our study aims to identify the clinical and epidemiological characteristics of viral meningitis in our environment and observe the differences with age.
Materials and methodsRetrospective study of viral meningitis that required admission to our hospital between 2000 and 2008. We compare characteristics between groups of children (under 15 years) and adults (15 years or older).
ResultsThe viral meningitis prevalent in males is higher during the summer months and the agent most involved is enterovirus. Children are seen in the hospital with shorter time of onset and their average stay is less. In children, the analytical data show greater systemic disorder, while in adults the cerebrospinal fluid anomalies are more important.
ConclusionsThe viral meningitis in our environment is more common in males and in summer months. The clinical presentation and prodrome are similar in children and adults, although the average hospital stay is less in children of this age probably because the clinical outcome is shorter. The analytical data show that children have a higher systemic inflammation but lower CSF level, probably because lumbar puncture is performed earlier than in adults. Enteroviruses are common pathogens in both children and adults.
nuestro estudio pretende identificar las características clínicas y epidemiológicas de las meningitis víricas en nuestro medio, así como ver las diferencias existentes con la edad.
Material y métodosestudio retrospectivo de las meningitis víricas que han precisado ingreso en nuestro hospital entre los años 2000 y 2008. Se comparan las características entre los grupos de niños (menores de 15 años) y adultos (15 años o mayores).
Resultadoslas meningitis víricas predominan en varones, aumentan durante los meses de verano y el germen más implicado es el enterovirus. Los niños acuden al hospital con menor tiempo de evolución y su estancia media es menor. En la analítica los niños presentan datos de mayor alteración sistémica, mientras que en los adultos las alteraciones a nivel del líquido cefalorraquídeo son más importantes.
Conclusioneslas meningitis víricas en nuestro medio son más frecuentes en varones y en los meses de verano. La clínica de presentación y los pródromos son similares en niños y adultos, aunque la estancia media es menor en niños probablemente porque en estas edades la clínica tenga una evolución más recortada. Los datos analíticos reflejan que los niños presentan una mayor inflamación sistémica, pero menor a nivel del LCR probablemente porque la punción lumbar se lleve a cabo más precozmente que en adultos. Los enterovirus son los patógenos más frecuentes tanto en niños y adultos.
The analysis of cerebrospinal fluid (CSF) confirms the clinical suspicion of meningitis and orients us to its aetiology. Some authors believe that viral meningitis normally has less than 1000leukocytes per ml, is predominantly lymphocytic, CSF protein concentration is generally less than 130mg/dl and glycorrhachia is normal with a glycorrhachia/glycaemia ratio higher than 50%.1
Enteroviruses are the main pathogens involved in viral meningitis (85–90%).2 Risk factors for meningitis caused by these virus are young age, poor hygiene practices (such as lack of hand-washing) and contact (such as that from staff in schools and nurseries).3,4 Other viruses that are frequently involved are herpes simplex type 2 and the varicella-zoster virus.5 Viral meningitis epidemics caused by enteroviruses have been reported in the summer.6 Therefore, enteroviruses are the main cause of viral meningitis with epidemic and endemic patterns. Its incidence rate is under-diagnosed because it presents mild symptoms in a great number of cases and people do not go to hospital.7 In temperate climates such as that of Spain, infections caused by enteroviruses are more common during the summer and autumn months and the transmission mechanism is faecal-oral.
Other viruses that years ago produced a great many symptoms of meningitis, such as mumps, have been radically reduced due to effective vaccines coming on the market. However, they are reappearing in immigrant patients who have not been vaccinated.8
Our study goal was to identify the clinical and epidemiological characteristics of viral meningitis in our environment and observe the differences with age. The knowledge of differences that exist between children and adults could have diagnostic and prognostic implications according to the analytical data found. It would also allow us to increase the profitability of the aetiological search tests.
Materials and methodsA retrospective and descriptive study that included the patients admitted to the Hospital Complex of Toledo between 2000 and 2008 who were diagnosed with viral meningitis.
The cases were collected from the MDS (minimum data set) database of our hospital cases coded as DRG (diagnosis related groups) No. 21: Viral meningitis (DRG-21). Viral meningitis cases with primary brain involvement, together with meningitis in immunocompromised patients, were excluded.
The following parameters were collected: gender, age, sex, month and year of hospital admission, mean stay, days of symptom evolution until admission, existence of prodromal symptoms (arthralgias, myalgias, malaise and nausea) and possible death. The following analytical data were also collected: blood leukocytes, percentage of neutrophils in the blood, protein concentration, glycorrhachia, glycorrhachia/glycaemia percentage, CSF leukocytes and CSF lymphocyte percentage. Finally, data were gathered on the aetiological study of the virus by carrying out polymerase chain reaction studies for viruses in the herpes family and enteroviruses in CSF, and serological ones for the parotitis virus in the blood.
The patients were divided into two age groups: children who were under the age of 15 years and adults who were 15 years or older. This age division was used because it is the age used at our hospital to decide whether to admit to Paediatrics or to admit to Neurology or Internal Medicine.
With regards to meningitis, the symptoms were divided into various groups:
- 1.
Have headache only.
- 2.
Have headache and fever.
- 3.
Have headache, fever and vomiting.
The statistical package used was SPSS version 15.0. The data description was expressed using means and standard deviations, with a statistically significant difference being considered as P<0.05.
Variable normality was verified using the Kolmogorov–Smirnov test. We used Student's t-test to compare means, or the non-parametric test for independent samples if the variable did not follow normality. In the correlation between the different analytical and demographic variables, we used the Pearson or Spearman test, according to their normality or not.
The different demographic and analytical variables in subjects with viral meningitis were compared between two age groups: children and adults.
ResultsDuring the 9 years analysed, 136 patients were identified on the database with viral meningitis. The average age was 22.01 years old and there was a male predominance of 69.8%. There were no deaths due to viral meningitis.
The cases of viral meningitis in children <15 years of age were 46 (33.8%), while there were 90 patients over that age (66.1%). The mean age among children was 5.9 years old, while among adults it was 30.2 years of age. In both groups, the male predominance continued – children 76.0% and adults 66.6% –; the differences found between the groups were not statistically significant. The mean stay was clearly less in the children's group (3.67 days) compared to the adults (6.59 days), with some clearly significant differences (P<0.000).
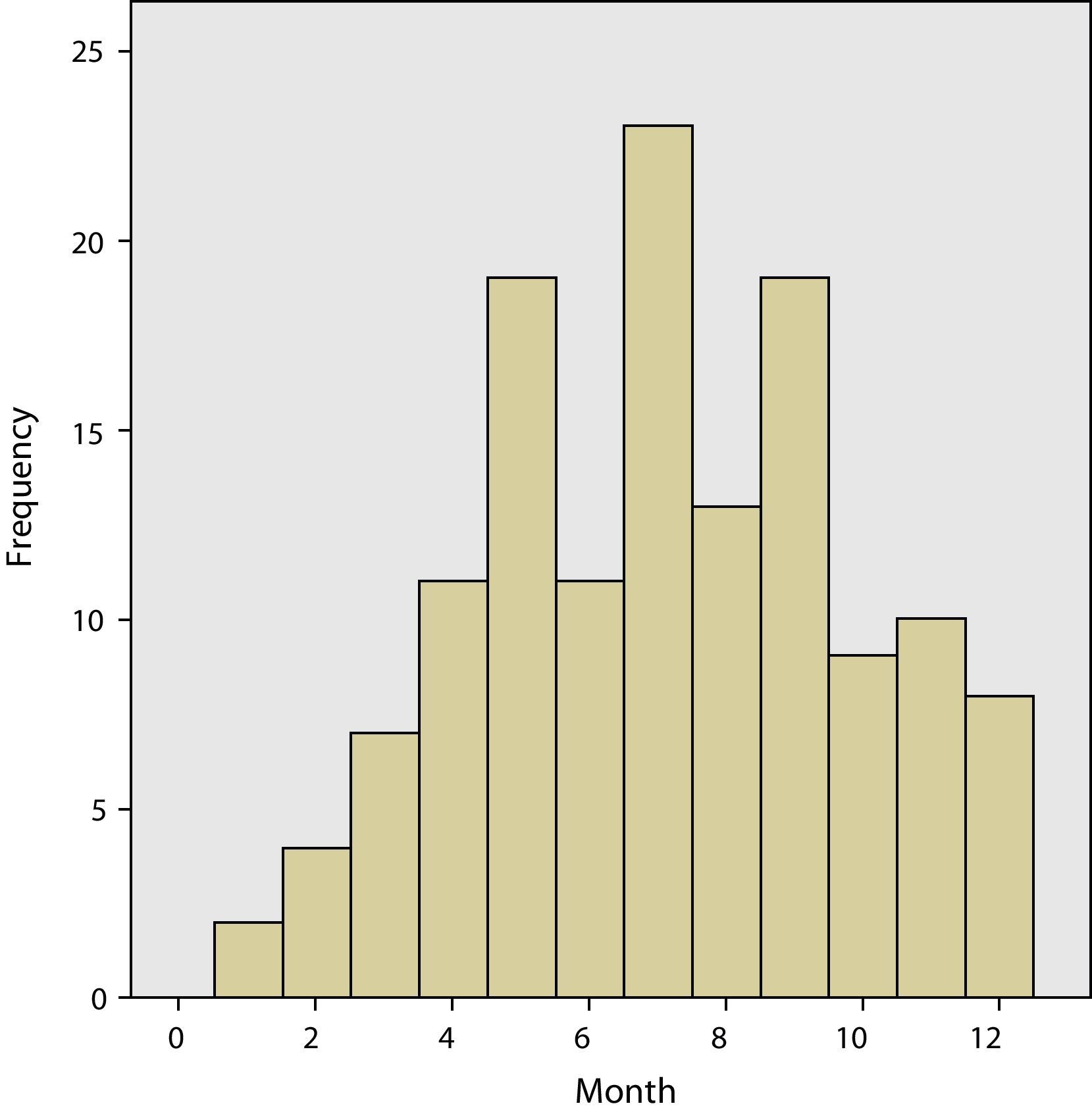
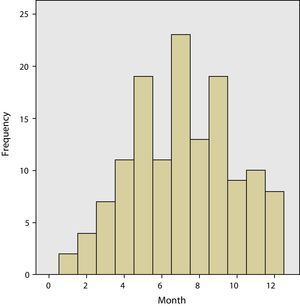
The grouping of the meningitis cases by months allowed us to see the greater incidence rate in the summer months compared to the winter months (Fig. 1). The incidence rate between children and adults was not analysed separately. Similarly, there were significant differences in the days of parameter evolution of meningeal symptoms before admittance, children 1.83 days and adults, 2.93 (P<0.0). The presence of prodromes before meningitis was also greater in adults (18.8%) than in children (13.0%), but there were no statistically significant differences. The clinical presentation was similar in children and adults. In both groups, the majority presented headache, fever and vomiting, with 54.3% in children and 52.2% in adults, with no significant differences found. The isolated presence of headache without fever appeared in only one adult patient.
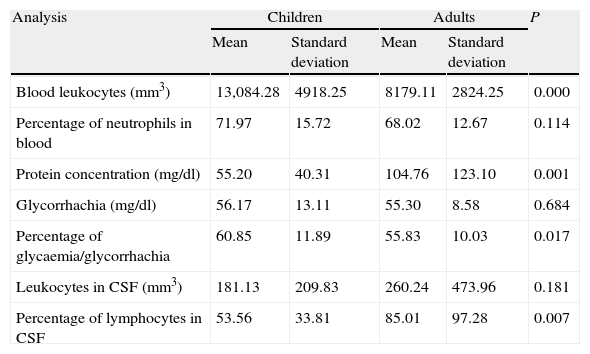
Comparison of means in the analytical variables between adults and children showed that there are greater numbers of blood leukocytes, as well as a higher glycorrhachia/glycaemia ratio, in children. However, protein concentration and percentage of lymphocytes in the CSF are less, as seen in Table 1.
The means, standard deviations and statistical significance between the different analytic variables for children compared to those of adults are shown.
| Analysis | Children | Adults | P | ||
| Mean | Standard deviation | Mean | Standard deviation | ||
| Blood leukocytes (mm3) | 13,084.28 | 4918.25 | 8179.11 | 2824.25 | 0.000 |
| Percentage of neutrophils in blood | 71.97 | 15.72 | 68.02 | 12.67 | 0.114 |
| Protein concentration (mg/dl) | 55.20 | 40.31 | 104.76 | 123.10 | 0.001 |
| Glycorrhachia (mg/dl) | 56.17 | 13.11 | 55.30 | 8.58 | 0.684 |
| Percentage of glycaemia/glycorrhachia | 60.85 | 11.89 | 55.83 | 10.03 | 0.017 |
| Leukocytes in CSF (mm3) | 181.13 | 209.83 | 260.24 | 473.96 | 0.181 |
| Percentage of lymphocytes in CSF | 53.56 | 33.81 | 85.01 | 97.28 | 0.007 |
If we focus on the analytical changes considered as typical in viral meningitis, we find the following results.
Protein concentration varied from 14 to 1185mg/dl. The levels of protein concentration considered as normal (<40mg/dl) appeared in 20 children and 8 adults (43.4% and 8.8%, respectively). Therefore, children present a protein concentration within the normal range to a greater degree.
The glycorrhachia/glycaemia ratio ranges from 34.7% to 94.2%. Considering a percentage of less than 50% as unusual in viral meningitis, we see that 6 children and 27 adults presented this abnormality (corresponding to 13% and 30%, respectively). Therefore, a greater percentage of adults present this reduced ratio.
The leukocytes in the CSF varied between 20 and 4320mm3. Considering the presence of more than 1000 leukocytes per mm3 in the CSF as atypical, we found it in 1 child and 2 adults (2.1% and 2.2%, respectively). Lastly, the percentage of lymphocytes in the CSF ranged from 0% to 100%. Taking less than 50% as abnormal, we found that 22 children and 18 adults presented it, which means 47.8% and 20%, respectively; that is, that children have a greater percentage of viral meningitis cases with a predominance of polymorphonuclear cells.
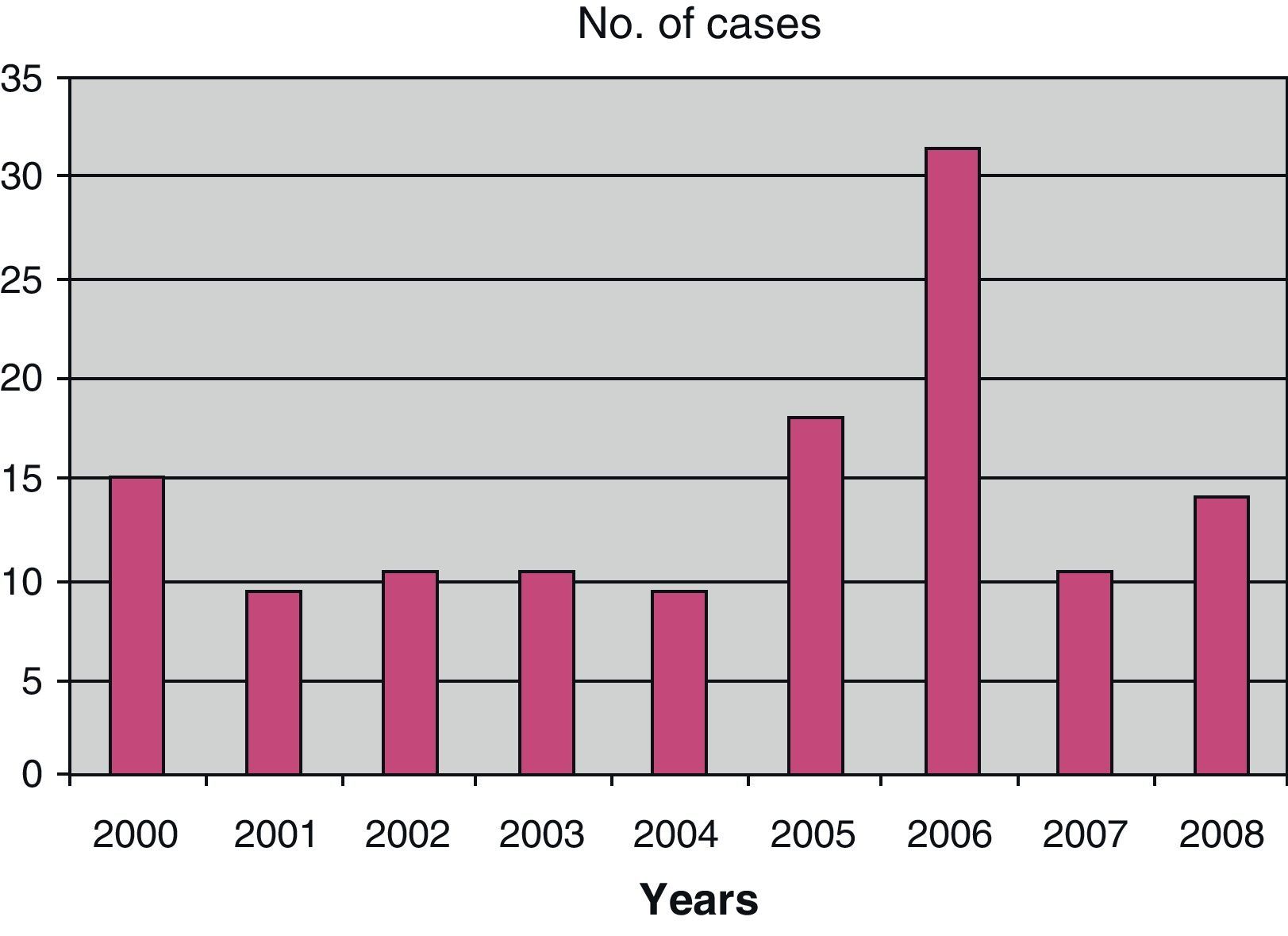
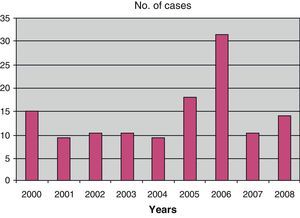
With respect to the distribution of viral meningitis cases over the years, we see that there were only 10 cases in 2001 and 2004, while the greatest number of cases was in 2006, with 33 (Fig. 2).
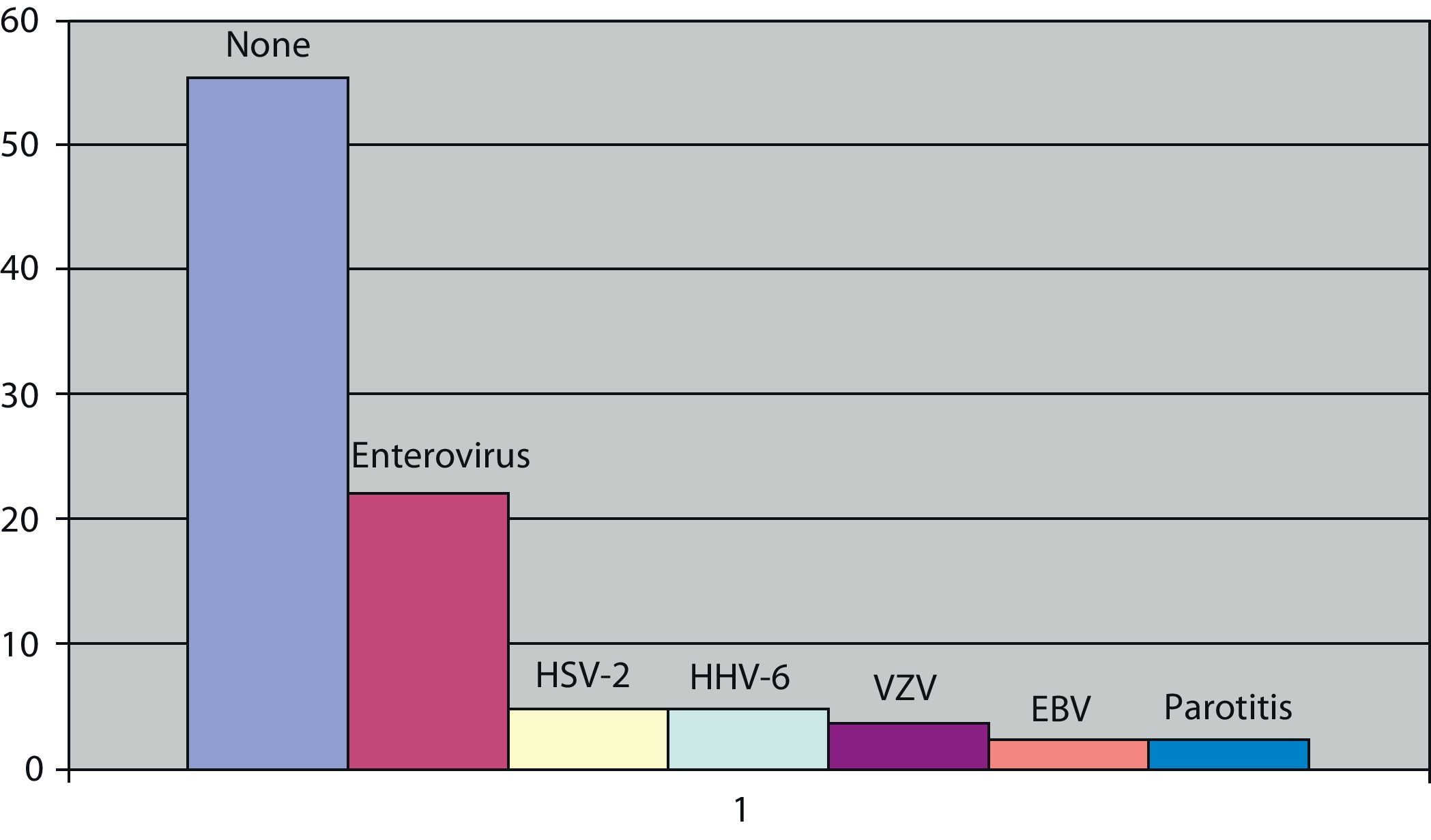
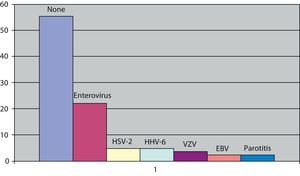
The causal germ in viral meningitis was analysed in 81 subjects (59.5% of cases). It was negative in 55.5% of the cases studied. The viruses most frequently identified were enteroviruses with 18 cases (22.2%) of those studied, followed by the herpes simplex type 2 virus and the human herpesvirus 6 with 4 cases each (4.9%), the chicken pox virus with 3 cases (3.7%) and the Epstein–Barr virus and mumps, which had 2 subjects in each (2.4%) (Fig. 3). With respect to the differences between children and adults, there were no differences in those with enteroviruses, which were still the most frequent in both groups. We could not analyse the data on the remaining viruses, due to their scarce number; however, the 2 cases of mumps and the 4 cases of human herpesvirus 6 were in children, while the herpes simplex type 2 was found in adults.
DiscussionThere is a clear male predominance in viral meningitis. The greater number of males is probably related to the predisposition of the enteroviruses in affecting the male gender, as has been demonstrated in other studies.5 This predominance is maintained in adult ages, which would explain why the enteroviruses are also the most frequently involved viruses at these ages, as we demonstrated in our study.
The mean hospital stay for children is less than that of adults, which implies that the clinical course in younger ages is more benign, with the symptoms being resolved quicker than in adults. However, other types of causes that could contradict this statement, such as the burden of care at the time of discharge by the various specialists who treat adults, have not been studied.
The incidence of viral meningitis is greater during the summer months than in winter, which could be explained by the predisposition that the enteroviruses have for producing meningitis during those dates. We have also seen that the incidence rate varies widely from one year to the next. More specific studies have shown that epidemics are produced by enteroviruses.9
In children, despite presenting a clinical presentation and prodromes similar to those of adults, the days of evolution until hospital admittance are fewer. This is probably because the symptoms in children are of more concern and medical services are consulted sooner.
Our data analyses show that children present a greater number of blood leukocytes and a higher glycorrhachia/glycaemia ratio in comparison to adults, while protein concentration and percentage of lymphocytes in the CSF are lower. This implies that there is a greater systemic response in children, while the inflammatory response is less on a CSF level. The way children react to meningeal infection could be different, although differences could also be explained because CSF analysis is carried out at an earlier stage, as previously mentioned, given that children normally go to the emergency department with a much shorter evolution time.
In our study, the appearance of atypical data for viral meningitis varies according to age group. In a recent study, the differentiation between viral and bacterial meningitis was established according to the following parameters: blood leukocytes >15,000mm−3, leukocytes in CSF >1800mm−3, percentage of neutrophils >80%, protein concentration >2.3g/l in adults and 1.2g/l in children and glycorrhachia/glycaemia ratio <0.33.10 That study agrees with ours, although indirectly, in that children present a lower protein concentration.
The lymphocyte percentage in CSF is less in children and is related to the duration of the symptoms before carrying out a lumbar puncture, because (as we already know) there can be a predominance of polymorphonuclear cells during the first hours of viral meningitis. Other authors have described similar data with a percentage of lymphocytes greater than 50% in those with a clinical presentation of more than 48h of evolution.11
The causal agent was not found in more than 50% of cases studied looking for the aetiology of viral meningitis. In other studies, this figure is even greater.5 However, the low percentage of enteroviruses in our study compared to others2,12 could be explained because a systematic study of this virus and the accompanying clinical presentation (parotid swelling, rash, elevated transaminases or presence of activated lymphocytes) was not undertaken, which led us to look for a virus that is different to the enterovirus. The use of immunological techniques would help in the diagnosis of viral processes such as mumps. We found that enteroviruses were the most frequent pathogens, not only in adults but also in children. The herpes simplex virus type 2 was more frequent in adults, as has been seen in other studies with a greater number of cases. Children seem to have a predominance of the parotitis virus and human herpesvirus 6, although the reduced number of our sample did not allow us to perform complex statistical analysis.
As has been demonstrated in recent studies, the presence of an on-call neurologist gives a better quality of care not only to patients that come from the emergency department13 but to those admitted from other departments.14 However, this quality may sometimes not be seen by the patients themselves.15
We should also be on the look-out for other new imported diseases16 that cause viral meningitis symptoms. This is due to the increase of the immigrant population in endemic areas, and we must develop diagnostic techniques to identify these viruses.17
Our study presents several limitations. To start with, its retrospective character has meant that the aetiology was not studied in all cases and some parameters were not collected, which would have given us more information as well as allowing us to perform more extensive analytical studies. In addition, although nearly all the adult patients were admitted to neurology, a small percentage was admitted to geriatrics and internal medicine.
To conclude, we could say that the viral meningitis in our environment is more frequent in males and during the summer months. The clinical presentation and prodromes are similar in children and adults, although the mean hospital stay is shorter in children, probably because evolution is much shorter at these ages. The analytical data reflect that children present greater systemic inflammation, but less at CSF level, probably because the lumbar puncture is carried out earlier than in adults. Enteroviruses are the most frequent pathogens, both in children and adults; we were not able to extract more conclusions from the rest of the viruses due to their scarce percentage.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Jiménez Caballero PE, et al. Análisis descriptivo de las meningitis víricas en nuestro hospital. Características diferenciales entre niños y adultos. Neurología. 2011; 26: 468–73.