Parkinson's disease is a neurodegenerative disorder characterised by balance problems, muscle rigidity, and slow movement due to low dopamine levels and loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The endocannabinoid system is known to modulate the nigrostriatal pathway through endogenous ligands such as anandamide (AEA), which is hydrolysed by fatty acid amide hydrolase (FAAH). The purpose of this study was to increase AEA levels using FAAH inhibitor URB597 to evaluate the modulatory effect of AEA on dopaminergic neuronal death induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
MethodsOur study included 4 experimental groups (n=6 mice per group): a control group receiving no treatment, a group receiving URB597 (0.2mg/kg) every 3 days for 30 days, a group treated with MPTP (30mg/kg) for 5 days, and a group receiving URB597 and subsequently MPTP injections. Three days after the last dose, we conducted a series of behavioural tests (beam test, pole test, and stride length test) to compare motor coordination between groups. We subsequently analysed immunoreactivity of dopaminergic cells and microglia in the SNpc and striatum.
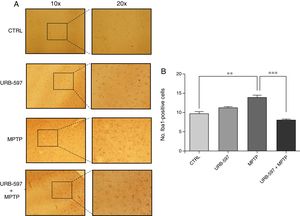
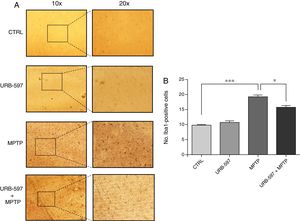
ResultsMice treated with URB597 plus MPTP were found to perform better on behavioural tests than those receiving MPTP only. According to the immunohistochemistry study, mice receiving MPTP showed fewer dopaminergic cells and fibres in the SNpc and striatum. Animals treated with URB597 plus MPTP displayed increased tyrosine hydroxylase immunoreactivity compared to those treated with MPTP only. Regarding microglial immunoreactivity, the group receiving MPTP showed higher Iba1 immunoreactivity in the striatum and SNpc than did the group treated with URB597 plus MPTP.
ConclusionOur results show that URB597 exerts a protective effect since it inhibits dopaminergic neuronal death, decreases microglial immunoreactivity, and improves MPTP-induced motor alterations.
La enfermedad de Parkinson es un desorden neurodegenerativo caracterizado por problemas de equilibro, rigidez muscular y lentitud para realizar movimiento, debido a la pérdida de neuronas dopaminérgicas de la sustancia nigra pars compacta (SNpc) y la reducción de los niveles de dopamina. Se sabe que el sistema endocannabinoide modula el funcionamiento de la vía nigroestriatal, a través de ligandos endógenos como anandamida (AEA), que es hidrolizado por la hidrolasa amida de ácidos grasos (FAAH). El objetivo de este trabajo consiste en aumentar los niveles de AEA, a través de la inhibición de FAAH por URB-597 y evaluar la modulación que ejerce AEA en la muerte neuronal dopaminérgica inducida por 1-metil-4-fenil-1,2,3,6-tetrahidropiridina (MPTP).
MétodosSe incluyeron 4 grupos experimentales con una n=6, el primero fue el grupo control sin tratamiento, un grupo al cual se le administró (0,2mg/kg) URB-597 cada 3.er día durante 30 días, un grupo tratado con MPTP (30mg/kg) por 5 días y un grupo inyectado con URB-597+MPTP. Tres días después de la última administración de los grupos experimentales, se llevó a cabo la aplicación de los siguientes paradigmas conductuales: prueba de la barra vertical, barra inclinada y longitud de la zancada para comparar la coordinación motriz. Posteriormente, se analizó la inmunorreactividad de las células dopaminérgicas y de microglía en SNpc y cuerpo estriado (CE).
ResultadosLos resultados muestran que el grupo tratado con URB-597 previo a la administración de MPTP, presentó un mejor desempeño en la realización de las pruebas conductuales comparados con los ratones que recibieron la administración de MPTP. Los hallazgos del análisis inmunohistoquímico de las células dopaminérgicas muestran que el grupo tratado con MPTP presenta una disminución en el número de células y fibras dopaminérgicas tanto en la SNpc y CE. En los animales tratados con URB-597 previo a la administración de MPTP se observó un aumento en la inmunorreactividad de tirosina hidroxilasa en comparación al grupo tratado con MPTP. Con respecto a la inmunorreactividad de las células de microglía, el grupo tratado con MPTP presentó una mayor inmunorreactividad a Iba-1 en el CE y la SNpc comparado con el grupo tratado con URB-597 previo a la administración de MPTP.
ConclusiónLos resultados obtenidos muestran que el URB-597 genera un efecto protector, al inhibir la muerte neuronal dopaminérgica, y disminución en la inmunorreactividad microglial y una mejora en las alteraciones motoras causadas por MPTP.
Parkinson's disease (PD) is a slowly progressive neurodegenerative disease characterised by the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The resulting dysfunction in dopaminergic signalling causes the typical features of PD: muscle rigidity, bradykinesia, and postural instability.1 In both animal models of PD and human patients, death of dopaminergic neurons in the substantia nigra is accompanied by an increase in the number of microglia. Microglia are activated following neuronal damage and can also promote macrophage activation and infiltration from the periphery to the nervous system. Like activated microglia, macrophage infiltration contributes to neuronal damage. Patients with PD display significantly higher levels of major histocompatibility complex class II in areas such as the caudate nucleus, putamen, and SNpc. Microglial activation is the main cause of neurodegeneration secondary to oxidative stress in these regions.2 The available evidence suggests an association between the activation of microglia and their role in neurodegeneration. Microglial activation is accompanied by the presence of inflammatory mediators, such as proinflammatory cytokines.3–5 However, these findings are controversial: there is evidence that microglia may have a neuroprotective effect, for example, by secreting neurotrophic factors.
Few pharmacological treatment options are available for PD, and levodopa is not viable for all patients as it does not halt disease progression and may increase the risk of developing dyskinesia in the long term.6,7
The above underscores the need for new treatment strategies based on new pharmacological targets and neurotransmission systems involved in or modulating the activity of the basal ganglia. An example is the endocannabinoid system, which may protect against damage to the nigrostriatal system.8
Endocannabinoids are lipid-derived molecules which are synthesised on demand in the central nervous system by glial cells (astrocytes and microglia).9 The two main endocannabinoids are anandamide (AEA), hydrolysed by fatty acid amide hydrolase (FAAH), and 2-arachidonoylglycerol, hydrolysed by FAAH and monoacylglycerol lipase.10 FAAH inhibition through the administration of URB-597 decreases AEA hydrolysis, increasing AEA concentration in the brain.11 Genetic deletion of FAAH also increases AEA concentration.12 AEA is known to be involved in various pathological and physiological processes, including metabolic regulation; modulation of pain, stress, and anxiety; cognition and memory; inflammation; and tumour progression.13 The purpose of the study was to increase endocannabinoid tone through URB-597-mediated FAAH inhibition in order to assess the modulatory effects of AEA on dopaminergic neuronal death induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
Material and methodsThis experimental study included 24 male C57BL/6 mice with a body weight of 20-25g. Mice had ad libitum access to food and water and were kept under controlled light (12:12 light/darkness cycles) and temperature conditions (23-25°C).
Mice were randomly allocated to four different groups (n=6 per group) according to the treatment received:
- a.
Control group: no drug was administered to mice in this group.
- b.
URB-597 group: mice received intraperitoneal injections of URB-597 (Sigma-Aldrich, MO, USA) dosed at 0.2mg/kg every 2 days for 30 days.
- c.
MPTP group: mice received 30mg/kg/day MPTP (Sigma-Aldrich, MO, USA) for 5 days.
- d.
URB-597+MPTP group: mice received the same treatment as in the URB-597 group, plus MPTP dosed at 30mg/kg/day for 5 days after completing treatment with URB-597.
All experiments observed the ethical standards established in the official Mexican guidelines on the use and care of experimental animals (NOM-062-ZOO-1999) and the Guide for the care and use of laboratory animals published by the National Institutes of Health (NIH Publication No. 8023, 1978).
Behavioural testsEach group underwent behavioural tests 3 days after the last dose was administered.
Beam testThis test uses an acrylic beam measuring 1m long and placed at an angle of 15°, which mice have to climb. During the test, the mice's home cages were placed at the upper end of the beam. Before starting treatment, mice were trained for 2 days using a 12-mm beam; the test was performed on a 6-mm beam. Mice were placed at the lower end of the beam; we recorded the time they took to reach the cage at the upper end. We set a maximum time of 120s, after which mice not reaching the upper end were manually removed from the beam and placed inside their cages; these mice were assigned a score of 120s. Results are expressed as the mean total time (in seconds) required to complete the task.14
Stride length testTo assess decreased stride length, a typical feature of hypokinetic gait in PD, we used an illuminated corridor measuring 60cm long, 7cm wide, and 12cm high, which led to a dark cage; the corridor was placed on a sheet of paper. All four paws were inked. Mice were placed at the beginning of the corridor leading to the dark cage, leaving paw prints on the paper as they walked; a millimetre ruler was subsequently used to measure stride length.15
Pole testThis test assesses bradykinesia, or slowness of spontaneous and automatic movement, measuring the time it takes a mouse to perform a turn and descend a vertical pole, as described by Matsuura et al.16 We used a pole of 50cm length and 1cm diameter. Mice completed the task five times, at 60s intervals. Time was limited to a maximum of 300s for the first attempt and 120s for all subsequent attempts. We calculated the mean of the best three scores for each mouse.
Immunohistochemical determination of dopaminergic and microglial cellsThree days after the last administration, mice were anaesthetised (100mg/kg ketamine+15mg/kg xylazine, intraperitoneal administration) and received intracardiac perfusion with 4% paraformaldehyde in 0.1M phosphate buffer solution (PBS). Following perfusion, brains were extracted and placed in a fixation solution for 24hours. They were subsequently washed three times with 0.1M PBS. Brains were cut into 35-μm coronal slices using a vibratome (VT1000E, Leica Microsystems, Wetzlar, Germany) to obtain the regions corresponding to the striatum (Bregma 1.70-0.14mm) and SNpc (Bregma –2.06 to –2.54mm), as per Paxinos and Franklin's stereotaxic coordinates.17 We collected six slices from each brain, with 175-μm spaces between slices. Using the above-mentioned coordinates, the slices were selected at the same level from every animal to perform a uniform analysis, using the basic principle of fractionation from the rostrocaudal region. We obtained the mean number of marker-positive cells per field (445μm) at 40× magnification (Leica Q5001W, Leica Imaging Systems, Cambridge, UK).
Morphological analysis was performed with free-floating sections. The selected slices were washed four times in 0.1M PBS for 5minutes each time; sodium citrate buffer (pH 6.4) was subsequently added for 10minutes at 37°C. Tissue samples were then washed with 0.1M PBS (4×5min). Tissue samples were exposed to the inactivation of endogenous peroxidase using a 3% H2O2 solution for 20minutes at room temperature. Samples were subsequently washed with 0.1M PBS (4×5min) and incubated in blocking solution (0.1M PBS, 0.03% Triton X-100, and 10% normal goat serum) for an hour at room temperature. The solution was replaced and primary antibodies were added for marking microglial (Iba1; Abcam ab5076) and dopaminergic cells (TH; Ab152 Merck Millipore); incubation lasted 48hours at 4°C. Primary antibodies were subsequently removed; samples were washed four times with 0.1% Triton X-100 in PBS. We added secondary antibodies (anti-rabbit IgG; BA-1000, Vector Laboratories) (1:500 dilution) in a solution of 0.1M PBS and 5% normal goat serum; slices were incubated in the dark for 90minutes at room temperature. Samples were then washed in 0.1M PBS four times for 5minutes each time and then incubated in ABC complex (PK-6000, Vector Laboratories) in 0.1M PBS in the dark for 30minutes at room temperature. Slices were washed several times and developed with the DAB kit (Vector Laboratories, SK-4100). Tissue samples were mounted on slides; once dry, they were covered with coverslips using Entellan (Merck). The images of immunoprocessed tissue samples were analysed with a Leica image analysis system (Q5001W, Leica Imaging Systems; Cambridge, UK).
Statistical analysisDifferences between treatment groups were calculated with the Kruskal–Wallis test, using the Mann–Whitney U-test, and the GraphPad Prism 5 software. Statistical significance was set at P<.05.
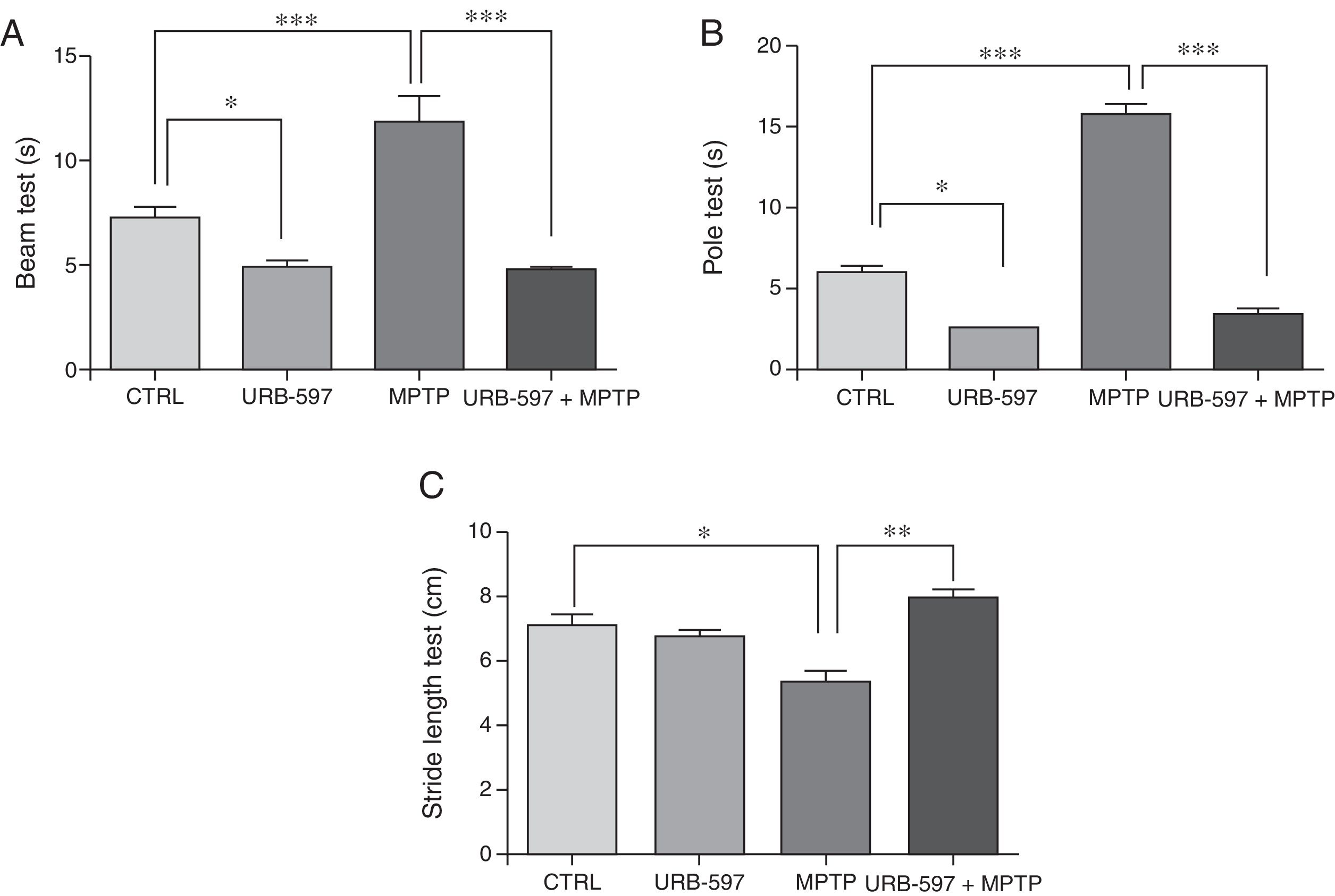
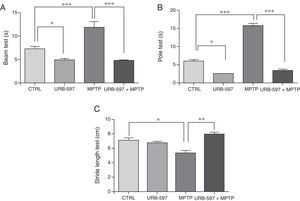
ResultsBehavioural testsBeam testMotor deficits, a typical feature of PD, were evaluated with the beam test in all groups. Control mice required a mean of 7.3seconds to reach the upper end of the beam, compared to 11.85seconds in the MPTP group; this difference is statistically significant (Fig. 1A). Before receiving MPTP, mice in the URB-597+MPTP group showed a latency of 4.79seconds; differences between this and the MPTP group were significant. Mice in the URB-597 group also showed a significant decrease in the time taken to reach the upper end of the beam.
Behavioural tests. Beam test (A) and pole test (B) results are expressed in seconds (time taken to complete the task). Stride length test (C) results are expressed in centimetres. Data are expressed as the mean±SD. Beam test: ***P<.001, control vs. MPTP. ***P<.001, MPTP vs. URB-597+MPTP. *P<.05, control vs. URB-597. Pole test: ***P<.001, control vs. MPTP. ***P<.001, MPTP vs. URB-597+MPTP. *P<.05, control vs. URB-597. Stride length test: *P<.05, control vs. MPTP. **P<.01, MPTP vs. URB-597+MPTP.
Animals treated with MPTP required a mean of 15.8seconds to complete this task. The control group required significantly less time (6seconds). Before receiving MPTP, mice in the URB-597+MPTP group showed a considerable decrease in the time taken to complete the task compared to mice in the MPTP group (Fig. 1B). No significant differences in the time taken to complete the task were observed between the URB-597 group and the control group.
Stride length testMean stride length in the control group was 7.13cm. Mice receiving MPTP showed a significantly shorter stride length, at 5.38cm (Fig. 1C). Before receiving MPTP, mice in the URB-597+MPTP group took significantly longer strides than mice in the MPTP group (Fig. 1B). No differences in stride length were observed between the URB-597 group and the control group (Fig. 1C).
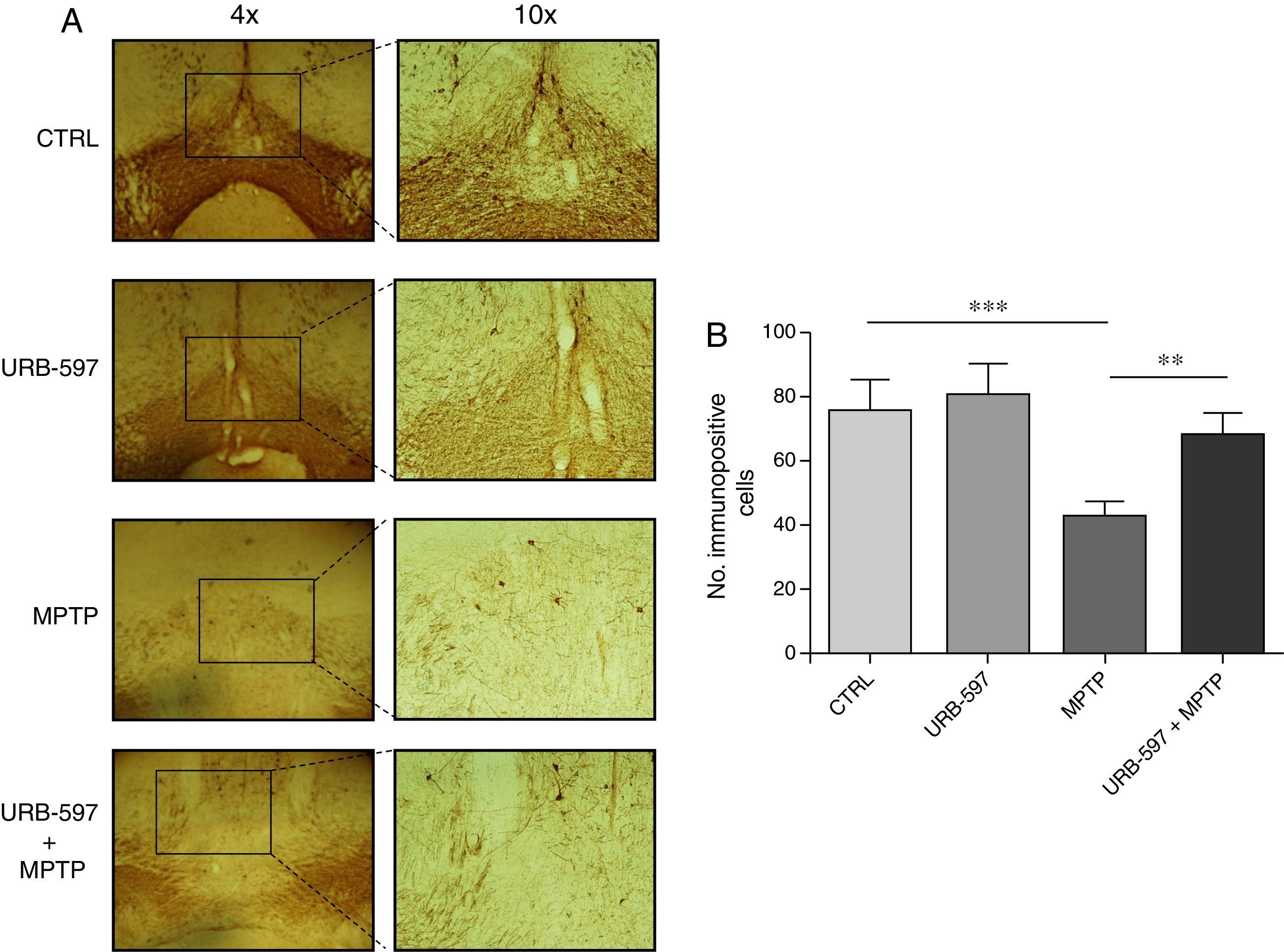
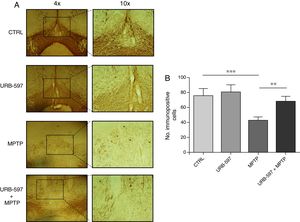
Immunohistochemical determination of tyrosine hydroxylase in the SNpc and the striatumMice in the MPTP group showed a 69.4% decrease in the number of tyrosine hydroxylase-positive dopaminergic neurons in the SNpc compared to control mice (Fig. 2).
Quantitative analysis of tyrosine hydroxylase-positive dopaminergic neurons in the SNpc. (A) Tyrosine hydroxylase-positive dopaminergic neurons in each study group. (B) Quantitative analysis; data are expressed as the mean±SD. ***P<.001, control vs. MPTP. **P<.01, MPTP vs. URB-597+MPTP.
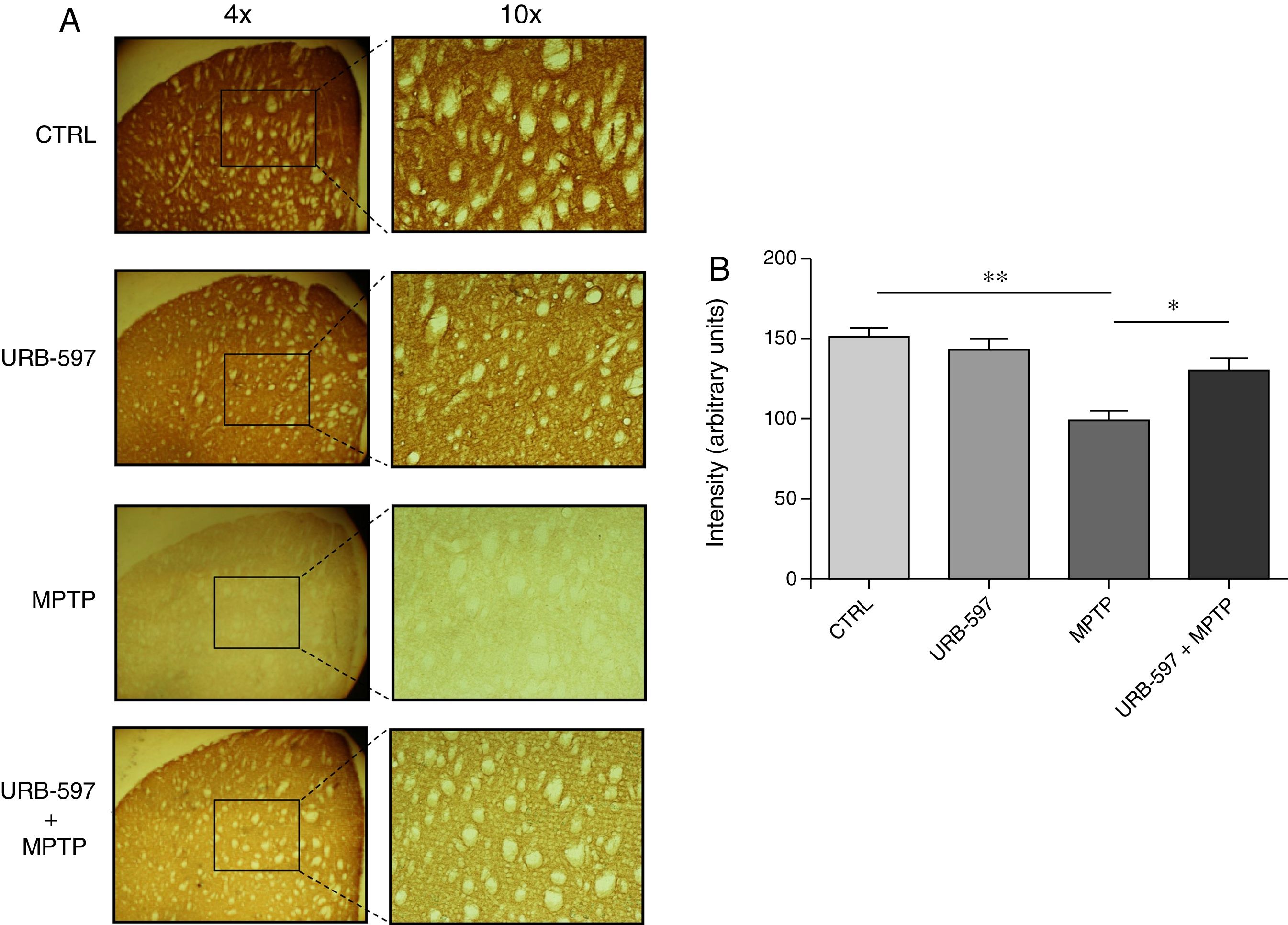
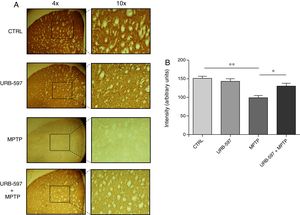
Mice in the MPTP group showed significantly fewer dopaminergic fibres (a 63% decrease) in the striatum than did control mice. In contrast, mice in the URB-597+MPTP group showed a 12% decrease compared to controls; this difference was not significant. Significant differences were observed between the URB-597+MPTP group and the MPTP group (Fig. 3).
Quantitative analysis of tyrosine hydroxylase-positive dopaminergic neurons in the striatum. (A) Tyrosine hydroxylase-positive dopaminergic fibres in each study group. (B) Quantitative analysis; data are expressed as the mean±SD. **P<.01, control vs. MPTP. *P<.05, MPTP vs. URB-597+MPTP.
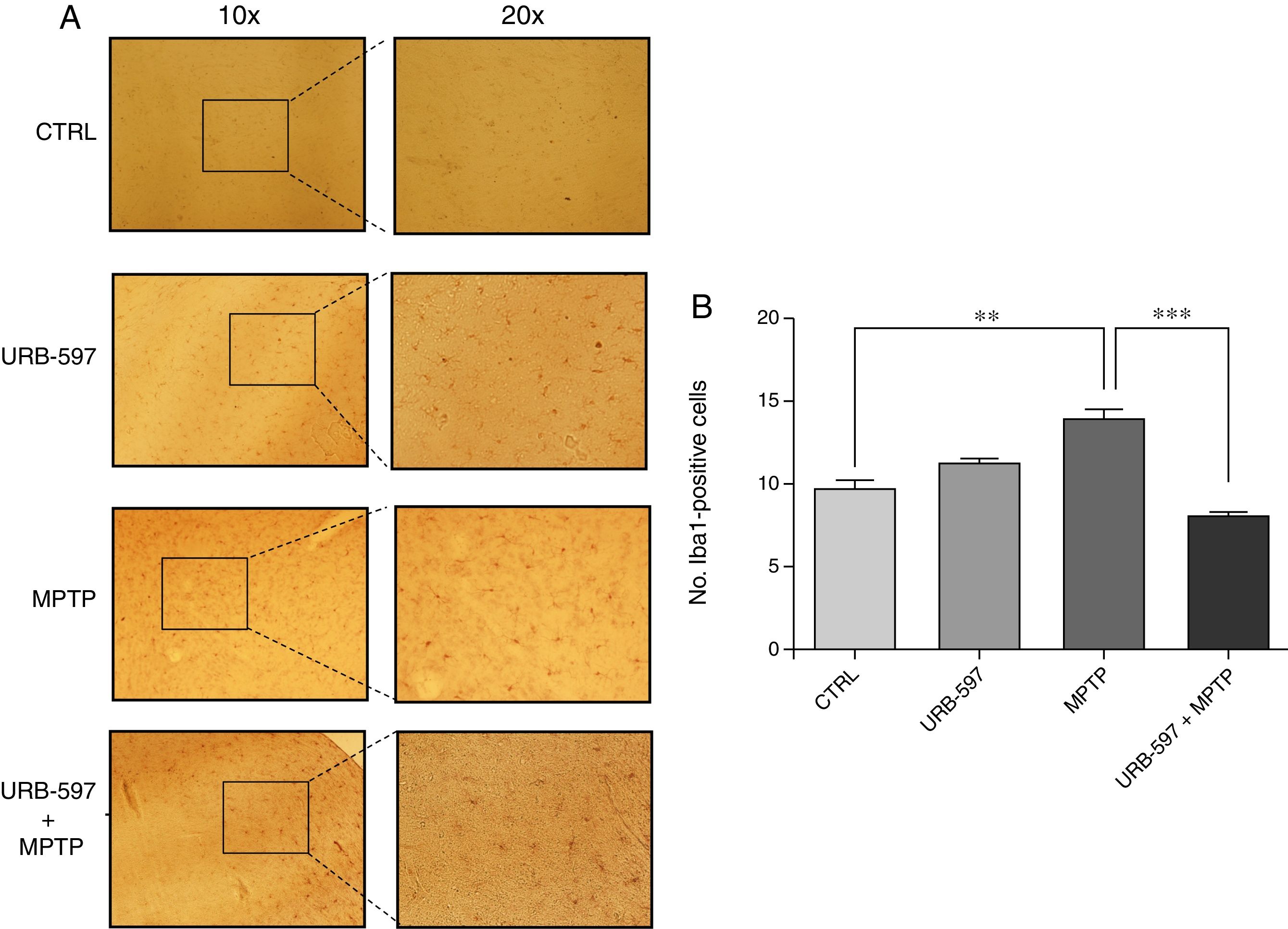
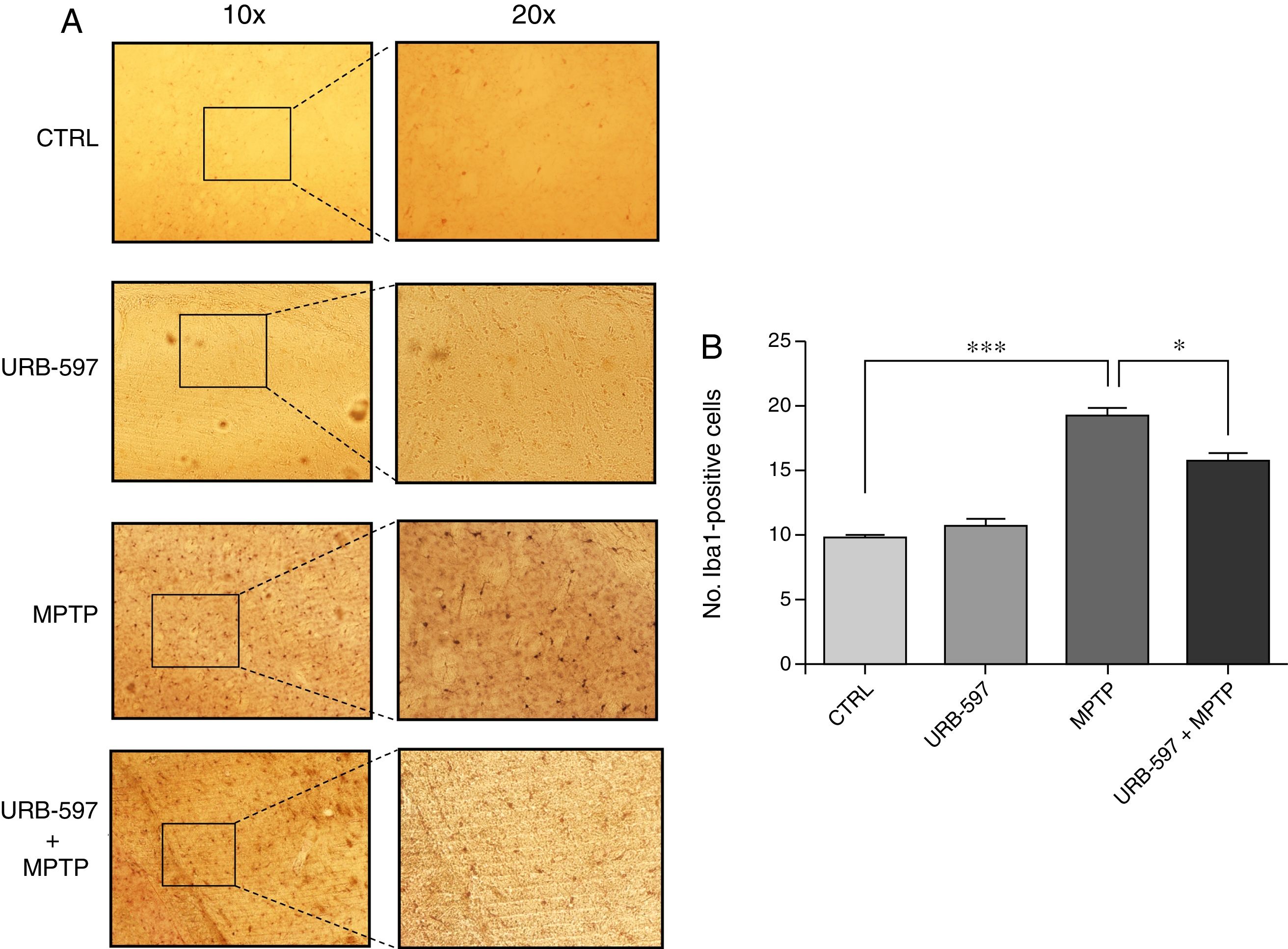
Fig. 4 shows the histology images of the SNpc from all groups. No Iba1-positive cells were observed in the control and URB-597 groups. The group receiving MPTP, in contrast, displayed numerous Iba1-positive cells; Iba1 positivity decreased when URB-597 was administered before MPTP. Differences between these groups were statistically significant.
Fig. 5 shows the histology images of the striatum from all groups. Mice in the MPTP group showed significantly more Iba1-positive cells than control mice. Mice receiving URB-597 before MPTP showed reduced microglial immunoreactivity compared to mice treated with MPTP only; both groups show statistically significant differences compared to the control group. This suggests that URB-597 decreases MPTP-induced immunoreactivity. No significant differences in Iba1 positivity were observed between the URB-597 group and the control group.
DiscussionEvaluating locomotor activity is an effective method for determining MPTP-induced damage to the nigrostriatal pathway. Multiple indicators can be assessed, including balance, muscle rigidity, and locomotor function in animal models of parkinsonism.18 Before receiving MPTP, mice underwent training to adapt to the tasks included in each test. URB-597 is known for its anxiolytic, antidepressive, antihypertensive, and analgesic effects in rodents, and particularly in models of thermal and inflammatory pain. It does not present the typical pharmacological profile of cannabinoid agonists, specifically the activation of the cannabinoid receptor type 1 (CB1).19 The effects of URB-597 are caused by increased endocannabinoid tone through the inhibition of FAAH.
The CB1 receptor is expressed in brain areas associated with emotions, such as the prefrontal cortex, thalamus, and hypothalamus. In a 2012 article by Mclaughlin and Gobbi,20 inhibition of AEA hydrolysis was observed to have anxiolytic effects during the tail suspension test, which shows the role of endocannabinoids in emotion. As shown in the beam test, URB-597 has anxiolytic effects during the performance of this task. This is consistent with the results of a 2016 study by Kregiel et al.,21 who found that URB-597 promoted emotional learning in a sample of rats receiving random positive and negative stimulation; the animals displayed optimistic judgement bias in a behavioural test. The pole test may generate a pessimistic attitude (anxiety) due to the height of the pole. Mice receiving URB-597 were less frightened and were therefore more confident during the performance of this test, resulting in quicker completion of the task. AEA modulation is known to induce positive behaviour in mice; this effect may be inhibited by CB1 receptor antagonists.21 The anxiolytic effects of URB-597 were also observed in our experiment, since mice seemed to be less anxious to conclude the task.
A 2010 study by Eisenstein et al.22 showed that animals receiving URB-597 exclusively showed greater motor hyperactivity during behavioural tests; this may be due to the anxiolytic effects caused by increased endocannabinoid tone. Endocannabinoids interact with multiple neurotransmission systems involved in movement; cannabinoid agonists therefore have positive motor effects.23
In our study, administration of URB-597 before MPTP reduced motor symptoms, as seen in the results of motor tests. The increase in AEA levels is a known compensatory mechanism in PD. A study by Pisani et al.24 found increased levels of AEA in the CSF of 16 patients with PD compared to control CSF samples from elderly patients.
The anti-inflammatory effects of cannabinoids are mediated by the modulation of cytokines such as tumour necrosis factor alpha, IL-12, IL-1, IL-6, and IL-10.23 AEA levels increase in the event of trauma, kainic acid-induced seizures, or 6-OHDA toxicity, confirming the neuroprotective role of this endocannabinoid through inhibition of proinflammatory cytokine production.25
Our results show that URB-597 reduces immunoreactivity in microglia, decreases dopaminergic cell death, and improves motor capacity in mice treated with MPTP. Inflammation plays a major role in pathological processes; modulating microglial function is therefore essential. The literature shows that microglia is associated with a lack of dopaminergic fibres and with motor symptom severity in PD; reduced numbers of microglia may therefore be associated with less severe symptoms and greater numbers of dopaminergic fibres or neurons.26
In our study, mice receiving MPTP showed greater numbers of Iba1-positive cells than control mice. Certain intrinsic factors induce microglial proliferation under pathological conditions.27 The formation of microglial processes and arborisations promotes fast, constant cytoplasmic transformation.28 Although we did not evaluate the degree of microglial activation, our study shows that URB-597 can have a positive effect on microglial reactivity. Future studies should aim to determine the temporal profile of microglial activation.
Among the possible aetiologies of PD, inflammatory processes mediated by CNS glial cells have been found to be largely responsible for dopaminergic neuron degeneration in the SNpc. In 1988, McGeer et al.29 observed increased numbers of microglial cells in the nigrostriatal pathway in post mortem brain tissue. Increased microglial activation results in the release of inflammatory mediators responsible for dopaminergic neuronal damage and death in the nigrostriatal pathway.
During inflammatory processes, the CB2 receptor seems to inhibit inflammation. Some studies have shown that increased expression of CB2 receptors and CB2 receptor agonists reduces neuroinflammation in different models of parkinsonism and Huntington disease; this receptor has therefore been proposed as a pharmacological target for the inhibition of neuroinflammation.30 A model of 6-OHDA-induced dopaminergic damage with a selective CB2 receptor agonist showed that the severity of damage to the nigrostriatal pathway decreases with increased CB2 receptor expression.31 Another study observed increased CB2 receptor expression after the administration of MPTP and lipopolysaccharides; some authors have therefore suggested that increased CB2 receptor expression during inflammation and selective agonism of this receptor may result from the blocking of microglial differentiation to a neurotoxic phenotype (M1) and inhibition of inflammatory factors when microglia are activated.32 In our study, mice treated with MPTP exclusively showed a greater decrease in microglial immunoreactivity than mice receiving URB-597 prior to MPTP. This suggests that URB-597 may reduce microglial activation triggered by neuronal damage. Other studies have shown that in the presence of anti-inflammatory mediators (e.g., IL-4, IL-13, or IL-10), microglial cells change from an M1 to an M2 phenotype, which is associated with cell repair processes and characterised by long ramifications and expression of anti-inflammatory markers. This morphology is also typical of resting microglia.2 Other studies report similar results to our own, showing that long-term use of URB-597 may reduce microglial immunoreactivity, abnormal microglial activation, and age-related neuroinflammation.33 In cultures of lipopolysaccharide-treated microglia, URB-597 has been found to decrease the activity of inducible nitric oxide synthase and cyclo-oxygenase 2, reducing the production of such reactive oxygen species as prostaglandin E2.34 An in vitro study by Murphy et al.33 showed that cannabinoids and endocannabinoids may reduce microglial activation induced by IFN-γ and lipopolysaccharides.
In conclusion, our laboratory findings support the neuroprotective capacity of URB-597, which protected mice against MPTP-induced motor impairment; administration of URB-597 before damage induction inhibited dopaminergic neuronal death and microglial immunoreactivity.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Viveros-Paredes JM, Gonzalez-Castañeda RE, Escalante-Castañeda A, Tejeda-Martínez AR, Castañeda-Achutiguí F, Flores-Soto ME. Efecto del inhibidor de amida hidrolasa de ácidos grasos en el daño neuronal dopaminérgico inducido por MPTP. Neurología. 2019;34:143–152.