Unilateral Gamma Knife™ stereotactic radiosurgery on the ventral-intermediate nucleus of the thalamus is a minimally invasive neurosurgical option for refractory tremor. We describe the experience of Gamma Knife™ thalamotomy (GKT) in patients with essential tremor (ET) and tremor-dominant Parkinson's disease (PD) at our specialised stereotactic neurosurgery unit.
MethodsWe reviewed the cases of patients treated with GKT between January 2014 and February 2018 with a minimum of 12 months’ follow-up. We analysed clinical and demographic variables, indication, radiation dose, effectiveness (based on subscales of the Fahn-Tolosa-Marin [FTM] scale and the Movement Disorders Society-Unified Parkinson's Disease Rating Scale [MDS-UPDRS] motor score), and adverse events.
ResultsThirteen patients were registered, 6 with a diagnosis of tremor-dominant PD, four with refractory ET, and three with ET and PD. Median age was 78 years (range, 62-83), with seven patients aged over 75 years. Four patients were receiving anticoagulants and two had history of stroke. The maximum radiation dose administered was 130 Gy. Mean (standard deviation) follow-up duration was 30.0 (14.5) months. Significant tremor improvement was observed on the FTM subscales: 63.6% at 12 months and 63.5% at the end of follow-up; MDS-UPDRS tremor items showed improvements of 71.3% at 12 months and 60.3% at the end of follow up. Eleven patients reported significant improvements in quality of life, and 3 reported mild and transient adverse effects.
ConclusionsThis is the largest series of patients with essential and parkinsonian tremor treated with GKT and followed up in the long term in Spain. GKT can be safe and effective in the long term in patients with refractory tremor, including in elderly patients and those receiving anticoagulants.
La radiocirugía estereotáctica con Gamma Knife® (GK), sobre el núcleo ventral intermedio-medial del tálamo (VIM), unilateral es una opción neuroquirúrgica mínimamente invasiva para el temblor refractario. Se describe la experiencia de talamotomía con GK (TGK) en pacientes con temblor esencial (TE) y enfermedad de Parkinson (EP) de predominio tremórico de una unidad especializada en cirugía estereotáctica.
MétodosSe revisan los pacientes tratados con TGK desde enero de 2014 hasta febrero de 2018. Se analizan variables clínico-demográficas, indicación, dosis empleada, eficacia (mediante subescalas de Fahn-Tolosa-Marin (FTM) y MDS-UPDRS motora) y efectos adversos (EA).
ResultadosSe registraron 13 pacientes, seis con diagnóstico de EP de predominio tremórico, cuatro con TE refractario y tres casos de TE + EP. La mediana de edad fue 78 años (62-83), con siete pacientes > 75 años. Cuatro pacientes anticoagulados y dos con antecedentes de ictus previo. La dosis máxima de radiación aplicada fue 130 Gy. La media de seguimiento fue 30,0 (14,5) meses. Se observó una mejoría significativa del temblor en las subescalas de FTM del 63,6% a 12 meses y del 63,5% al final del seguimiento y en items de temblor de MDS-UPDRS del 71,3% a 12 meses y del 60,3% al final del seguimiento. Once pacientes refirieron mejoría significativa en su calidad de vida. Tres pacientes refirieron EA leves y transitorios.
ConclusionesSe presenta la mayor serie de pacientes con TE y parkinsoniano tratados con TGK en España con seguimiento a largo plazo. La TGK puede ser un tratamiento seguro y con eficacia mantenida en temblor refractario, incluso en edad avanzada o en tratamiento anticoagulante.
Tremor is one of the most frequent reasons for neurological consultation and the most prevalent movement disorder. Although it is most frequently caused by a benign tumour, it tends to worsen with age and with progression of the underlying disease, even becoming disabling and refractory to medical treatment.1,2 The 2 main causes of pathological tremor are essential tremor (ET) and tremor-dominant Parkinson’s disease (PD).3 Bilateral deep brain stimulation (DBS) of the ventral intermediate nucleus (VIM), thalamus, and subthalamic nucleus has been shown to be effective for treating refractory tremor with known risks, the most important being inherent to the surgical process, in adequately selected patients diagnosed with ET and PD.4,5 Gamma Knife® (GK) stereotactic radiosurgery targeting the unilateral VIM of the thalamus for tremor suppression is a minimally invasive surgical treatment option that has received growing attention in recent years,6,7 and may be especially valuable for patients in whom DBS is formally contraindicated due to advanced age or comorbidity.8 This study describes the long-term experience of a stereotactic surgery unit in unilateral GK thalamotomy (GKT) in patients with medication-refractory ET and tremor-dominant PD. Finally, we compare this surgical option with other procedures currently available to treat tremor.
MethodsWe retrospectively reviewed all patients with tremor treated with unilateral GKT at our centre from January 2014 to February 2018. We collected clinical and demographic data such as age, sex, diagnosis, symptom progression time, number of oral treatments used, indication for GKT, and follow-up time. Diagnosis of ET, PD, or both was established by a movement disorders specialist, following the validated diagnostic criteria.9,10 GKT was indicated for patients with ET presenting disabling tremor in the dominant hand and treatment resistance or intolerance to antitremor drugs. Treatment resistance to antitremor drugs was defined as poor control of tremor after the use of 2 first-line drugs (propranolol and primidone) or, in the event that one of these was contraindicated, one first-line drug and at least one second-line drug (topiramate, gabapentin, alprazolam, zonisamide, etc). In the case of PD, patients considered ideal candidates were those with severe asymmetric tremor causing a significant decrease in quality of life, regardless of whether the most affected side was the dominant side, and in whom tremor was not controlled with optimised oral antiparkinsonian treatment. Presence or absence of motor fluctuations was not a requirement for indicating this treatment. Patients with dementia were excluded.
In all cases, a Leksell Gamma Knife® was used to target the unilateral VIM; the target area was located according to an imaging protocol using a 3T MRI scanner; the procedure involved participation of a multidisciplinary team including a neuroradiologist, a medical physicist, a radiation oncologist, a neuropsychologist, neurologists, and neurosurgeons experienced in stereotactic radiosurgery. We used the system described by Taillerach as modified by Régis and Ohye, located the intercommissural line, and used a rectangular calculation diagram. We obtained mean coordinates of 3.5-4 mm above the intercommissural line (Z), 7-8 mm anterior to the posterior commissure (Y), and 11 mm lateral to the third ventricular wall (X). The localisation of the VIM was compared to the Wahren–Schaltenbrand atlas coordinates,11 calculated using our own stereotactic software (Estereonauta®). We made the 2 calculations separately in a stereotactic brain MRI study with a slice thickness of 1.2 mm for T1 and 2 mm for T2 sequences, obtaining axial and coronal images. We also located the internal capsule in the MRI study and correlated the location with the results of a 3T MRI tractography. The maximum radiation used was 130 Gy, using a single 4 mm collimator.
Effectiveness was calculated with validated clinical scales, applied at baseline, at 12 months, and at the end of the follow-up period. In 4 of the 13 patients included, baseline scores were calculated after assessment of footage recorded at baseline by a movement disorders specialist (JRPS). In patients with ET, effectiveness was evaluated using the Fahn-Tolosa-Marin (FTM) tremor rating scale,12 based on the items assessing postural and kinetic tremor in the dominant hand, tremor in writing and drawing (spiral A), and the ability to bring fluids to the mouth or to drink. In patients with PD, we assessed items referring to tremor (resting tremor, postural tremor, kinetic tremor, and persistence of tremor) in the target hemi-body from the motor part (III) of the latest version of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS).13 In cases diagnosed with concomitant ET and PD, both subscales were used. Quality of life was assessed using the visual analogue scale (VAS) from the Euro-QoL-5D healthcare questionnaire.14 All patients were administered a clinical global impression scale15 at 12 months after GKT and at the end of the follow-up period, which was adapted to include the following values: 1 = significant improvement or resolution of tremor, with complete functional recovery; 2 = moderate improvement or decrease in tremor, with partial functional recovery; 3 = mild improvement or decrease in tremor, with no functional recovery; 4 = no changes; 5 = mild worsening; 6 = moderate worsening; 7 = significant worsening.
Lastly, we reviewed the adverse effects reported during follow-up: at follow-up consultations, patients were interviewed about the possible appearance of sensory symptoms, loss of strength, impaired speech production, or gait disorders.
We calculated the mean and standard deviation of the different scale items at baseline, at 12 months, and at the end of the follow-up period. We performed a statistical analysis of FTM and MDS-UPDRS scores at baseline, at 12 months, and at the end of follow-up, using the paired-samples Wilcoxon test. Statistical significance was set at P < .05. Results are expressed as means (standard deviation) and medians (range).
ResultsWe included a total of 13 patients diagnosed with tremor-dominant PD (6 patients), ET refractory to conventional medical treatment (4 patients), and long-standing ET plus PD with shorter progression time and more recent diagnosis (3 patients). Seven patients were of advanced age (< 75 years; maximum of 83 years), 4 were receiving anticoagulant treatment, and 2 had history of stroke. Three patients presented more than one contraindication for DBS (age < 75 years, anticoagulant treatment, and/or history of stroke). Five patients declined treatment with DBS after being properly informed of the expected benefits and risks of both procedures (Table 1). Three patients were informed about the possibility of treatment with high-intensity focused ultrasound (HIFU), but finally opted for GK treatment for financial reasons. Mean follow-up time was 30.0 (14.5) months (range, 12-60 months). Latency to tremor improvement was 3 months (range, 1-6 months) after the procedure.
Clinical and demographic characteristics.
| Total number of patients | 13 | |
| Age, years (median, range) | 78 | 62-83 |
| Sex | ||
| Men | 9 | 69% |
| Women | 4 | 31% |
| Diagnosis | ||
| ET | 4 | 31% |
| PD | 6 | 46% |
| ET + PD | 3 | 23% |
| ET progression time, years (median, range) | 31 | 6-68 |
| PD progression time, years (median, range) | 9.5 | 3-11 |
| Antitremor drugs used (median, range) | 5 | 3-7 |
| Levodopa equivalent dose, mg (median, range) | 800 | 300-1000 |
| Target side | ||
| Right VIM | 4 | 31% |
| Left VIM | 9 | 69% |
| Reasons for indicating GKT | ||
| Age > 75 years | 7 | 54% |
| Anticoagulation | 4 | 31% |
| History of stroke | 2 | 15% |
| Rejection of DBS | 5 | 38% |
| Follow-up time in months (mean, SD) | 30.0 | 14.5 |
DBS: deep brain stimulation; ET: essential tremor; GKT: Gamma Knife® thalamotomy; PD: Parkinson’s disease; SD: standard deviation; VIM: ventral intermediate nucleus.
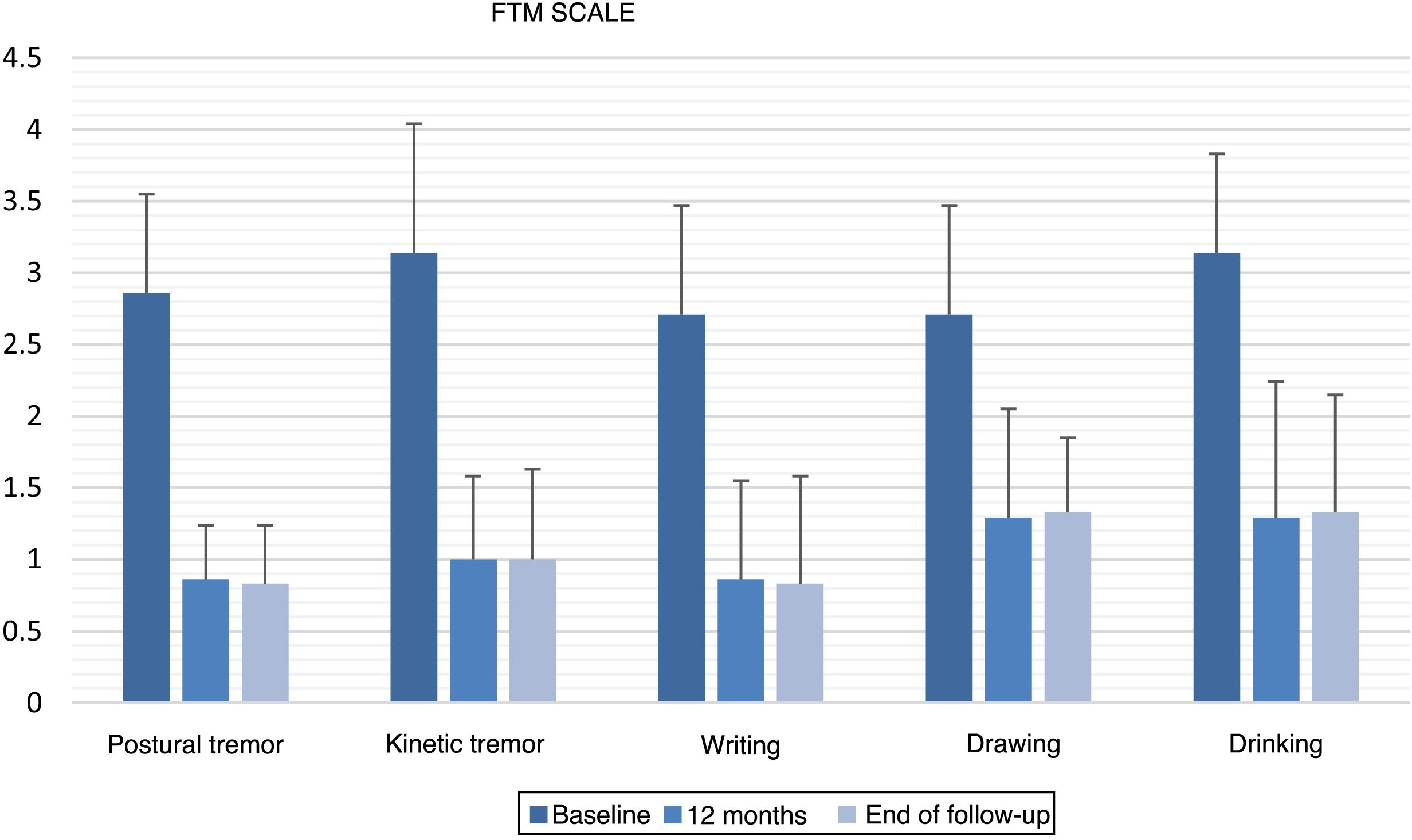
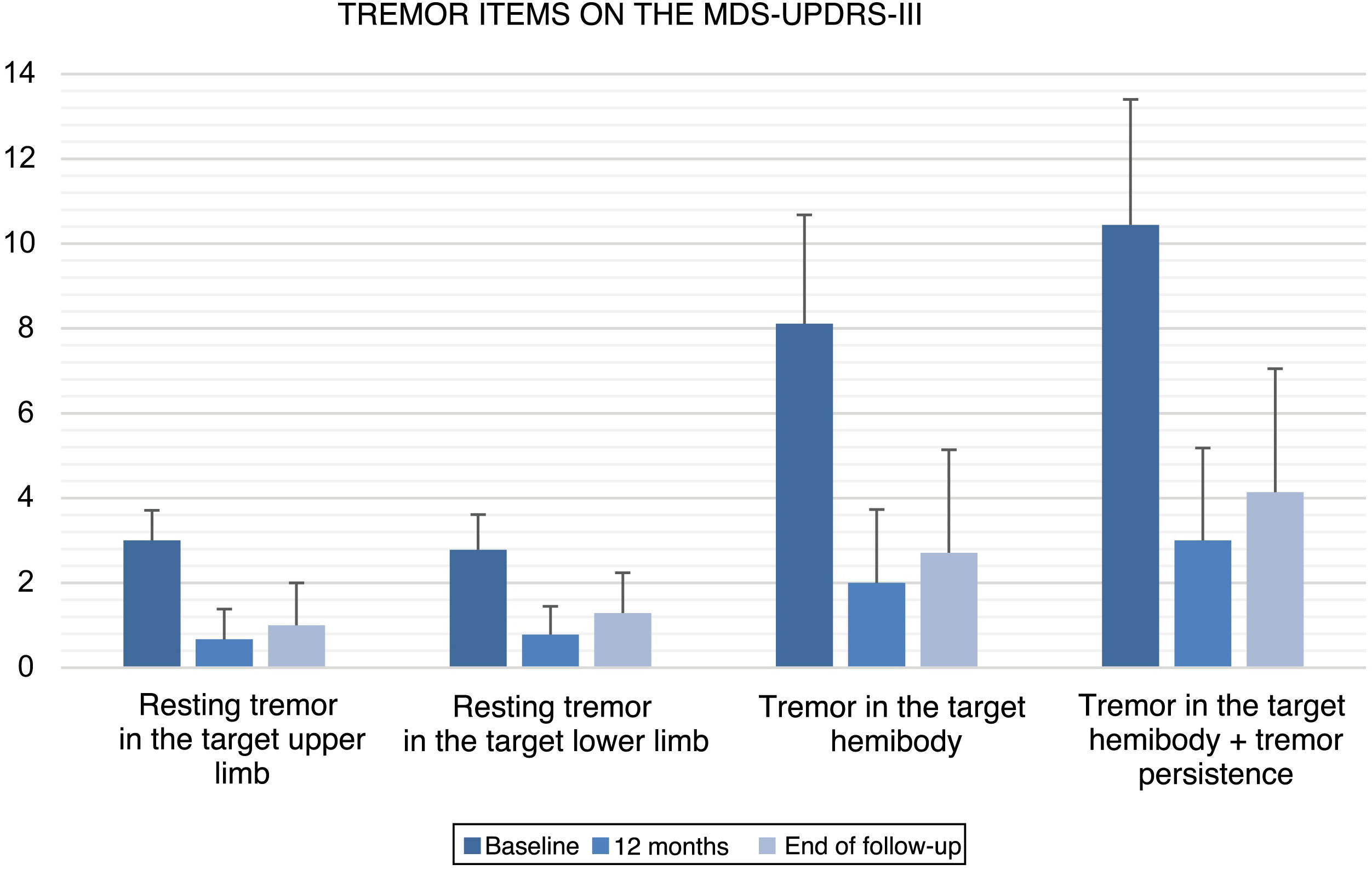
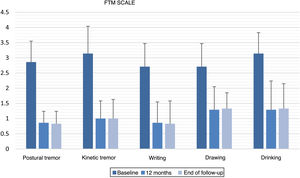
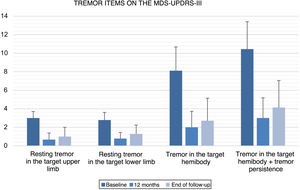
All items of the FTM scale, motor MDS-UPDRS, and VAS of the EuroQoL-5D questionnaire showed significant improvements versus baseline at 12 months after GKT and at the end of follow-up (Table 2). No statistically significant changes were observed between the scores obtained at 12 months and at the end of follow-up. Fig. 1 and 2 show the mean scores and standard deviations obtained for items from the FTM scale and MDS-UPDRS, respectively.
FTM, MDS-UPDRS, and VAS scores at baseline, 12 months after GKT, and the end of follow-up.
| Baseline | 12 m | P (B vs 12 m) | End of follow-up | P (B vs E) | P (12 m vs E) | |
|---|---|---|---|---|---|---|
| Fahn-Tolosa-Marin tremor rating scale (n = 7, ET) | ||||||
| Postural tremor in the dominant hand | 2.86 (0.69) | 0.86 (0.38) | .014 | 0.83 (0.41) | .02 | 1 |
| Kinetic tremor in the dominant hand | 3.14 (0.90) | 1.00 (0.58) | .017 | 1.00 (0.63) | .026 | 1 |
| Writing | 2.71 (0.76) | 0.86 (0.69) | .011 | 0.83 (0.75) | .02 | 1 |
| Drawing (spiral A) | 2.71 (0.76) | 1.29 (0.76) | .015 | 1.33 (0.52) | .023 | .317 |
| Drinking | 3.14 (0.69) | 1.29 (0.95) | .026 | 1.33 (0.82) | .039 | .317 |
| Motor part of the MDS-UPDRS (n = 9; PD) | ||||||
| Resting tremor in the target upper limb | 3.00 (0.71) | 0.67 (0.71) | .007 | 1.00 (1.00) | .027 | .157 |
| Resting tremor in the target lower limb | 2.78 (0.83) | 0.78 (0.67) | .007 | 1.29 (0.95) | .026 | .083 |
| Tremor in the target hemibody | 8.11 (2.57) | 2.00 (1.73) | .008 | 2.71 (2.43) | .027 | .102 |
| Tremor in the target hemibody + persistence | 10.44 (2.96) | 3.00 (2.18) | .008 | 4.14 (2.91) | .027 | .109 |
| VAS (EuroQoL-5D) (n = 13) | 59.23 (7.60) | 80.38 (13.91) | .002 | 80.00 (15.49) | .005 | .059 |
All values are expressed as mean (standard deviation). P-values are calculated with the paired-samples Wilcoxon test.
12 m: 12 months; B: baseline; E: end of study; ET: essential tremor; GKT: Gamma Knife® thalamotomy; PD: Parkinson’s disease; VAS: visual analogue scale.
In patients with ET (n = 7), the intensity of postural tremor and kinetic tremor in the dominant hand was reduced by 70% and 68.2%, respectively, at 12 months after GKT (P = .014; P = .017) and by 71% and 68.2% at the end of follow-up (P = .020; P = .026). Regarding specific manual tasks, writing and spiral drawing improved by 68.4% and 52.6% at 12 months (P = .011; P = .015) and by 69.4% and 50.9% at the end of follow-up (P = .020; P = .023). Regarding the ability to bring fluids to the mouth, we observed improvements of 59.1% at 12 months (P = .026) and 57.6% at the end of follow-up (P = .039). The overall improvement in the FTM items assessed amounted to 63.6% at 12 months and 63.5% at the end of follow-up.
In patients with PD (n = 9), resting tremor in the target upper and lower limbs was reduced by 77.7% and 71.9%, respectively, at 12 months (P = .007; P = .007) and by 66.7% and 53.6% at the end of follow-up (P = .027; P = .026). Regarding tremor scores in the target hemibody (resting tremor, postural tremor, kinetic tremor in the target upper limb, and resting tremor in the target lower limb), we observed an improvement of 75.3% at 12 months (P = .008) and 66.6% at the end of follow-up (P = .027). Hemibody tremor in the motor part of the MDS-UPDRS presented overall improvements of 71.3% at 12 months (P = .008) and 60.3% at the end of follow-up (P = .027). We observed a slight worsening at the end of follow-up with regard to the situation at 12 months after GKT, which was not statistically significant (P = .109; Table 2).
The 11 patients who showed a sustained objective clinical improvement also reported an improvement in quality of life. The remaining 2 patients reported no changes in their quality of life. With respect to baseline, the VAS showed an improvement of +21.2 points at 12 months after GKT (P = .002), which was maintained at the end of follow-up (+20.8; P = .005).
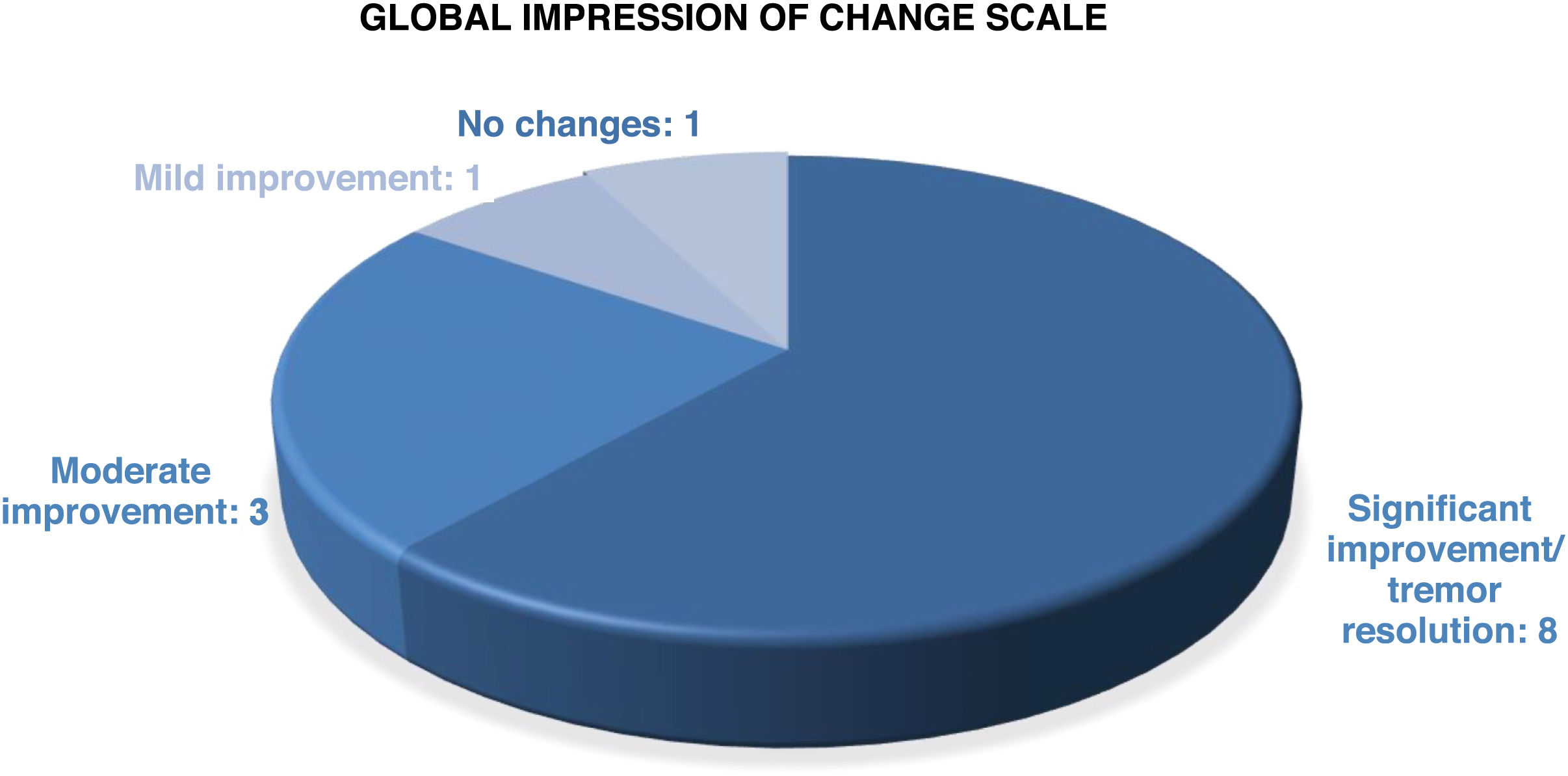
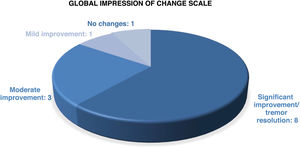
According to the clinical global impression scale (Fig. 3) applied at 12 months after GKT and at the end of follow-up, 11 patients (84.6%) showed a sustained improvement in all scores on tremor scales. Tremor disappeared or symptoms were minimal in 8 patients (61.5%), 6 with ET and 2 with PD. Improvement was moderate in 3 patients (23.1%). An 83-year-old patient with ET since the age of 15 presented a slight improvement in tremor that led to no changes in her quality of life. One patient with ET + PD presented improvement at 3 months after GKT but tremor worsened at 6 months, returning to the baseline status.
Three patients reported mild or transient adverse events, or both. One patient reported transient paraesthesia in the target hand for 2 months, which subsequently disappeared; another patient presented minor cognitive complaints or episodes of forgetfulness, with no changes in the follow-up neuropsychological test with regard to baseline. One patient presented depression with a favourable response to antidepressants. No severe or important adverse effects were observed in any case.
An 81-year-old patient with ET and history of stroke, under treatment with anticoagulants, was referred from an HIFU reference centre as she was not considered a good candidate for that treatment due to her clinical history; she underwent GKT with good results and no adverse effects at 24 months.
A 69-year-old patient diagnosed with PD of 10 years’ progression and treated with a levodopa equivalent daily dose of 900 mg was followed-up for 54 months after GKT; he showed no adverse effects, with a 76.9% decrease in tremor (from 13 to 3 points) in the target hemibody, according to MDS-UPDRS scores at the end of follow-up.
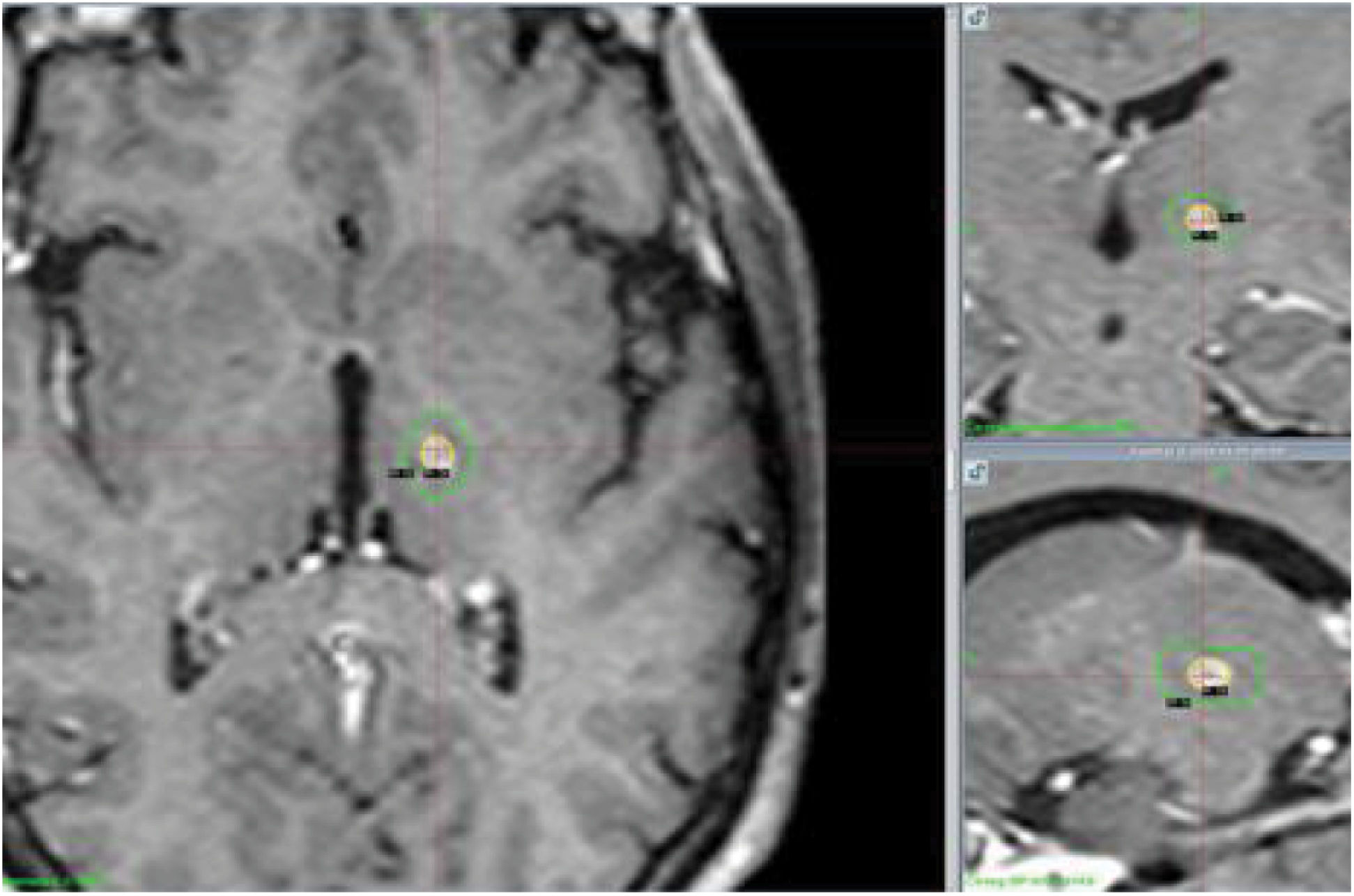
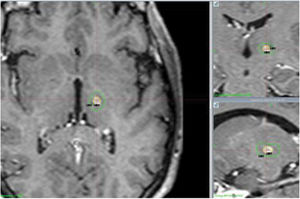
Regarding the findings of the follow-up MRI studies performed at 12 months, lesions were oval-shaped and their centres coincided with the target location in all follow-up studies. The mean volume of T1-hypointense and T2-hyperintense tissue with contrast enhancement was 104 mm3, with a median of 91.5 mm3 (Fig. 4).
DiscussionWe present the largest Spanish series of patients with ET and tremor-predominant PD treated with GKT, assessing effectiveness and safety over a mean follow-up period of 30 months.
GK stereotactic radiosurgery is an ablative, non-incisional, or minimally invasive procedure not requiring open cranial surgery, based on the precise irradiation of well-delimited brain volumes.16 Since the description of the technique by Leksell in the 1950s, it has been used in the treatment of brain tumours, arteriovenous malformations, epilepsy, and trigeminal neuralgia. Since the first GK thalamotomy of a patient with ET in 1992,17 34 articles including 1389 patients with tremor treated with GK have been published,8 with 3 prospective studies being noteworthy.18–20 No randomised clinical trial has been performed. Approximately 82% of patients treated with GKT due to tremor present a clinical improvement8; this is consistent with the results obtained in our series (84.6%). Furthermore, the decrease in tremor observed in our series at 12 months after GKT, 63.3% in patients with ET and 71.3% in patients with PD, is consistent with those reported in other studies.21,22 Furthermore, we observed sustained effectiveness in reducing tremor both in ET and in PD, which was comparable to that observed at 24 months in the study by Ohye et al.19 In the case of patients with PD, we observed a slight worsening in parkinsonian tremor at the end of follow-up with respect to the situation at 12 months; while this difference was not statistically significant, it may reveal a tendency towards reduced effectiveness of the ablative treatment in the long term, or progression of the disease.
Series with larger patient samples describe complications in 1.6% to 16.7% of cases.8 The most severe complications are loss of strength in the side contralateral to the lesion, dysarthria, and dysphagia. When the first GKT procedures were performed, the rate of complications was very high, and high-dose radiation (from 150 Gy) was determined to be unnecessarily toxic.23,24 Thanks to the learning curve, tested protocols have been established for small-volume surgery in healthy tissue, such as the VIM in patients with ET, with doses between 90 and 130 Gy. Two cases of thalamic haemorrhage have been described (out of 1389 reported procedures; 0.14%), one at 14 months20 and the other at 90 months in an 85-year-old patient receiving anticoagulants.25 In our series, we observed no such complications, despite including patients of advanced age, receiving anticoagulants, and with history of stroke. The use of doses no higher than 130 Gy and our centre’s experience in treatment with GK are relevant factors contributing to the level of safety observed. The current level of evidence on stereotactic radiosurgery with GK in the treatment of tremor refractory to pharmacological treatment is class IV.8,18,19
The limitations of our study include its retrospective design, the small patient sample, and the lack of blinding of clinical assessments. We are also unable to rule out future late-onset complications, which have very exceptionally been reported in the literature. A retrospective study of 28 patients with ET with a median follow-up time of 54 (range, 37-152) months reported no late-onset complications.22 The radiation dose used in that study was 130-150 Gy. In our hospital, radiation never exceeds 130 Gy.
Few studies have directly compared GK and other neurosurgical techniques in the treatment of ET.26–28 In conclusion, in neurosurgical treatment of ET, DBS of the VIM is a non-lesional, reversible, incisional technique, frequently requiring bilateral craniotomy. In contrast, thalamotomy of the VIM using GK, radiofrequency, and more recently HIFU are ablative, non-incisional, unilateral techniques; very limited experience is available regarding their bilateral performance, given the potential risks of bilateral thalamotomy.29 DBS is considered the first-line neurosurgical treatment option for ET considering its demonstrated effectiveness in reducing tremor bilaterally, assuming known risks such as cerebral haemorrhage (in 0.5%-1.5% of patients) and infection of the surgical wound (1.7%-5.4%).27 A similar decrease in tremor has been observed using HIFU, GK, and radiofrequency in unilateral thalamotomy,27 with a higher level of evidence at 12 months for HIFU than for GK and radiofrequency, as randomised clinical trials have been conducted for the former technique.30,31 A recent meta-analysis32 on unilateral thalamotomy of the VIM to treat ET reports rates of severe permanent adverse effects such as hemiparesis in patients undergoing radiofrequency (9.3%), GK (1.8%), and HIFU (1.2%). In prospective studies, a total of 18.7% of patients presented permanent adverse reactions, most of which were mild (paraesthesias). One study revealed that targeting the cerebellothalamic tract may be an effective approach with a lower rate of adverse effects in patients undergoing HIFU.33
The advantages of HIFU over GK are the intraoperative control possible and the immediate symptom improvement, whereas with GK, such control is not possible and improvements occur later (at 3-6 months). The advantages of GK over HIFU are the lower cost, the lack of a need for a transcranial window (HIFU requires a minimum skull thickness) or hair shaving, and the possibility of performing the procedure without suspending anticoagulation. Furthermore, the mechanism of GK is believed to involve not only the lesion but also a neuromodulation area surrounding the necrotic core and subnecrotic area; this, together with the absence of an acute lesion, may explain the lower frequency of such adverse effects as ataxia or paraesthesias than with other techniques.24,27,32 Regarding long-term evidence, some retrospective studies indicate sustained effectiveness and safety with GK22,34–36; less evidence is available for HIFU due to its recent appearance.37 In elderly patients with comorbidities and receiving anticoagulation, the safety profile would favour GK27,32; this is reflected in our series.
A comparative cost-effectiveness analysis of DBS, HIFU, and GK in the treatment of ET28 revealed no significant differences in the projected cost of HIFU with regard to GK; cost-effectiveness results favoured HIFU over DBS and GK. However, the results of this study are difficult to extrapolate to our setting.
Indication of GKT in patients with PD is more controversial, as PD is a neurodegenerative disease that progresses bilaterally and axially, and thalamotomy does not improve bradykinesia or gait.19 Therefore, we should mention that GKT should be indicated for patients with asymmetric tremor-predominant PD refractory to antiparkinsonian drugs at optimal doses, with or without motor complications, and presenting mild bradykinesia, as well as in cases of contraindication or rejection of DBS. Given the phenotypic heterogeneity of PD, current research is focusing on other therapeutic targets for non-incisional stereotactic surgery, such as the subthalamic nucleus38,39 and the internal globus pallidus.40
ConclusionsOur findings and the literature lead us to conclude that Gamma Knife® stereotactic radiosurgery performed unilaterally on the VIM at doses of 130 Gy at experienced centres presents sustained effectiveness and safety for refractory ET and PD-associated tremor, even in patients of advanced age, receiving anticoagulation, or with history of stroke, which are contraindications for DBS.
Randomised studies are needed to confirm the effectiveness and safety of GKT in patients with refractory ET and PD-associated tremor, as well as comparative studies to assess the effectiveness, safety, and cost of DBS, HIFU, and GKT.
FundingThis study has received no private or public funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.