Consumption of energy drinks has increased in recent years, mainly among preadolescents, adolescents, and young adults; 30%-70% of adolescents are frequent consumers.1,2 Energy drinks frequently contain high doses of caffeine, combined with such other substances as taurine, guarana, and sugar.3 These beverages have no proven therapeutic effects. On the contrary, consumption of energy drinks is associated with a number of adverse reactions, especially in children and young adults; these include seizures, cardiovascular problems, diabetes, behavioural alterations, and even sudden death.3–8 Knowing the possible adverse effects of energy drinks and ruling out their consumption is essential for proper differential diagnosis of neurological symptoms and to prevent recurrences and potential worsening of secondary symptoms.
We present the case of an 8-year-old boy with no relevant personal or family history who began to experience paraesthesia around the oral commissure, followed by clonus and commissure deviation, resulting in difficulty speaking and retaining saliva. The patient showed no alterations in the level of consciousness and had no other symptoms. He had experienced similar isolated episodes in the previous 2 months; episode frequency had increased over the previous week, with 2 episodes occurring on the day of the consultation, leading to his visit to the emergency department. Symptoms alternately affected both commissures, with episodes occurring predominantly at night and resolving spontaneously within 5minutes. The last episode was associated with paresis of the left arm and the right side of the face.
The patient did not present fever or any other symptom of infection, and was not taking any medication. No substance use was reported except for energy drinks, which he consumed on a nearly daily basis. According to the patient's parents, the episodes started when the patient began consuming energy drinks. Consumption had increased in the previous week (500-mL cans of Monster Energy®, each containing 5mg caffeine per kg of the patient's weight).
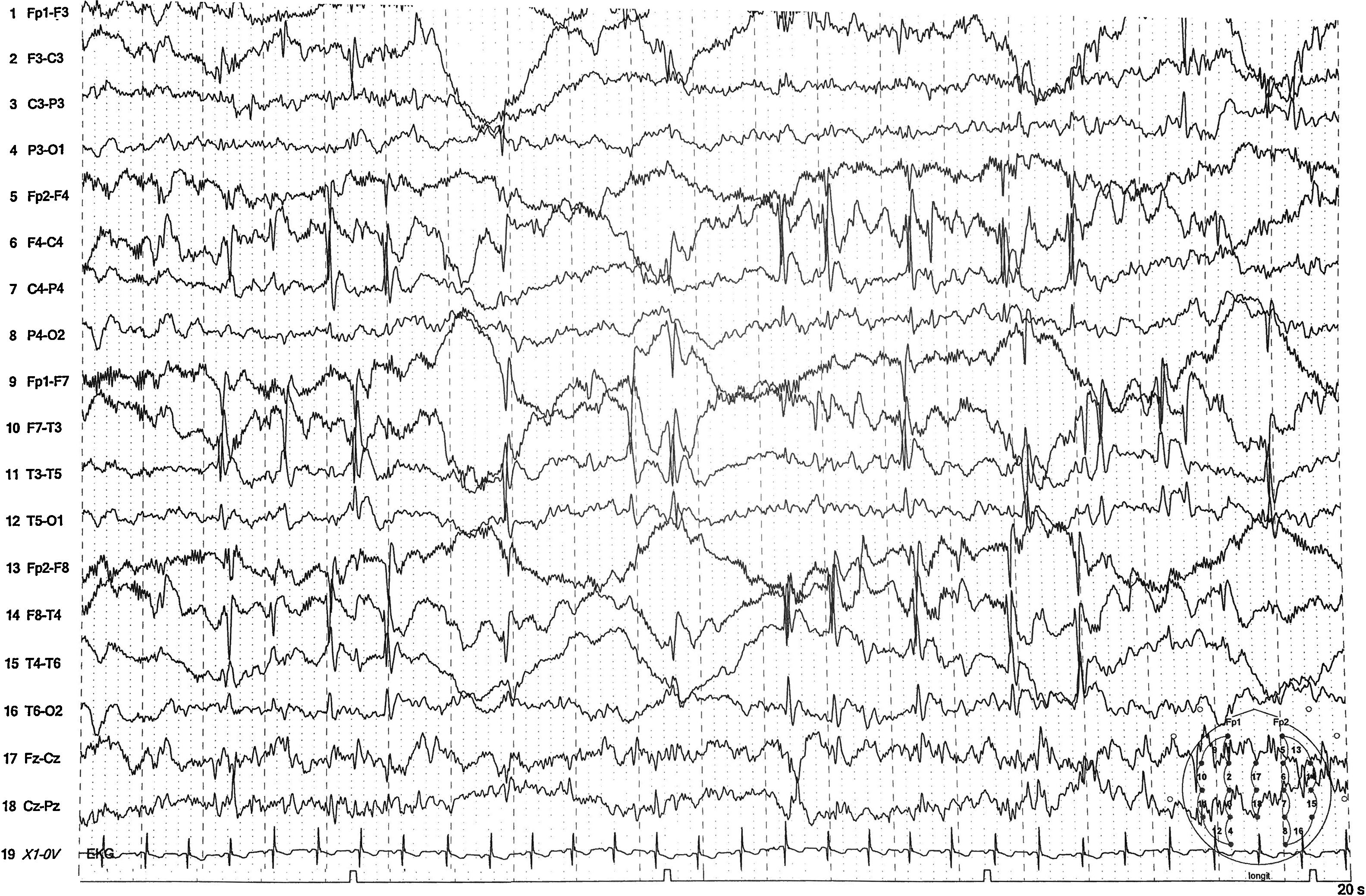
The baseline neurological examination revealed no alterations. Normal results were also obtained from a blood test, including a complete blood count, blood gas analysis, and biochemical analysis (ions were within normal ranges). A urine analysis tested negative for toxins, and the electrocardiogram showed no alterations. The patient was admitted for observation and to undergo further testing. He experienced no additional episodes during hospitalisation. An electroencephalography (Fig. 1) showed bihemispheric epileptiform activity, predominantly during non-REM sleep, compatible with Rolandic epilepsy. The patient was discharged; discontinuation of energy drink consumption was recommended. He was followed up at the neuropaediatric department, displaying no further episodes in the 2 months after admission. After that period, he began to experience isolated episodes; a brain MRI scan revealed no alterations, and the patient started treatment with oxcarbazepine.
Video-EEG recording revealing epileptiform activity, frequently independent and in the centrotemporal region of both hemispheres, becoming more marked when the patient fell asleep and during non-REM sleep, with high persistence. Centrotemporal epileptiform activity is mixed with slow, irregular activity. Sensitivity: 15μV/mm; high-frequency filter: 70Hz; time constant: 0.3s.
Energy drink consumption is becoming more prevalent among adolescents and young adults. Consumption starts at increasingly younger ages, as in our patient.9
The main active components of these beverages are caffeine, taurine, and such herbal supplements as guarana or ginseng. Although data are scarce, several of these substances are thought to be involved in the pathogenesis of seizures. Caffeine, a natural stimulant and a non-selective adenosine receptor antagonist, has proconvulsant effects both in healthy individuals, when administered at high doses, and in caffeine-sensitive patients.10,11 Taurine, an essential amino acid, may also have proconvulsant properties as the seizure threshold decreases in cases of sustained interaction between taurine and GABAA receptors.12 Guarana is a plant that contains such methylxanthines as caffeine and theophylline.
Although caffeine tolerance varies, most individuals experience toxic effects with doses higher than 200mg, or 3mg/kg in children. Caffeine consumption in children and adolescents should not exceed 2.5mg/kg/day or 100mg/day, respectively; the maximum recommended dose for adults is 400mg/day.2
In the United States, 73% of children and adolescents are frequent caffeine consumers.13 Adolescents consume a mean of 60-70mg/day,3 mostly from soft drinks; caffeine obtained from energy drinks has increased in recent years and amounts to up to 10% of total caffeine intake.13
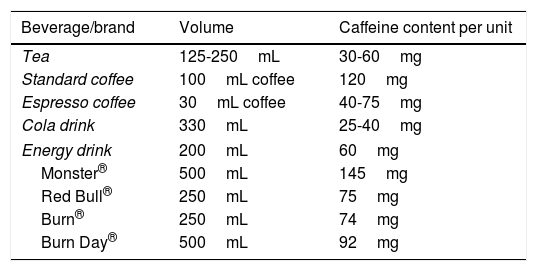
Energy drinks contain a mean of 70-80mg caffeine per can (3 times more than cola); caffeine content may be even higher, as in the energy drink consumed by our patient (Table 1). Caffeine content is not specified on the packaging of many of these beverages. Furthermore, each gram of guarana extract contains 40-80mg caffeine, but manufacturers are not obliged to specify this additional caffeine content.3 Therefore, a single can of these beverages is very likely to contain more caffeine than the maximum recommended daily dose.14
Caffeine content of various widely consumed beverages.
| Beverage/brand | Volume | Caffeine content per unit |
|---|---|---|
| Tea | 125-250mL | 30-60mg |
| Standard coffee | 100mL coffee | 120mg |
| Espresso coffee | 30mL coffee | 40-75mg |
| Cola drink | 330mL | 25-40mg |
| Energy drink | 200mL | 60mg |
| Monster® | 500mL | 145mg |
| Red Bull® | 250mL | 75mg |
| Burn® | 250mL | 74mg |
| Burn Day® | 500mL | 92mg |
Consumption of energy drinks is associated with a wide range of severe adverse reactions,7 including first-time seizures,6–8 also in paediatric patients15; seizures resolve after discontinuation of energy drink consumption. The adverse effects of these beverages may be underestimated, as patients are rarely asked about energy drink consumption. It is also difficult to differentiate between dose-dependent episodes in healthy individuals and episodes triggered by decreased seizure threshold in caffeine-sensitive patients.
Although we cannot confirm a causal relationship between consumption of energy drinks and onset of partial seizures in the context of benign childhood epilepsy, these beverages may have triggered the episodes. Identifying the involvement of energy drink consumption in episodes of seizures may be a diagnostic challenge; a high level of clinical suspicion is therefore necessary. Children and adolescents are frequent consumers of these beverages but are not fully aware of the associated risks.
Raising awareness of the adverse effects of these substances among consumers and healthcare professionals is essential. Further studies should aim to determine the safety of energy drink consumption.
Please cite this article as: Butragueño Laiseca L, Toledo del Castillo B, Miranda Herrero MC. Bebidas enérgicas como desencadenante de crisis convulsivas en pacientes pediátricos: a propósito de un caso. Neurología. 2019;34:343–345.