot spot) contralateral to the more affected limb | 5 consecutive daily sessions per week for 2 weeks | Real iTBS>sham iTBS | |
| Spasticity (modulation of M1 resting-state functional connectivity) | Boutière et al.46 | iTBS | 13 SPMS and 4 RRMS (EDSS: 4.0-7.0)9: real iTBS8: sham iTBS | Parallel | 20 bursts of 3 stimuli at 50Hz repeated at 5Hz every 10s (600 stimuli) at 80% AMT | 192s | M1 (lower limb hot spot) contralateral to the more affected limb | 13 consecutive daily sessions | Real iTBS>sham iTBS |
| Motor performance (primarily spasticity and fatigue) | Mori et al.45 | iTBS+ET | 30 RRMS (EDSS: 2.0-6.0)10: real iTBS+ET10: sham iTBS+ET10: real iTBS | Parallel | 10 bursts of 3 stimuli at 50Hz repeated at 5Hz every 10s (600 stimuli) at 80% AMT | 200s | M1 (lower limb hot spot) contralateral to the more affected limb | 5 consecutive daily sessions per week for 2 weeks | Real iTBS+ET>sham iTBS+ET or real iTBS only |
| LUT dysfunction | Centonze et al.47 | Conventional rTMS | 10 RRMS (EDSS: 3.0-5.5) | 20 trains of 50 stimuli (1000 stimuli) at 5Hz at 100% RMT (train duration, 10s; interval, 40s) | 16min | M1 (lower limb hot spot) contralateral to the more affected limb | 5 consecutive daily sessions per week for 2 weeks | LUT dysfunction improved with rTMS | |
| Gait | Burhan et al.50 | Conventional rTMS | 1 RRMS (EDSS not administered) | – | Burst of stimuli (number of bursts not specified) at 6Hz at 90% RMT (1200 stimuli) | 20min | Left DLPFC (point F3 in the international 10-20 EEG system) | 1 initial session; 3 days later, 3 consecutive daily sessions | 3 sessions of rTMS>1 session rTMS |
| Motor performance (manual dexterity) | Koch et al.48 | Conventional rTMS | 8 RRMS with cerebellar symptoms (EDSS not evaluated), 7 healthy controls | Crossover | 18 trains of 50 stimuli (900 stimuli) at 5Hz at 100% RMT (train duration, 10s; interval, 40s) | 15min | Upper limb area of the M1 region contralateral to the dominant upper limb | 2 consecutive daily sessions | Real rTMS>sham rTMS only in patients with MS |
| Elzamarany et al.49 | Conventional rTMS | 24 RRMS/SPMS with dysmetria6 RRMS+8 SPMS: 2 rTMS4 RRMS+6 SPMS: 1 sham rTMS+1 real rTMS (EDSS: 1.5-6.5) | Parallel | 18 trains of 50 stimuli (900 stimuli) at 5Hz at 100% RMT (train duration, 10s; interval, 10s). Divided into 3 series of 300 stimuli | 45min | M1 (hand hot spot) contralateral to the dominant hand | 2 consecutive daily sessions | Real rTMS>sham rTMS | |
| Cognitive performance (working memory) | Hulst et al.51 | Conventional rTMS | 13 RRMS+4 SPMS (EDSS: 1.5-4.5), 11 healthy controls | Crossover | 60 trains of 50 stimuli (3000 stimuli) at 10Hz at 110% RMT (train duration, 5s; interval, 25s) | 30min | Right DLPFC (peak voxel of activation, according to fMRI neuronavigation) | 1 session | Real rTMS>sham rTMS only in patients with MS |
AMT: active motor threshold; DLPFC: dorsolateral prefrontal cortex; EDSS: Expanded Disability Status Scale; ET: exercise therapy; fMRI: functional magnetic resonance imaging; iTBS: intermittent theta burst stimulation; LUT: lower urinary tract; MS: multiple sclerosis; RMT: resting motor threshold; RRMS: relapsing-remitting multiple sclerosis; rTMS: repetitive transcranial magnetic stimulation; SPMS: secondary progressive multiple sclerosis. Adapted from Iodice et al.39
Centonze et al.43 studied whether rTMS modifies spasticity in MS, testing high-frequency (5Hz) and low-frequency (1Hz) protocols and sham stimulation applied to the primary motor cortex (M1) in 19 patients diagnosed with relapsing-remitting MS (RRMS) according to the McDonald criteria,53 scoring between 3.0 and 6.0 on the Expanded Disability Status Scale (EDSS),54 and presenting spasticity predominantly or exclusively affecting one leg. Patients initially received a single session of high- or low-frequency rTMS to assess the effects of each treatment on the H/M amplitude ratio (a reliable neurophysiological marker of spinal cord excitability), H-reflex and M potential amplitudes, MEP amplitude and latency, and central motor conduction time. In a subsequent trial, daily sessions of rTMS were performed over a period of 2 weeks with the aim of inducing clinical improvements in lower limb spasticity, which was quantified neurophysiologically by measuring the H/M amplitude ratio and the H-reflex and M potential amplitudes, and clinically with the Modified Ashworth Scale (MAS).55 To summarise the findings, we may infer that a single session of 5-Hz rTMS of the M1 region contralateral to the affected limb inhibited the H-reflex (reducing activity in the spinal cord) and increased MEP amplitude, hence reducing also the H/M amplitude ratio and indirectly increasing corticospinal excitability (by increasing MEP amplitude); 1-Hz rTMS had the opposite effect. Individual sessions did not affect spasticity. A significant improvement in lower limb spasticity was observed with daily 5-Hz rTMS over a period of 2 weeks. This improvement persisted for at least 7 days after the treatment period ended. However, no effect was achieved with sham rTMS. Centonze et al.43 conclude that excitatory rTMS may constitute a therapeutic tool for improving spasticity in patients with MS and that the benefits observed may be promoted by lasting modulation of complex spinal circuits, such as the circuit modulated by the corticospinal tract in the presynaptic control of Ia sensory afferents modulating the stretch reflex. The authors do not rule out the possibility of transcallosal pathways also being involved in this modulation, or the activation of other sensory circuits during the induction of MEPs with rTMS, and underline the need for further studies to verify this hypothesis.
Intermittent theta burst stimulation reduces spasticity in multiple sclerosisIn another trial, Mori et al.44 studied whether iTBS can modulate lower limb spasticity in patients with MS. The study included 20 patients diagnosed with RRMS (McDonald criteria) and scoring 3.0-6.0 on the EDSS, with spasticity predominantly or exclusively affecting one leg, who were pseudo-randomised to receive daily sessions of either real or sham iTBS for 2 weeks. Spasticity was evaluated before and after treatment by measuring the soleus muscle H/M amplitude ratio and with the MAS. Patients receiving real iTBS presented a significant reduction in the H/M amplitude ratio after one week of treatment, and a reduction in MAS score after the second week; the reduction in the H/M amplitude ratio persisted for 2 weeks after the treatment was complete, and the reduction in MAS score persisted for one week. The authors conclude that iTBS is a safe, non-invasive, well tolerated, and feasible treatment, and constitutes a promising tool for treating spasticity in MS.44
Intermittent theta burst stimulation increases the effects of physiotherapy on spasticityGiven the benefit of exercise therapy (ET), and the fact that iTBS induces long-term changes in cortical excitability and can reduce spasticity in disabled patients with MS, Mori et al.45 studied whether the combination of both treatments may improve motor disability in these patients. The researchers conducted a double-blind, sham-controlled clinical trial of iTBS, recruiting 30 patients with RRMS (McDonald criteria) who scored between 2.0 and 6.0 on the EDSS and presented spasticity predominantly or exclusively affecting one leg. Patients were randomly allocated to receive one of 3 interventions: real iTBS+ET, sham iTBS+ET, or iTBS only. Before and after the 2-week treatment period, the researchers assessed spasticity (MAS and the 88-item MS Spasticity Scale), fatigue (Fatigue Severity Scale), independence in the basic activities of daily living (Barthel index), and health-related quality of life (54-item MS Quality of Life Scale). Real iTBS+ET reduced scores on the MAS, the 88-item MS Spasticity Scale, and the Fatigue Severity Scale; increases (representing improvement) were recorded in Barthel index and 54-item MS Quality of Life Scale (physical composite) scores. Intermittent TBS alone only reduced MAS scores, with patients receiving sham iTBS+ET showing no significant changes. The researchers conclude that iTBS+ET is a promising therapeutic approach for motor rehabilitation of patients with MS, as it can have a favourable impact on both negative (fatigue) and positive symptoms (spasticity).45
Intermittent theta burst stimulation reduces spasticity in multiple sclerosis by modulating resting-state functional connectivity of the primary motor cortexGiven the premise that iTBS of the M1 region generates a transient improvement in lower limb spasticity in patients with MS, Boutière et al.46 designed a study to assess whether iTBS-induced modulation of spasticity is promoted by functional reorganisation of the M1 regions ipsilateral and contralateral to stimulation. Patients were randomly allocated to receive real or sham iTBS for 5 weeks, and followed up with resting-state functional MRI (fMRI) at 3 time points. The study included a total of 17 patients (13 with secondary-progressive MS [SPMS] and 4 with RRMS, according to the 2010 revised McDonald criteria56) scoring between 4.0 and 7.0 on the EDSS and ≥2 on the MAS, who presented spasticity predominantly or exclusively affecting one lower limb, were not receiving corticosteroid treatment, had not presented any relapse in the 2 months prior to study inclusion, and had not received botulinum toxin in the 6 months prior to inclusion. All patients participated in a standardised functional rehabilitation programme, with sessions 5 days per week for 5 weeks. Spasticity was evaluated at 3 time points: immediately before the first session of treatment (W0), the day after the last iTBS session (W3), and at the end of the 5-week rehabilitation programme (W5). This study used a visual analogue scale to evaluate spasticity (scored from 0, no spasticity, to 100, worst possible spasticity), with patients rating their perceived degree of spasticity over the previous 24 hours. Raw score changes were calculated at each time point for each patient, by subtracting the baseline score (VASW0) from the scores at W3 and W5; changes were defined as ΔVASW3-W0 and ΔVASW5-W0. Each patient also underwent MRI studies at W0, W3, and W5. According to the hypothesis that iTBS of the M1 region induces functional changes in the motor cortices ipsilateral and contralateral to stimulation, the degree k was calculated for each of the paracentral lobules (anatomical automatic labelling atlas region corresponding to the real targeted area). The laterality index of the stimulated and non-stimulated paracentral lobules was calculated to evaluate the potential modulation of interhemispheric balance.46
At the end of the iTBS treatment period, patients from the group receiving real stimulation showed greater improvements in spasticity than those in the sham group, according to VAS scores (P=.026). Intermittent TBS had a significant effect on interhemispheric balance of the degree of connectivity between the stimulated and contralateral M1 regions between W0 and W3 (P=.008). Another noteworthy finding was that the changes in interhemispheric balance were correlated with the improvement in spasticity (rho=0.56, P=.015). Other findings were as follows: (1) the combination of repeated sessions of iTBS of the M1 region and physical rehabilitation has a greater effect on spasticity than rehabilitation alone; (2) the improvement in spasticity induced by iTBS is associated with transient, bilateral functional reorganisation of the homologous M1 areas; and (3) iTBS induces an imbalance in the degree of connectivity of the 2 primary motor cortices, in favour of the contralateral M1 region. In other words, the stimulated M1 region has fewer connections to other brain regions than does the non-stimulated contralateral M1 region. The study's main limitation is the small sample used. While there is a need for studies with larger samples to confirm these findings, the study suggests that iTBS is a promising method for managing spasticity in MS, and provides important evidence regarding the underlying cerebral mechanisms.46
Lower urinary tract dysfunctionCentonze et al.47 evaluated the effects of 5-Hz rTMS of the M1 region in 10 patients with RRMS (McDonald criteria) scoring 3.0-5.5 on the EDSS and presenting lower urinary tract symptoms involving either bladder filling or voiding. The researchers found that this treatment, administered 5 consecutive days per week for 2 weeks, improved the voiding phase but had no effect on the filling phase. This suggests that increasing corticospinal excitability may be useful for improving the contraction of the detrusor muscle and/or relaxation of the external urethral sphincter in patients with MS and bladder dysfunction. This is the only study to date to provide evidence supporting the hypothesis that rTMS may affect bladder activity in these patients. These findings suggest that the increase in corticospinal excitability after excitatory rTMS promotes detrusor muscle contraction and/or external urethral sphincter relaxation, rather than increasing sphincter activity.
Manual dexterityRepetitive transcranial magnetic stimulation of the M1 region improves manual dexterity in patients with multiple sclerosis and cerebellar symptomsKoch et al.48 studied the effects of 5-Hz rTMS of the left M1 region in 8 patients with RRMS (McDonald criteria) presenting cerebellar symptoms (dysmetria, dysarthria, intention tremor, balance and gait disorders, and adiadochokinesia) and 7 healthy controls; patients with cerebellar symptoms were selected on the basis of the hypothesis that cerebellar lesions cause impairment of manual dexterity.57 Manual dexterity was evaluated with the 9-hole pegboard task.58 The researchers found that 5-Hz rTMS significantly improved manual dexterity in these patients, with a significant reduction in the time taken to complete the task with the dominant (right) hand immediately after stimulation (P<.01) and 10 minutes later (P<.01), but not 20 minutes later (P>.1); no changes were observed in controls. The transient effect induced by rTMS was observed only in patients who presented dysmetria immediately after stimulation and 10 minutes later.48
As these patients did not present pyramidal tract dysfunction, the improvement observed in the cerebellar symptoms may be explained by modulation of cortico-pontine-cerebellar projections.59,60 It may also be the case that 5-Hz rTMS counteracts the reduction in cerebellar conduction secondary to demyelination, which probably contributes to the loss of manual dexterity. Therefore, administering this technique to the M1 region may be useful in treating cerebellar functional impairment in patients with MS. However, as the improvements observed were only transient, there is a need for further research with additional sessions of rTMS to verify whether persistent clinical benefits may be achieved.48
Repetitive transcranial magnetic stimulation of the M1 region improves manual dexterity in patients with RRMS and SPMSAddressing the premises that the motor cortex receives an excitatory input from the cerebellum, which is reduced in patients with cerebellar lesions, and that the cortical facilitation induced by rTMS can counteract the reduction in excitatory cerebellar drive to the motor cortex, Elzamarany et al.49 conducted a study to determine whether improvements in manual dexterity (measured with the 9-hole pegboard test and the EDSS cerebellar functional system score54) are observed immediately after and one month after high-frequency rTMS of the M1 region in patients with RRMS and SPMS. They recruited 24 patients with RRMS or SPMS (McDonald criteria) who presented ataxia and scored 1.5-6.5 on the EDSS, and 10 healthy controls. Patients were randomly allocated to receive 2 sessions of real 5-Hz rTMS on 2 consecutive days (group A) or one session of real 5-Hz rTMS followed by a session of sham rTMS the following day (group B). Healthy controls (group C) received 2 sessions of real 5-Hz rTMS on 2 consecutive days. Manual dexterity was measured with the 9-hole pegboard test.49
Patients in group A showed significantly better 9-hole pegboard test performance (P=.002) and EDSS cerebellar functional system score (P=.000), both immediately after the second session of rTMS and one month later. Patients with RRMS presented greater improvements than those with SPMS. Participants from groups B and C showed no improvement in either test.
The mechanism underlying the improvements observed in manual dexterity in this study may be explained by direct facilitation of the motor cortex, which is inhibited in patients with cerebellar lesions. A previous study reported increased post-excitatory inhibition after rTMS of the motor cortex in patients with cerebellar lesions61; this suggests that cerebellar lesions activate inhibitory cortical interneurons or disrupt a normally tonic cerebellar excitation of the motor cortex. However, further study is needed to verify whether this improvement varies according to the cause of the cerebellar lesions (e.g., hereditary, inflammatory, or vascular lesions). Additional studies should also compare the effectiveness of rTMS against that of other treatments including drugs and physiotherapy. Finally, the authors suggest using such neuroimaging techniques as diffusion spectrum imaging and fMRI to more accurately identify the mechanism underlying the improvements observed after rTMS.49
GaitGiven the evidence from studies reporting considerable impairment of gait velocity, cadence, and step length in patients with MS,54,62,63 Burhan et al.50 tested the effects of high-frequency (6Hz) rTMS of the prefrontal cortex on gait parameters in a 51-year-old white man with a 4-year history of RRMS and residual disabling symptoms consisting of upper and lower limb spastic weakness, diplopia, nystagmus, and some cognitive deficits (mainly affecting attention). An MRI study showed significant white matter involvement, including the left prefrontal and parietal regions, which are involved in attention. The patient initially underwent one session of rTMS, and 3 days later, 3 consecutive daily sessions. The international 10-20 EEG lead localisation system was used to locate the F3 point, which generally corresponds to the left dorsolateral prefrontal cortex (DLPFC).64 Data on gait parameters were recorded at 2 time points: (1) before (baseline 1) and immediately after the initial single session of rTMS; and (2) before (baseline 2) and immediately after the 3 consecutive daily sessions of rTMS. No statistically significant differences were observed in ambulation time or gait velocity before and after the single session of rTMS, whereas cadence did show a significant difference (t(2)=−4.99; P<.05). Statistically significant differences were observed before and after the 3 consecutive daily sessions of rTMS for ambulation time, (t(2)=8.32; P<.05), gait velocity (t(2)=−4.59; P<.05), and cadence (t(2)=−7.57; P<.05). Analysis of the data found that the variability in stride time (defined as the time between the first contact of 2 consecutive footfalls of the same foot) decreased after the initial single session of rTMS, from 5.02% to 4.6% of coefficient of variation. However, variability increased (from 4.64% to 5.34% of coefficient of variation) after the patient received the 3 consecutive daily sessions of rTMS.50
In this case report, modulation of left DLPFC excitability by high-frequency rTMS caused a significant change in gait parameters in a patient with MS, without adverse effects; this study is the first to report the benefits of excitatory rTMS of the left DLPFC on gait in patients with MS. The underlying mechanism probably involved an improvement in excitability in this region, which in turn influences the volitional aspect of gait. The prefrontal cortex is functionally connected to the caudate nucleus, and there is evidence that dopaminergic transmission in the latter structure increases as a result of prefrontal cortical stimulation with rTMS, which may explain this effect.65 While stride time variability decreased after one session of rTMS, it subsequently increased after the 3 consecutive daily sessions. This finding is of considerable importance due to its implications for the risk of falls: Montero-Odasso et al.66,67 report a direct correlation between stride time variability and risk of falls. This correlation may be associated with ambulation time or velocity, with greater speed involving a greater variability in stride time, and therefore greater fall risk. However, due to the short interval (3 days) between the initial single rTMS session and the 3 consecutive daily sessions, a new baseline situation was established, with longer ambulation time and lower gait velocity. The differences observed are difficult to interpret, and further research is needed on this subject. Furthermore, the treatment's long-term impact on gait could not be evaluated as the patient was not followed up.50
Working memory, cerebral activation, and functional connectivityHulst et al.51 designed the first study into the effects of high-frequency rTMS on cognitive performance in patients with MS, administering rTMS to the right DLPFC to evaluate the effects on working memory and task-related cerebral activation and connectivity (studied with fMRI). The study aimed to contribute information on the frequent presence of cognitive deficits, which affect up to 70% of patients with MS68 and for which no specific treatments are currently available. More specifically, problems with working memory are common,68,69 particularly in patients with high frontal lesion load.70,71 Functional MRI studies on working memory performance reveal frontal hyperactivation72–76 and increased frontal interhemispheric connectivity in patients with MS not presenting cognitive impairment, as compared to healthy controls; these findings are suggestive of functional reorganisation.
The researchers performed a randomised single-blind sham-controlled cross-over trial including a sample of 17 patients with clinically definite MS (13 with RRMS and 4 with SPMS, according to the 2005 revised McDonald criteria77) and 11 healthy controls. All participants underwent 3 experimental sessions (baseline, real rTMS, and sham rTMS) in an MRI scanner, with each session including an N-back task (3 task loads [N1, N2, N3] and a control condition [N0]). Repetitive TMS (10Hz) was administered to the right DLPFC prior to imaging. Subsequently, changes in whole-brain functional activation and functional connection with the right DLPFC were analysed. The stimulation site was defined as the peak voxel of activation of the right DLPFC (i.e., when all voxels were activated significantly more in all N-back task loads [1-back, 2-back, and 3-back combined], compared to the control condition; N123>N0 contrast). The N-back task for visuospatial working memory comprised 3 increasing task loads (N1, N2, and N3) and a control condition (N0).78 In each trial (2.8 seconds’ duration), a yellow dot would appear randomly on the computer monitor, at the left, right, top, or bottom of a grey diamond with 4 blue circles. The location on the diamond corresponded to 4 similar locations on an MRI-compatible response box, comprising 2 controllers with 2 buttons each (for the thumb and index finger of each hand), and data were collected on the computer used to present the stimuli. Under condition N0, participants were asked to respond immediately by pressing the corresponding button. In conditions N1, N2, and N3, patients had to indicate the position of the yellow dot one, 2, or 3 trials previously and simultaneously remember new locations as the task progressed. The main behavioural outcome variables were the total number of correct responses and the reaction time for each task load.
All participants underwent thorough neuropsychological tests specifically designed for research into working memory, which was evaluated with the digit span and letter-number sequencing tasks from the Wechsler Adult Intelligence Scale.79,80 Brain connectivity was calculated using a generalised psychophysiological interaction (GPPI) model to identify voxels (in the whole brain) associated with activation in a seed region in a specific psychological context; other task variables were controlled.81 The seed region was defined individually for each participant by calculating a 6-mm sphere around the baseline peak voxel of task-related activation in the right DLPFC in the standard space. The time series within the seed region was used as the physiological regressor. The psychological regressors used were the blood oxygen level-dependent responses of the 3N-back task contrasts (N1>N0, N2>N0, and N3>N0); therefore, 3 GPPI analyses were performed per session. Subsequently, all individual right DLPFC seed regions were combined in one common mask to perform the group analysis. The common mask was used to calculate differences in connectivity between groups and between sessions. In the initial analysis, no significant differences were observed between patients and controls for age, sex, dominant hand, premorbid intelligence quotient, or level of schooling. Repetitive TMS parameters (e.g., the rTMS intensities applied based on the resting motor threshold, and the sequencing of real and sham rTMS) also showed no significant differences between patients and controls. Slight differences were observed for measures of anxiety and depression. Fourteen of the 17 patients had subclinical scores on the Hospital Anxiety and Depression Scale (with the cut-off point for subclinical status established at ≤10). In the structural MRI study, no differences were observed between patients and controls for normalised whole brain volume, grey matter volume, or white matter volume. The rest of the study findings may be summarised as follows:
- ∘
Regarding task-related activation, a consistent task effect was found for the frontoparietal network bilaterally in both groups and for all task load conditions. At baseline, patients with MS showed greater task-related activation than controls in the frontal region (left DLPFC, N2>N0) and the right temporal pole (N3>N0) (data not shown in article) during the working memory task, suggesting a process of functional reorganisation that disappeared after the application of rTMS. This is consistent with the hypothesis that greater activation is a form of compensation for potential (subclinical) cognitive problems.71–73 At baseline, no intergroup differences were detected in N1>N0; furthermore, no differences were detected between groups in connectivity between the right DLPFC (stimulated area) and other brain areas. Patients with MS showed greater activation than controls in the left inferior parietal lobule during N1>N0. Frontal activation showed no differences between groups. After sham rTMS, patients with MS showed greater activation than controls in the left superior frontal gyrus and parietal regions during N2>N0 (data not shown in article).
- ∘
Accuracy in the N-back task only showed a significant improvement in patients with MS after real rTMS as compared to baseline for task loads N2 and N3 (P=.029 and P=.015, respectively). However, their accuracy after real rTMS was not significantly different from their accuracy after sham rTMS (P=.077). The authors suggest that this difference may have been statistically significant had the study used multiple stimulation sessions and a larger sample.5 However, the fact that the improvements recorded in accuracy and reaction time in the working memory task after real rTMS was only seen in patients with MS indirectly supports the theory that any alteration in brain activation from the healthy situation is unfavourable to subsequent functioning if MS later develops.82,83
- ∘
In patients with MS, increases were observed after real rTMS in task-related functional connectivity (N1>N0) between the right DLPFC and the right caudate nucleus, the right putamen, the left anterior cingulate gyrus, both paracingulate gyri, and the left frontal pole. Post hoc, the researchers studied the GPPI findings in greater depth, focusing on the significant differences observed in functional connectivity following real rTMS as compared to sham stimulation, in other words, within the cluster-corrected difference mask (real vs sham stimulation, contrast N1>N0). Connectivity parameter estimates were extracted for N1>N0, N2>N0, and N3>N0 for each individual fMRI analysis model. Changes induced by rTMS were defined as those differences observed vs baseline after administration of real rTMS but not after sham stimulation. Real and sham conditions were also compared. Finally, the post hoc analysis found that estimates of right DLPFC connectivity parameters were higher for greater task loads. Additionally, connectivity was significantly greater after real rTMS than after sham stimulation for all task loads (N1>N0: P<.001; N2>N0: P=.001; N3>N0: P=.003). As no differences in connectivity were observed in the control group, the post hoc analysis was only performed in patients with MS. The authors speculate that these changes may be associated with the normalisation of frontal activation, which tends to resemble that of healthy controls at baseline. In other words, the changes observed after real rTMS in the frontal region (reduced cerebral activation and increased functional connectivity) reflect an improvement in the efficiency of frontal processing during a working memory task. While these findings should be interpreted with caution, they may represent a change in neural network efficiency induced by rTMS in patients with MS, with brain function closer resembling that of healthy controls. Repetitive TMS is a potentially relevant technique in the cognitive rehabilitation of patients with MS.
While the preliminary evidence on the use of rTMS to treat MS seems promising, little is understood about the underlying mechanisms and pathways responsible for its therapeutic effect.40 Dr Túnez's research group recently published the results of several studies using the EAE model in rats, concluding that the model induces motor impairment.84–87 In addition to clinical changes, the model demonstrates the importance of oxidative stress and the possible involvement of the microbiota. The authors also observed neuroinflammation and apoptotic and necrotic cell death in the striatum of rats with EAE. ELF-EMF stimulation may attenuate motor symptoms, oxidative stress, and brain injury, due to the technique's ability to potentiate the antioxidant system via the Nrf2 pathway, which is closely involved in protection against 3-NP-induced neurotoxicity.11,86 Given these findings, ELF-EMF may be of considerable therapeutic interest in the rat EAE model. These researchers are currently exploring this hypothesis, reporting promising results for motor impairment in EAE, which may support the future implementation of ELF-EMF stimulation as a treatment for patients with MS.40 In their last article, the study group compared the effect of ELF-EMF stimulation to the effects of natalizumab, dimethyl fumarate, and dexamethasone on clinical scores and oxidative stress induced by a single dose of myelin oligodendrocyte glycoprotein administered to the tails of Dark Agouti rats. Both ELF-EMF stimulation and the pharmacological treatments attenuated the changes caused by the myelin oligodendrocyte protein, although the effect was more significant with the former treatment. These results further support the antioxidant and neuroprotective effects of TMS, which showed greater activity than the pharmacological treatments used.87
ConclusionsThe evidence from studies on TMS demonstrates this cutting-edge technique's potential as a reliable, safe, and effective treatment for some symptoms of MS. Application of excitatory electromagnetic pulses to the affected hemisphere enables the possibility of optimising the brain's functional activity, including the transmission of nerve impulses through the demyelinated corticospinal tract. Various studies into the use of TMS in patients with MS have demonstrated that the treatment significantly improves spasticity (rTMS and iTBS), fatigue (iTBS), lower urinary tract dysfunction (rTMS), manual dexterity (rTMS), gait alterations (rTMS), and cognitive disorders involving working memory (rTMS), although the absence of controlled trials replicating the results precludes establishing the exact level of evidence. These benefits are probably explained by the technique's capacity to promote regeneration of the brain through neuroplasticity. Túnez's study group report very interesting results on the use of ELF-EMF stimulation in a rat EAE model of MS (a valid model of the disease). The treatment was effective in attenuating motor impairment, decreasing astrocyte proliferation in response to the autoimmune attack, increasing brain cell density, and decreasing the number of pyknotic nuclei.85 It also decreases the level of oxidative stress in EAE, with rats showing optimisation of motor skills secondary to TMS, and improvements in oxidative and cell damage and in cell density.86 Furthermore, these researchers report greater antioxidant and neuroprotective activity than with pharmacological treatments.87
Finally, specific guidelines should be developed for the use of TMS as a supplementary treatment for MS and the identification of candidates for the treatment; this would guarantee the use of proper procedures and a favourable risk-benefit ratio. However, the most suitable treatment duration, time of intervention, and treatment protocol are still to be determined, and the main application of the treatment is in experimental studies. In the coming years, further well-designed prospective studies including larger samples and longer follow-up periods will surely provide a higher level of evidence to recommend the use of TMS as an adjuvant treatment alongside novel and existing treatments. In the foreseeable future, studies will probably be conducted based on the application of conventional rTMS to treat such disabling symptoms as fatigue. A fundamental issue to be resolved in the future is the great variability in the effectiveness of rTMS in different patients.
FundingThis study received no public or private funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: León Ruiz M, Sospedra M, Arce Arce S, Tejeiro-Martínez J, Benito-León J. Evidencias actuales sobre las potenciales aplicaciones terapéuticas de la estimulación magnética transcraneal en la esclerosis múltiple: Revisión sistemática de la literatura. Neurología. 2022;37:199–215.